Lingonberry Fruit Ethanol Extract Ameliorates DSS-Induced Ulcerative Colitis In Vivo and In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of LB Extract
2.3. Animals
2.4. Induction of Colitis
2.5. Disease Activity Index (DAI)
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Western Blotting Analysis
2.8. Hematoxylin and Eosin Staining (H&E Staining)
2.9. Immunohistochemistry (IHC)
2.10. Prostaglandin (PG)E2 Assay
2.11. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.12. Determination of Myeloperoxidase (MPO) Activity
2.13. Isolation and Culture of Peritoneal Macrophages
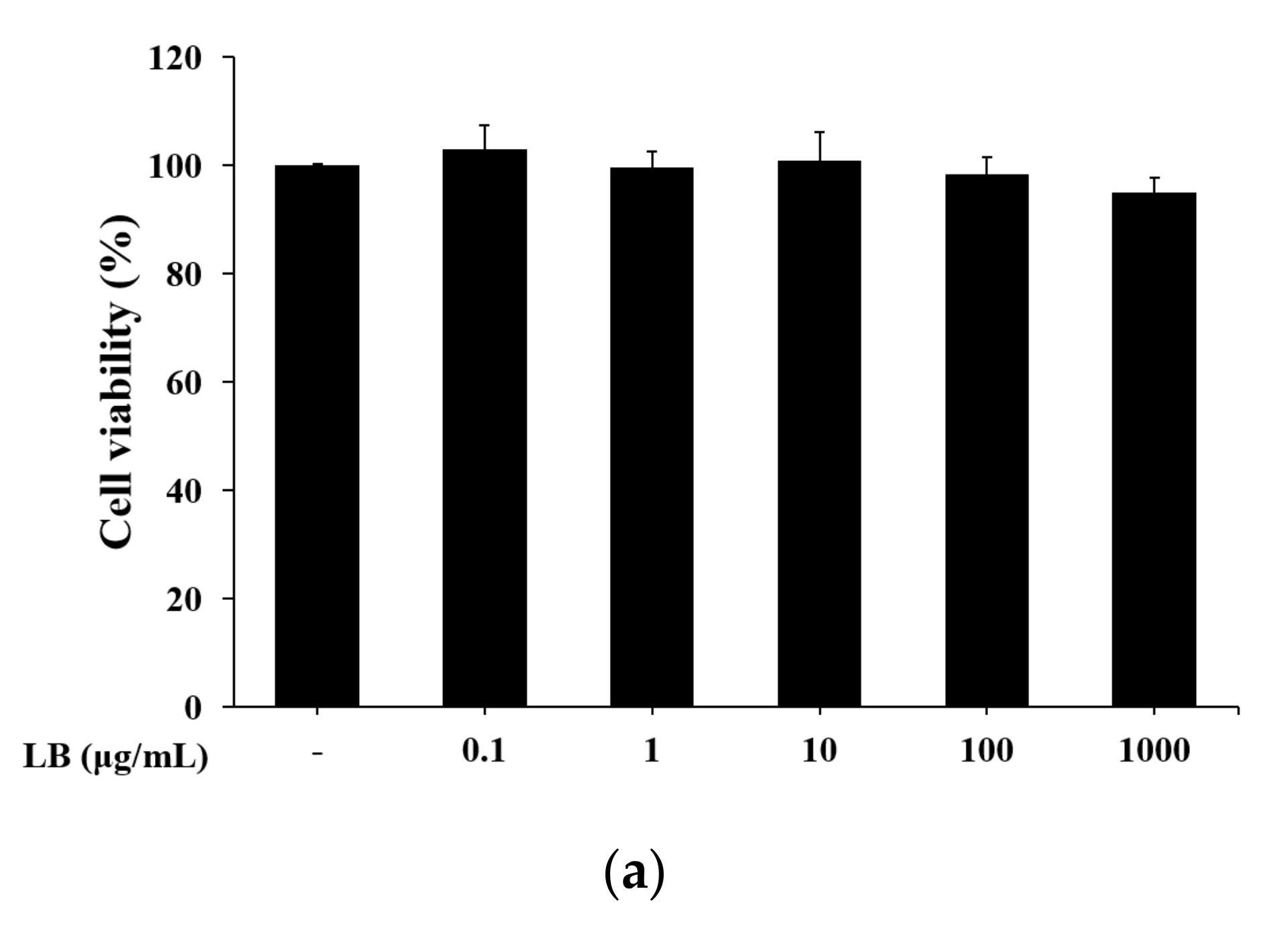
2.14. Cell Viability Assay
2.15. Measurement of Nitric Oxide Level
2.16. Statistical Analysis
3. Results
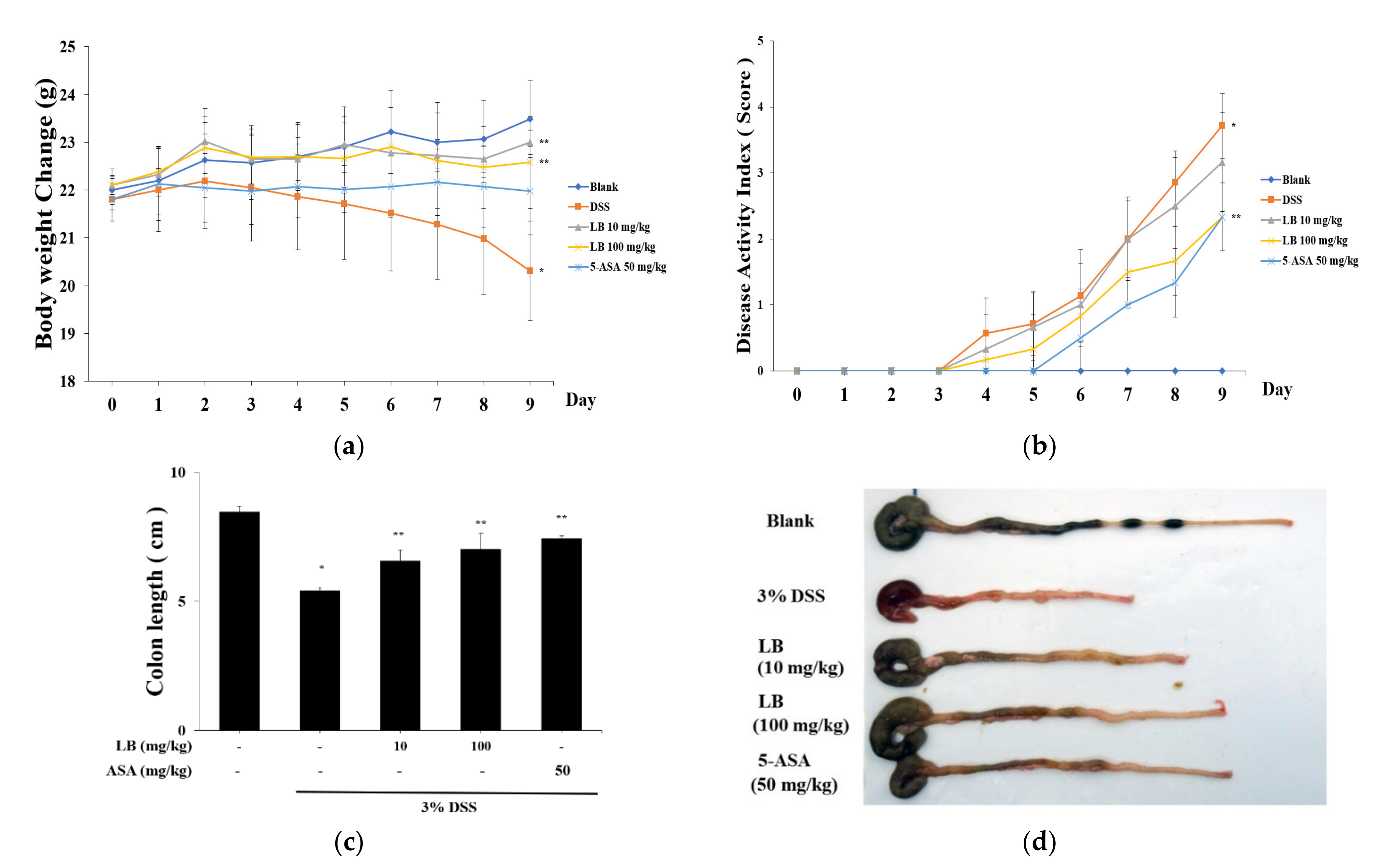
3.1. Effect of LB on Clinical Signs in DSS-Induced UC Mice
3.2. LB Administration Recovered Colon Length Shortening
3.3. LB Regulates the Histological Injury and MPO Activity
3.4. LB Decreases the Secretion of Inflammatory Cytokines in DSS-Induced UC Mouse Serum
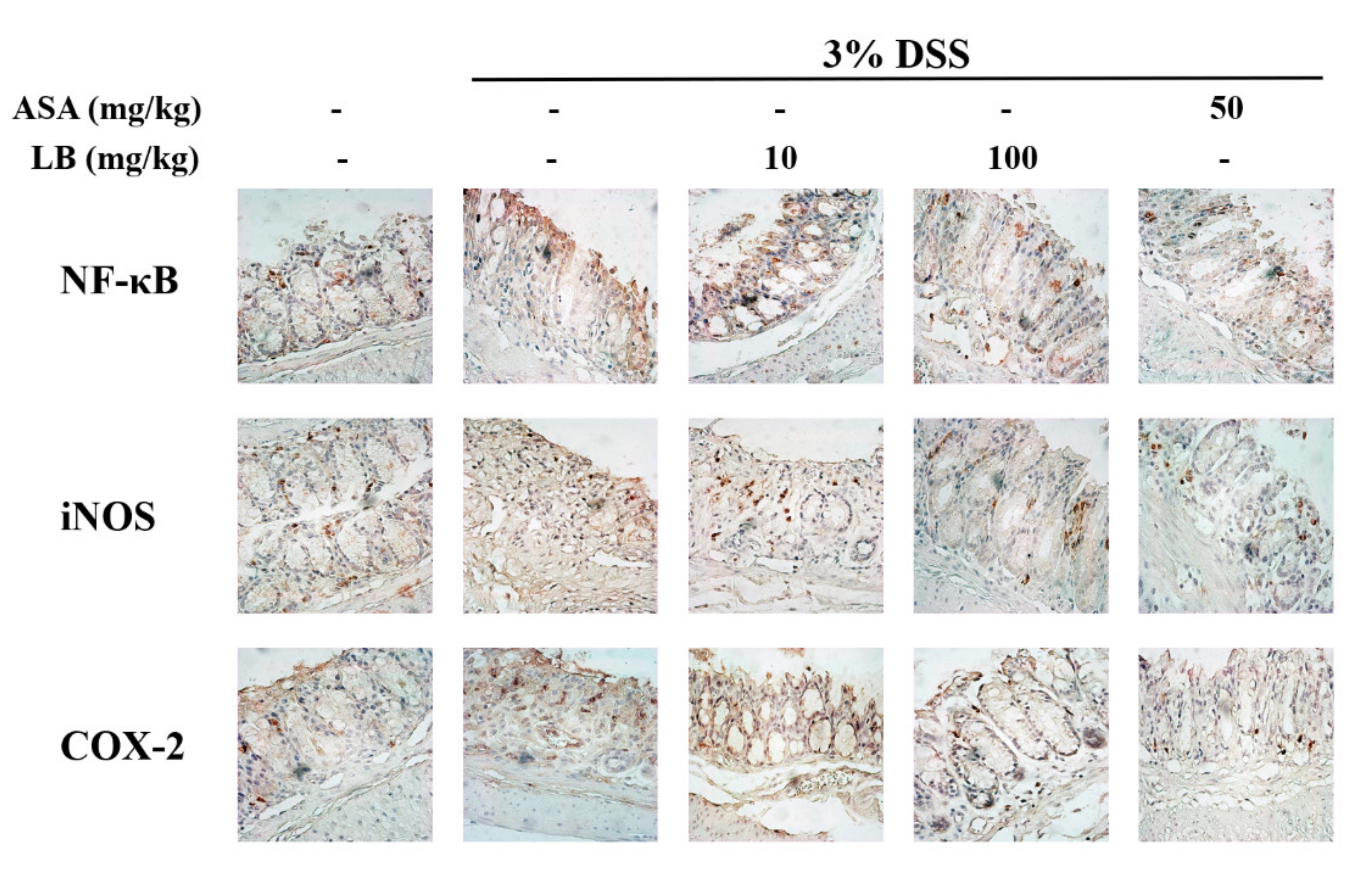
3.5. LB Attenuates the Expression of Cyclooxigenase-2(COX-2), NF-κB, and iNOS in DSS-Induced UC Mice
3.6. LB Regulates the mRNA Expression of Inflammatory Cytokines in LPS-Stimulated Peritoneal Mouse Macrophages
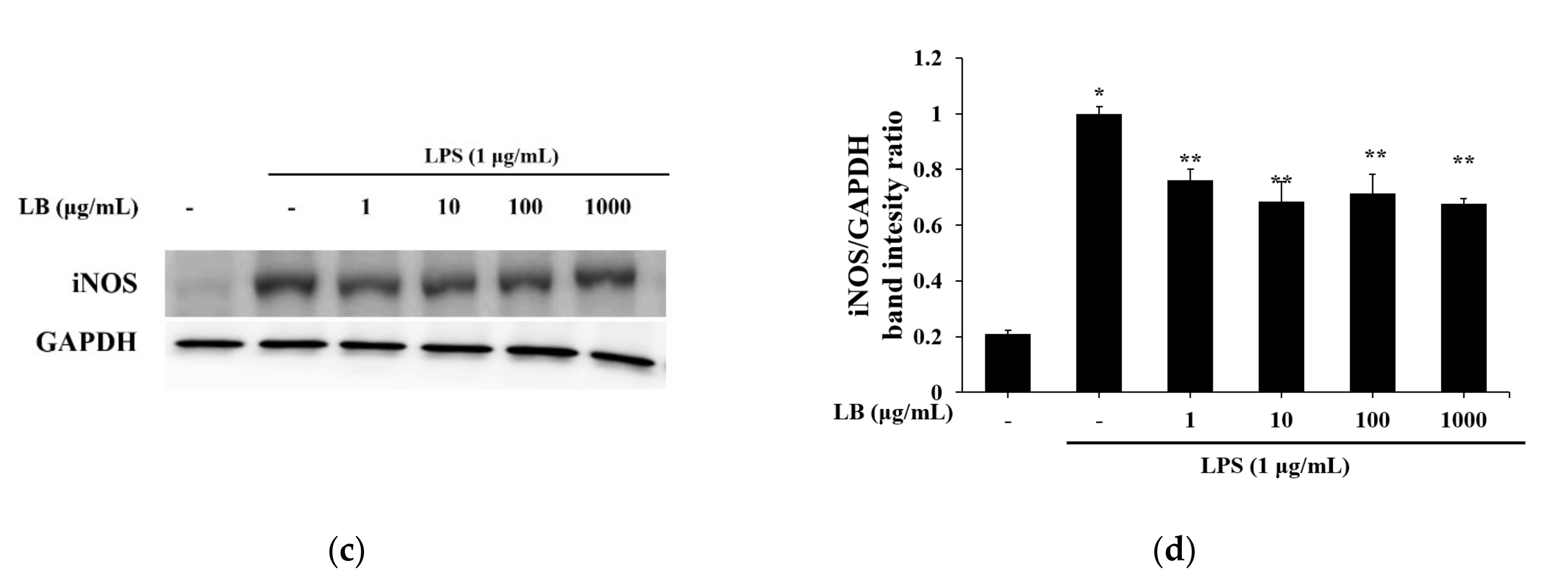
3.7. LB Regulates the NO Production in LPS-Stimulated Peritoneal Mouse Macrophages
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cosnes, J.; Gower-Rousseau, C.; Seksik, P.; Cortot, A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011, 140, 1785–1794. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Carding, S.R. Inflammatory bowel disease: Cause and immunobiology. Lancet 2007, 369, 1627–1640. [Google Scholar] [CrossRef]
- Kornbluth, A.; Sachar, D.B. Ulcerative colitis practice guidelines in adults (update): American College of Gastroenterology, Practice Parameters Committee. Am. J. Gastroenterol. 2004, 99, 1371–1385. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Zhang, S.; Wang, C.; Zhao, W.; Shen, H. Health-related quality of life in Chinese patients with mild and moderately active ulcerative colitis. PLoS ONE 2015, 10, e0124211. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, K.A.; Targan, S.R. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu. Rev. Med. 2000, 51, 589–598. [Google Scholar] [CrossRef]
- Yu, Y.R.; Rodriguez, J.R. Clinical presentation of Crohn’s, ulcerative colitis, and indeterminate colitis: Symptoms, extra intestinal manifestations, and disease phenotypes. Semin. Pediatric Surg. 2017, 26, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Gilardi, D.; Fiorino, G.; Genua, M.; Allocca, M.; Danese, S. Complementary and alternative medicine in inflammatory bowel diseases: What is the future in the field of herbal medicine? Expert Rev. Gastroenterol. Hepatol. 2014, 8, 835–846. [Google Scholar] [CrossRef]
- Jeon, Y.D.; Bang, K.S.; Shin, M.K.; Lee, J.H.; Chang, Y.N.; Jin, J.S. Regulatory effects of glycyrrhizae radix extract on DSS-induced ulcerative colitis. BMC Complementary Altern. Med. 2016, 16, 459. [Google Scholar] [CrossRef] [Green Version]
- Toiyama, Y.; Mizoguchi, A.; Yoshinaga, O.; Koike, Y.; Morimoto, Y.; Araki, T.; Uchida, K.; Tanaka, K.; Nakashima, H.; Hidi, M.; et al. Intravital imaging of DSS-induced cecal damage in GFP-transgenic mice using two-photon microscopy. J. Gastoenterol. 2010, 45, 544–553. [Google Scholar] [CrossRef]
- Ovaskainen, M.L.; Törrönen, R.; Koponen, J.M.; Sinkko, H.; Hellström, J.; Reinivuo, H.; Mattila, P. Dietary intake and major food sources of polyphenols in Finnish adults. J. Nutr. 2008, 138, 562–566. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef]
- Mane, C.; Loonis, M.; Juhel, C.; Dufour, C.; Malien-Aubert, C. Food grade lingonberry extract: Polyphenolic composition and in vivo protective effect against oxidative stress. J. Agric. Food Chem. 2011, 59, 3330–3339. [Google Scholar] [CrossRef]
- Heyman, L.; Axling, U.; Blanco, N.; Sterner, O.; Holm, C.; Berger, K. Evaluation of beneficial metabolic effects of berries in high-fat fed C57BL/6J mice. J. Nutr. Metab. 2014, 2014, 403041. [Google Scholar] [CrossRef]
- Ryyti, R.; Hamalainen, M.; Peltola, R.; Moilanen, E. Beneficial effects of lingonberry (Vaccinium vitis-idaea L.) supplementation on metabolic and inflammatory adverse effects induced by high-fat diet in a mouse model of obesity. PLoS ONE 2020, 15, e0232605. [Google Scholar] [CrossRef]
- Wang, S.Y.; Feng, R.; Bowman, L.; Penhallegon, R.; Ding, M.; Lu, Y. Antioxidant activity in lingonberries (Vaccinium vitis-idaea L.) and its inhibitory effect on activator protein-1, nuclear factor-κB, and mitogen-activated protein kinases activation. J. Agric. Food Chem. 2005, 53, 3156–3166. [Google Scholar] [CrossRef] [PubMed]
- Kivimäki, A.S.; Siltari, A.; Ehlers, P.I.; Korpel, R.; Vapaatalo, H. Lingonberry juice negates the effects of a high salt diet on vascular function and low-grade inflammation. J. Funct. Foods 2014, 7, 238–245. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Rupasinghe, H.P.V. Antioxidant and cytoprotective properties of partridgeberry polyphenols. Food Chem. 2015, 169, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Tuulia, O.; Anne, K.; Matti, M.; Tuula, S.; Riitta, K. Anticancer effects of Lingonberry and bilberry on digestive tract cancers. Antioxidants 2021, 10, 850. [Google Scholar]
- Mary, H.G.; Debora, E.; Kriya, L.D.; Mary, A.L. Comparative analysis of phenolic content and profile, antioxidant capacity, and anti-inflammatory bioactivity in wild Alaskan and commercial Vaccinium berries. J. Agric. Food Chem. 2014, 62, 4007–4017. [Google Scholar]
- Oqata, H.; Hibi, T. Cytokine and anti-cytokine therapies for inflammatory bowel disease. Curr. Pharm. Des. 2003, 9, 1107–1113. [Google Scholar] [CrossRef]
- Kihara, N.; de la Fuente, S.G.; Fujino, K.; Takahashi, T.; Pappas, T.N.; Mantyh, C.R. Vanilloid receptor-1 containing primary sensory neurones mediate dextran sulphate sodium induced colitis in rats. Gut 2003, 52, 713–719. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, T.; Ma, C.; Wang, S. Puerarin attenuates airway inflammation by regulation of eotaxin-3. Immunol. Lett. 2015, 163, 173–178. [Google Scholar] [CrossRef]
- Dharmani, P.; Chadee, K. Biologic therapies against inflammatory bowel disease: A dysregulated immune system and the cross talk with gastrointestinal mucosa hold the key. Curr. Mol. Pharmacol. 2008, 1, 195–212. [Google Scholar] [CrossRef]
- Strober, W.; Fuss, I.; Mannon, P. The fundamental basis of inflammatory bowel disease. J. Clin. Investig. 2007, 117, 514–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.H.; Xu, Z.L.; Dong, D.; He, S.A.; Yu, H. Protective effect of anthocyanins extract from blueberry on TNBS-induced IBD model of mice. Evid.-Based Complementary Altern. Med. 2011, 2011, 525462. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Targan, S.R. Biologic therapy of inflammatory bowel disease. Gastroenterology 2002, 122, 1592–1608. [Google Scholar] [CrossRef] [PubMed]
- Rufo, P.A.; Bousvaros, A. Current therapy of inflammatory bowel disease in children. Pediatric Drugs 2006, 8, 279–302. [Google Scholar] [CrossRef] [PubMed]
- Morita, I. Distinct functions of COX-1 and COX-2. Prostaglandins Other Lipid Mediat. 2002, 68, 165–175. [Google Scholar] [CrossRef]
- Wiercińska-Drapało, A.; Flisiak, R.; Prokopowicz, D. Effects of ulcerative colitis activity on plasma and mucosal prostaglandin E2 concentration. Prostaglandins Other Lipid Mediat. 1999, 58, 159–165. [Google Scholar] [CrossRef]
- Lauritsen, K.; Laursen, L.S.; Kjeldsen, J.; Bukhave, K.; Hansen, T.K.; Rask-Madsen, J. Effects of mesalazine on the formation of lipoxygenase and cyclooxygenase products. Adv. Exp. Med. Biol. 1995, 371, 1301–1306. [Google Scholar]
- Chen, C.C.; Sun, Y.T.; Chen, J.J.; Chiu, K.T. TNF-α-induced cyclooxygenage-2 expression in human lung epithelial cells: Involvement of the phospholipase C-γ2, protein kinase C-α, tyrosine kinase, NF-κB-inducing kinase, and I-κB kinase 1/2 pathway. J. Immunol. 2000, 165, 2719–2728. [Google Scholar] [CrossRef] [Green Version]
- Coskun, M. Intestinal epithelium in inflammatory bowel disease. Front. Med. 2014, 2014, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.S.; Lee, H.E.; Yang, H.K.; Kim, W.H. High lactate dehydrogenase 5 expression correlates with high tumoral and stromal vascular endothelial growth factor expression in gastric cancer. Pathobiology 2014, 81, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Kovacs-Nolan, J.; Zhang, H.; Ibuki, M.; Nakamori, T.; Yoshiura, K.; Turner, P.V.; Matsui, T.; Mine, Y. The PepT1-transportable soy tripeptide VPY reduces intestinal inflammation. Biochim. Biophys. Acta 2012, 1820, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Rose-John, S.; Waetzig, G.H.; Scheller, J.; Grötzinger, J.; Seegert, D. The IL-6/sIL-6R complex as a novel target for therapeutic approaches. Expert Opin. Ther. Targets 2007, 11, 613–624. [Google Scholar] [CrossRef]
- Locksley, R.M.; Killeen, N.; Lenardo, M.J. The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell 2001, 104, 487–501. [Google Scholar] [CrossRef] [Green Version]
- Abraham, C.; Medzhitov, R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology 2011, 140, 1729–1737. [Google Scholar] [CrossRef] [Green Version]




| Score | Weight Loss (%) | Stool Consistency | Gross Bleeding |
|---|---|---|---|
| 0 | None | Normal | Negative |
| 1 | 1–5 | Loose stool | Negative |
| 2 | 5–10 | Loose stool | Positive |
| 3 | 10–20 | Diarrhea | Positive |
| 4 | >20 | Diarrhea | Gross bleeding |
| Score | Remarks |
|---|---|
| 0 | Normal colonic mucosa |
| 1 | Loss of one-third of the crypts |
| 2 | Loss of two-thirds of the crypts |
| 3 | Lamina propria covered with single layer of epithelial cells with mild inflammation cell infiltration |
| 4 | Erosions and marked inflammatory cell infiltration |
| Gene | Forward | Reverse |
|---|---|---|
| Mouse IL-1β | CCTTCCAGGATGAGGACATGA | TGAGTCACAGAGGATGGGCTC |
| Mouse IL-6 | GAGGATACCACTCCCAACAGACC | AAGTGCATCATCGTTGTTCATACA |
| Mouse TNF-α | CAGGCGGTGCCTATGTCTC | CGATCACCCCGAAGTTCAGTAG |
| Mouse IFN-γ | ACACTGCATCTTGGCTTTGC | CCAGTTCCTCCAGATATCCA |
| Mouse iNOS | CAGCTGGGCTGTACAAAC | CATTGGAAGTGAAGCGATTCG |
| Mouse GAPDH | CCATCACCATCTTCCAGGAG | CCTGCTTCACCACCTTCTTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, Y.-D.; Lee, J.-H.; Kang, S.-H.; Myung, H.; Jin, J.-S. Lingonberry Fruit Ethanol Extract Ameliorates DSS-Induced Ulcerative Colitis In Vivo and In Vitro. Appl. Sci. 2021, 11, 7955. https://doi.org/10.3390/app11177955
Jeon Y-D, Lee J-H, Kang S-H, Myung H, Jin J-S. Lingonberry Fruit Ethanol Extract Ameliorates DSS-Induced Ulcerative Colitis In Vivo and In Vitro. Applied Sciences. 2021; 11(17):7955. https://doi.org/10.3390/app11177955
Chicago/Turabian StyleJeon, Yong-Deok, Ji-Hyun Lee, Sa-Haeng Kang, Hyun Myung, and Jong-Sik Jin. 2021. "Lingonberry Fruit Ethanol Extract Ameliorates DSS-Induced Ulcerative Colitis In Vivo and In Vitro" Applied Sciences 11, no. 17: 7955. https://doi.org/10.3390/app11177955
APA StyleJeon, Y.-D., Lee, J.-H., Kang, S.-H., Myung, H., & Jin, J.-S. (2021). Lingonberry Fruit Ethanol Extract Ameliorates DSS-Induced Ulcerative Colitis In Vivo and In Vitro. Applied Sciences, 11(17), 7955. https://doi.org/10.3390/app11177955







