Copper Nanowires for Transparent Electrodes: Properties, Challenges and Applications
Abstract
:1. Introduction
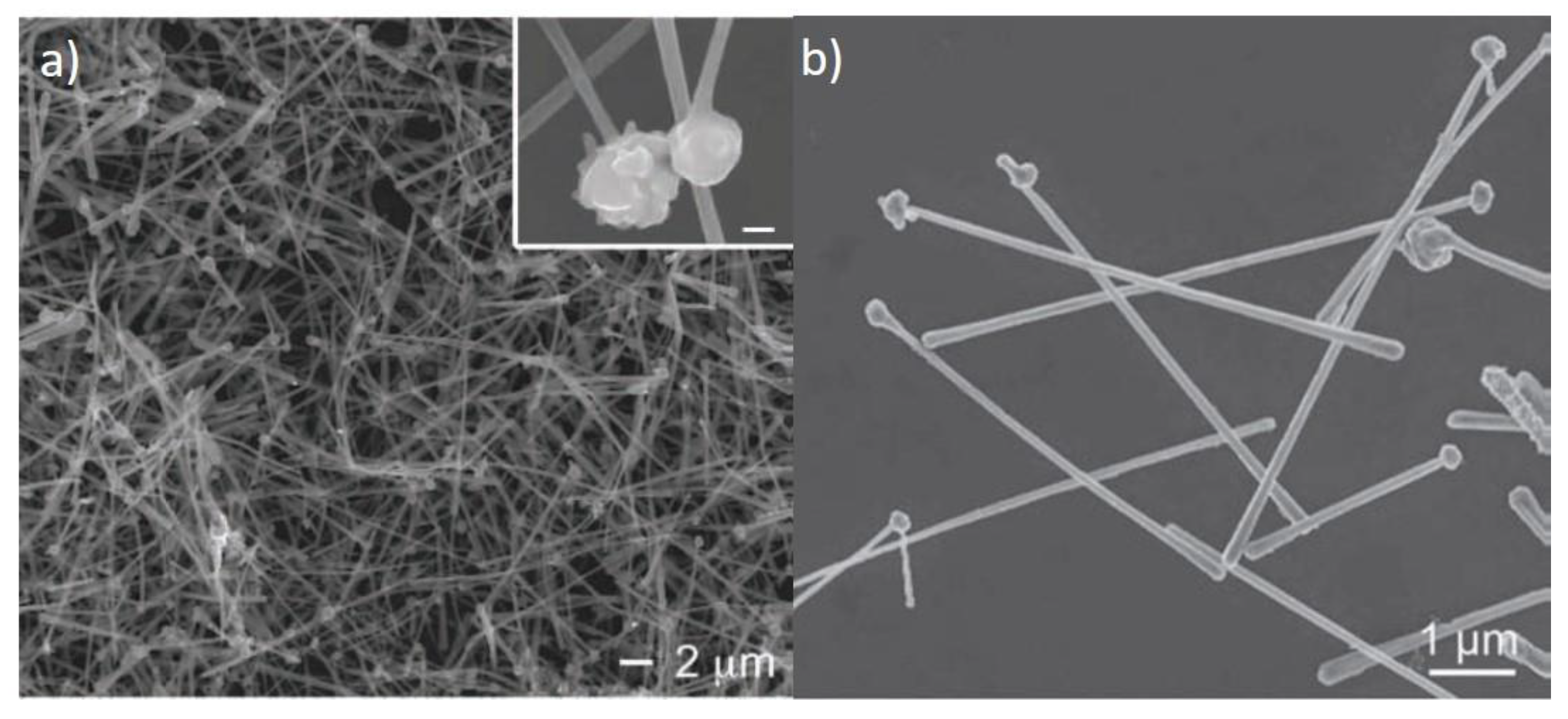
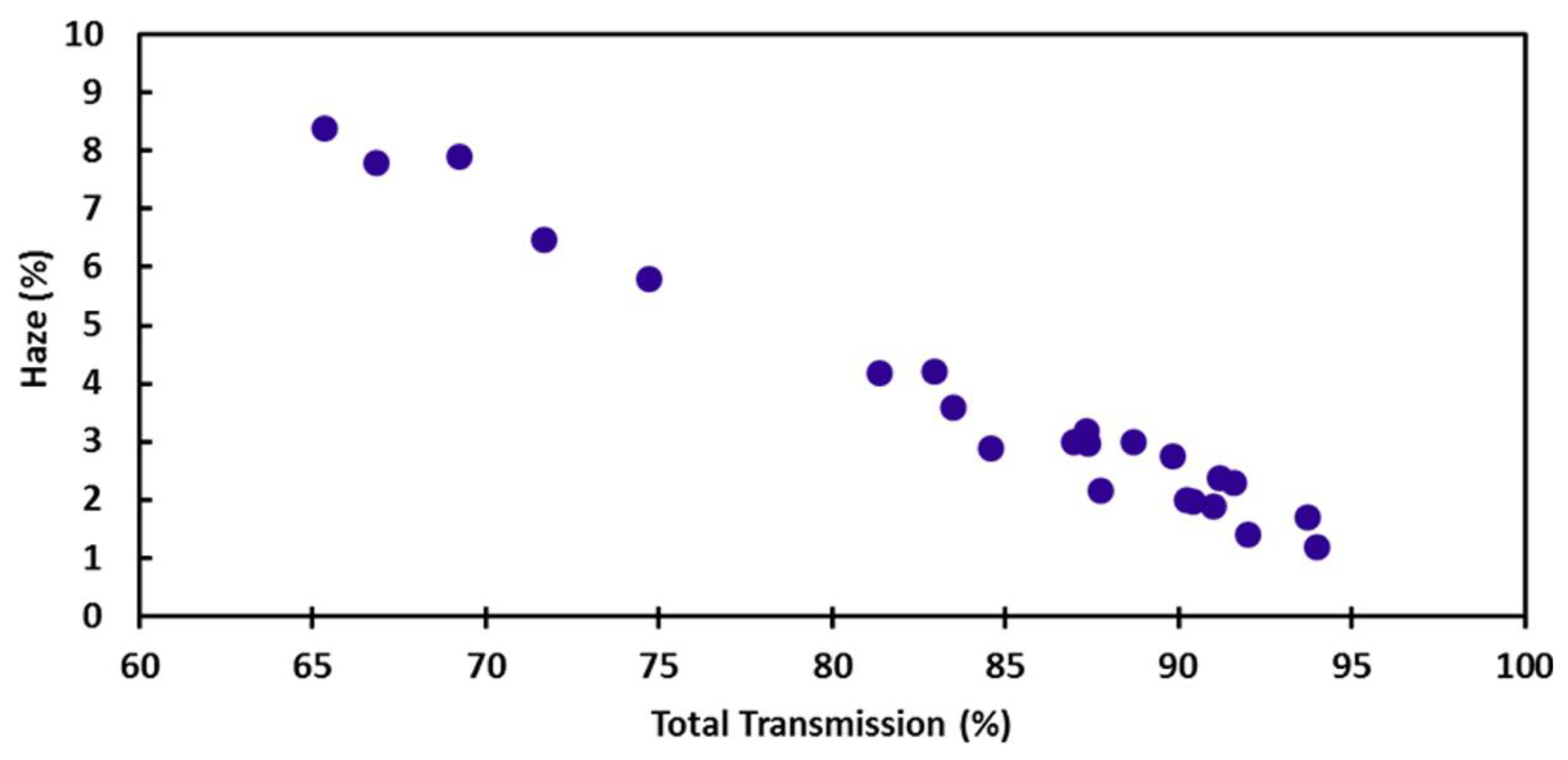
2. Electrical and Optical Properties of Cu NWs
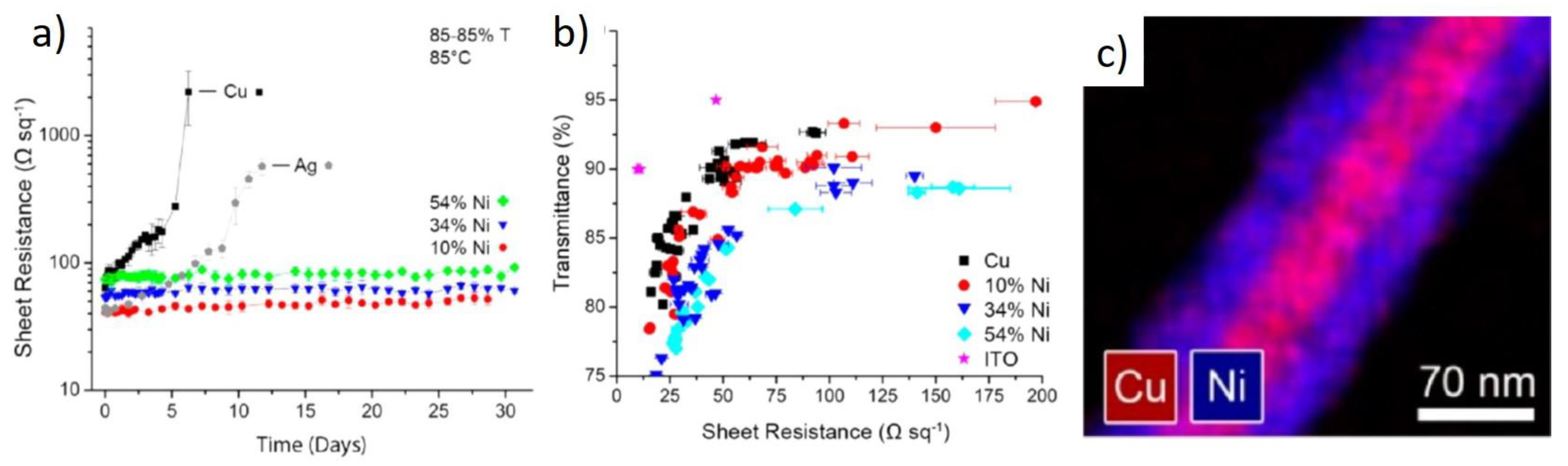
3. Oxidation Prevention
4. Joining of Nanowires
5. Mechanical Properties
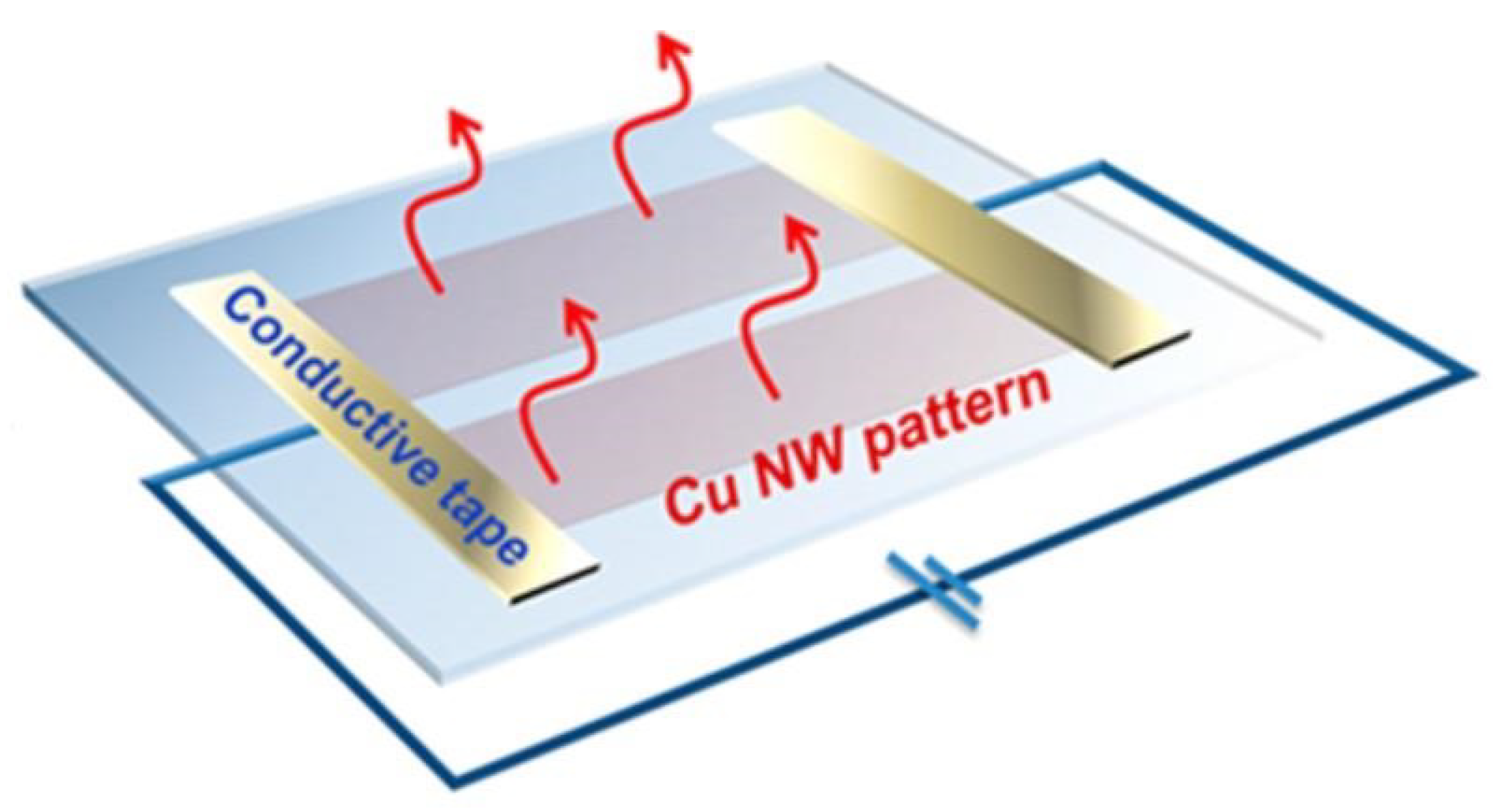
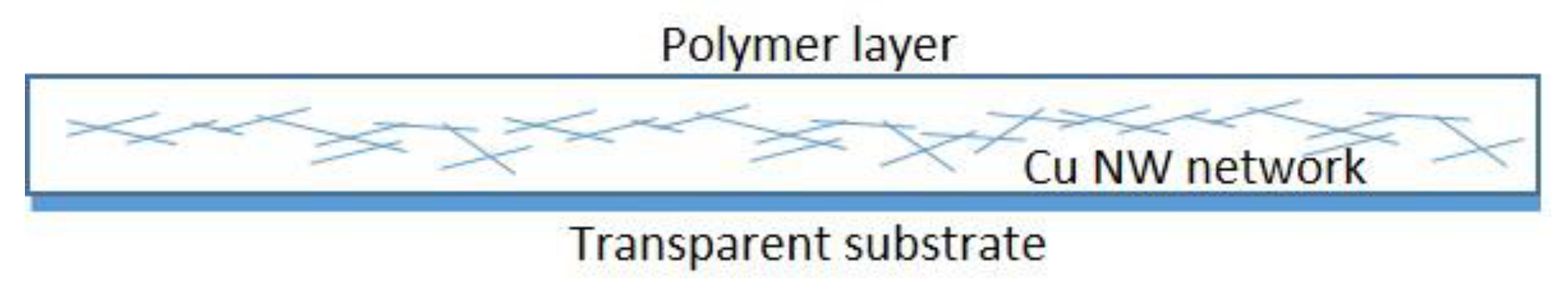
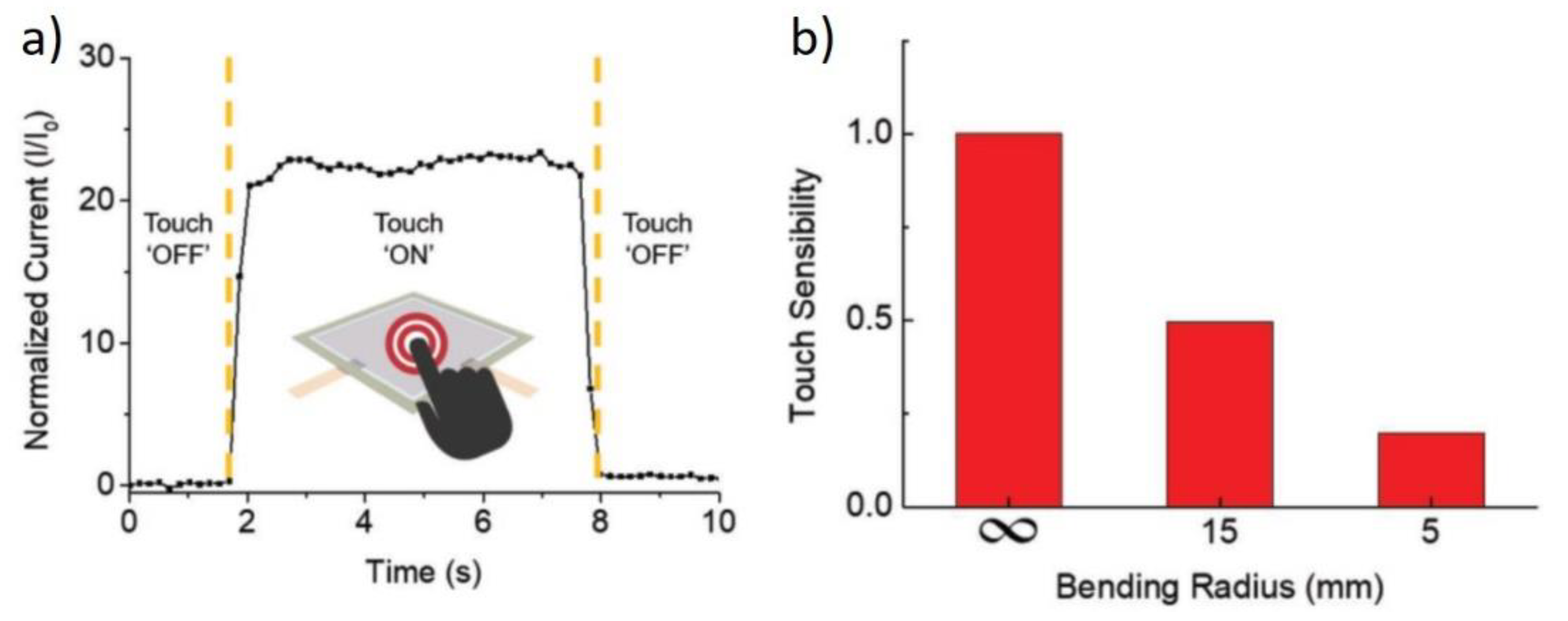
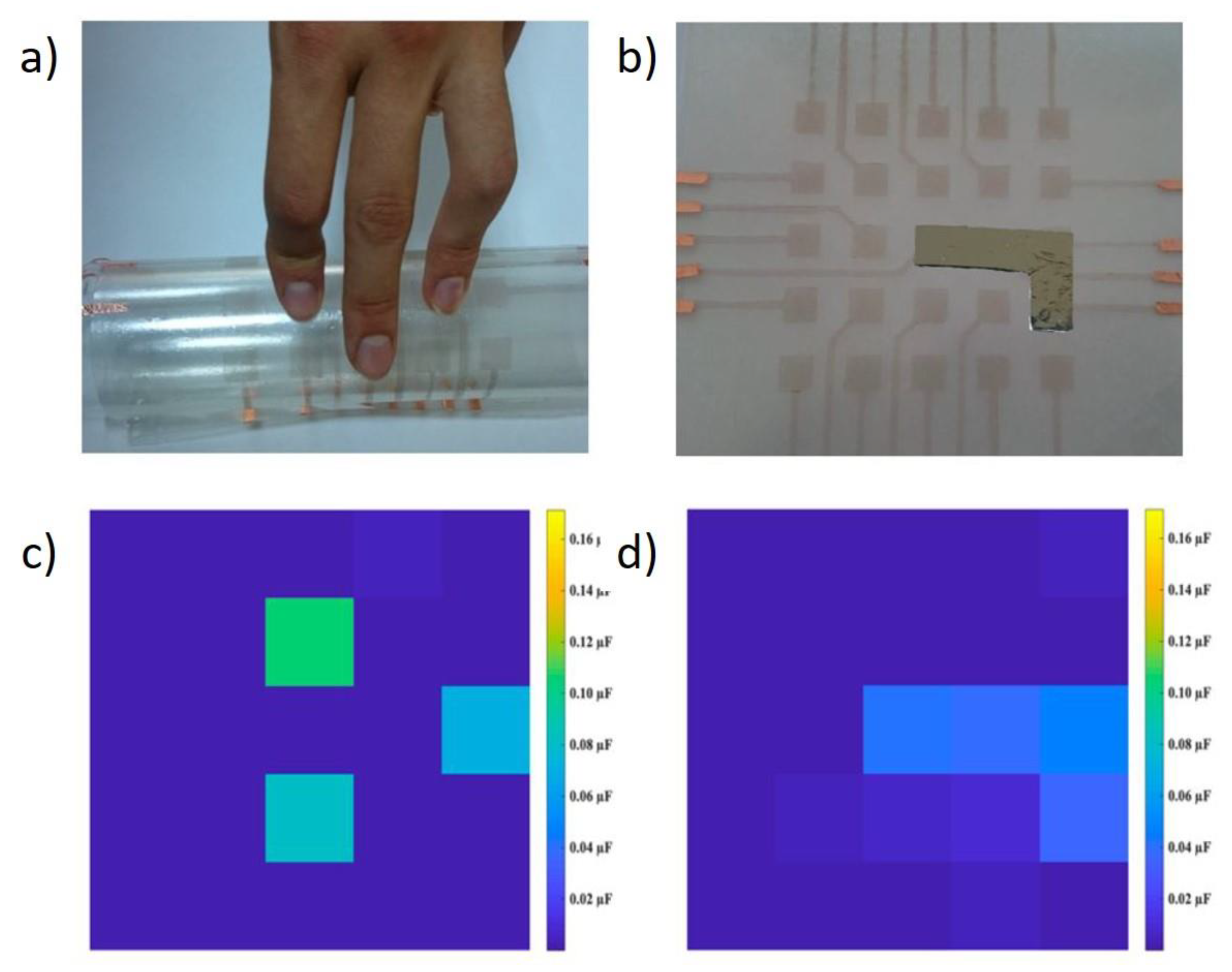
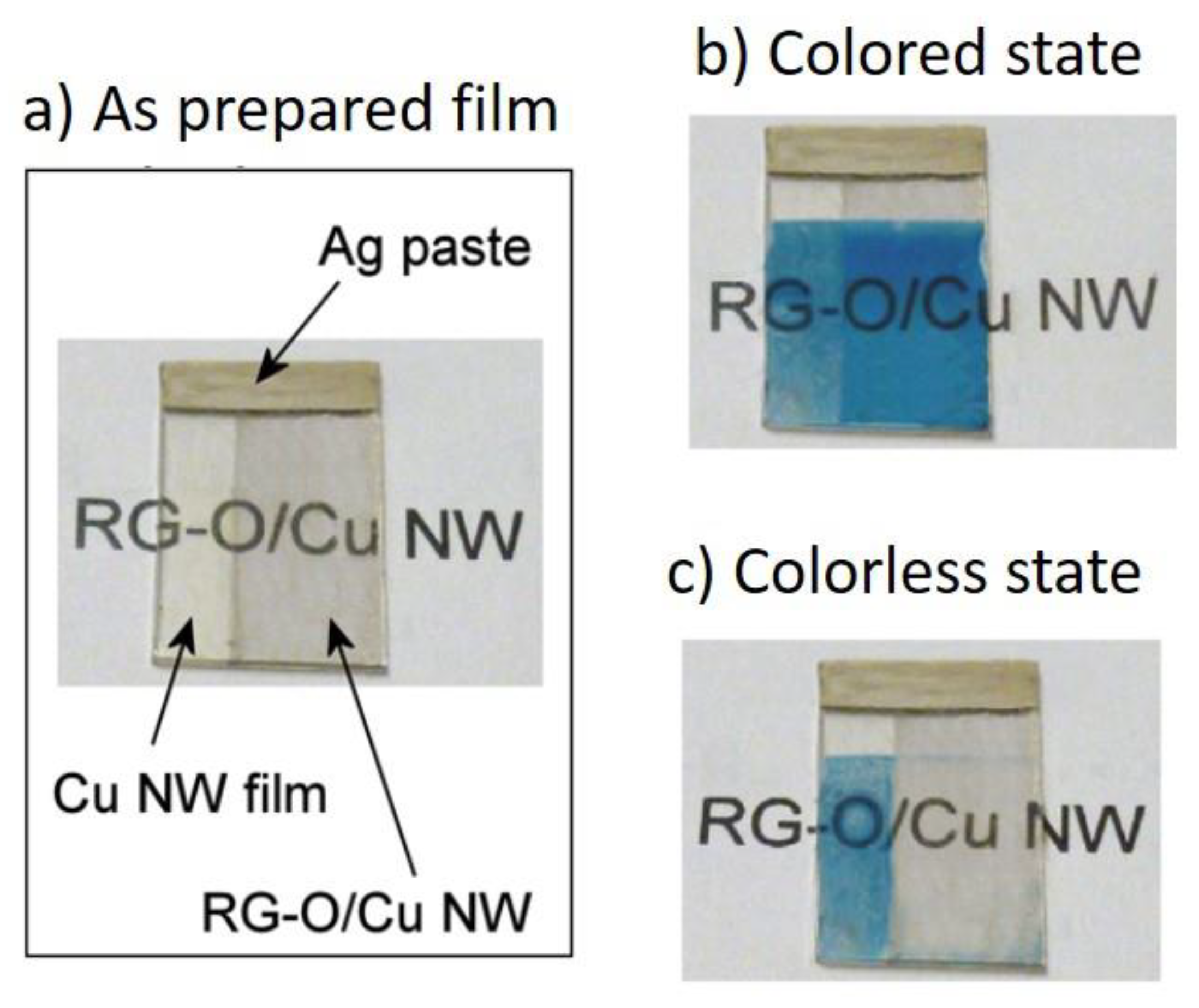
6. Applications of Copper Nanowires
7. Conclusions and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gordon, R.G. Criteria for Choosing Transparent Conductors. MRS Bull. 2000, 25, 52–57. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Cotterell, B.; Wang, W. The fracture of brittle thin films on compliant substrates in flexible displays. Eng. Fract. Mech. 2002, 69, 597–603. [Google Scholar] [CrossRef]
- Leterrier, Y.; Médico, L.; Demarco, F.; Månson, J.A.E.; Betz, U.; Escolà, M.F.; Kharrazi Olsson, M.; Atamny, F. Mechanical integrity of transparent conductive oxide films for flexible polymer-based displays. Thin Solid Film. 2004, 460, 156–166. [Google Scholar] [CrossRef]
- Rowell, M.W.; McGehee, M.D. Transparent electrode requirements for thin film solar cell modules. Energy Environ. Sci. 2011, 4, 131–134. [Google Scholar] [CrossRef]
- Barnes, T.M.; van de Lagemaat, J.; Levi, D.; Rumbles, G.; Coutts, T.J.; Weeks, C.L.; Britz, D.A.; Levitsky, I.; Peltola, J.; Glatkowski, P. Optical characterization of highly conductive single-wall carbon-nanotube transparent electrodes. Phys. Rev. B 2007, 75, 235410. [Google Scholar] [CrossRef]
- Geng, H.-Z.; Kim, K.K.; So, K.P.; Lee, Y.S.; Chang, Y.; Lee, Y.H. Effect of Acid Treatment on Carbon Nanotube-Based Flexible Transparent Conducting Films. J. Am. Chem. Soc. 2007, 129, 7758–7759. [Google Scholar] [CrossRef]
- Doherty, E.M.; De, S.; Lyons, P.E.; Shmeliov, A.; Nirmalraj, P.N.; Scardaci, V.; Joimel, J.; Blau, W.J.; Boland, J.J.; Coleman, J.N. The spatial uniformity and electromechanical stability of transparent, conductive films of single walled nanotubes. Carbon 2009, 47, 2466–2473. [Google Scholar] [CrossRef]
- Scardaci, V.; Coull, R.; Coleman, J.N. Very thin transparent, conductive carbon nanotube films on flexible substrates. Appl. Phys. Lett. 2010, 97, 023114. [Google Scholar] [CrossRef]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.-S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Ri Kim, H.; Song, Y.I.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De, S.; King, P.J.; Lotya, M.; O’Neill, A.; Doherty, E.M.; Hernandez, Y.; Duesberg, G.S.; Coleman, J.N. Flexible, Transparent, Conducting Films of Randomly Stacked Graphene from Surfactant-Stabilized, Oxide-Free Graphene Dispersions. Small 2010, 6, 458–464. [Google Scholar] [CrossRef]
- Becerril, H.A.; Mao, J.; Liu, Z.; Stoltenberg, R.M.; Bao, Z.; Chen, Y. Evaluation of Solution-Processed Reduced Graphene Oxide Films as Transparent Conductors. ACS Nano 2008, 2, 463–470. [Google Scholar] [CrossRef]
- Wang, X.; Zhi, L.; Müllen, K. Transparent, Conductive Graphene Electrodes for Dye-Sensitized Solar Cells. Nano Lett. 2008, 8, 323–327. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Higgins, T.M.; Lyons, P.E.; Doherty, E.M.; Nirmalraj, P.N.; Blau, W.J.; Boland, J.J.; Coleman, J.N. Silver Nanowire Networks as Flexible, Transparent, Conducting Films: Extremely High DC to Optical Conductivity Ratios. ACS Nano 2009, 3, 1767–1774. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Kim, H.S.; Lee, J.-Y.; Peumans, P.; Cui, Y. Scalable Coating and Properties of Transparent, Flexible, Silver Nanowire Electrodes. ACS Nano 2010, 4, 2955–2963. [Google Scholar] [CrossRef]
- Madaria, A.R.; Kumar, A.; Ishikawa, F.N.; Zhou, C. Uniform, highly conductive, and patterned transparent films of a percolating silver nanowire network on rigid and flexible substrates using a dry transfer technique. Nano Res. 2010, 3, 564–573. [Google Scholar] [CrossRef] [Green Version]
- Scardaci, V.; Coull, R.; Lyons, P.E.; Rickard, D.; Coleman, J.N. Spray Deposition of Highly Transparent, Low-Resistance Networks of Silver Nanowires over Large Areas. Small 2011, 7, 2621–2628. [Google Scholar] [CrossRef]
- USGS. Mineral Commodity Summaries 2021; USGS: Reston, VA, USA, 2021; p. 200.
- Rathmell, A.R.; Bergin, S.M.; Hua, Y.-L.; Li, Z.-Y.; Wiley, B.J. The Growth Mechanism of Copper Nanowires and Their Properties in Flexible, Transparent Conducting Films. Adv. Mater. 2010, 22, 3558–3563. [Google Scholar] [CrossRef]
- Rathmell, A.R.; Wiley, B.J. The Synthesis and Coating of Long, Thin Copper Nanowires to Make Flexible, Transparent Conducting Films on Plastic Substrates. Adv. Mater. 2011, 23, 4798–4803. [Google Scholar] [CrossRef]
- Rathmell, A.R.; Nguyen, M.; Chi, M.F.; Wiley, B.J. Synthesis of Oxidation-Resistant Cupronickel Nanowires for Transparent Conducting Nanowire Networks. Nano Lett. 2012, 12, 3193–3199. [Google Scholar] [CrossRef]
- Nam, V.B.; Lee, D. Copper Nanowires and Their Applications for Flexible, Transparent Conducting Films: A Review. Nanomaterials 2016, 6, 47. [Google Scholar] [CrossRef]
- Li, X.S.; Wang, Y.M.; Yin, C.R.; Yin, Z.X. Copper nanowires in recent electronic applications: Progress and perspectives. J. Mater. Chem. C 2020, 8, 849–872. [Google Scholar] [CrossRef]
- Zhao, S.; Han, F.; Li, J.; Meng, X.; Huang, W.; Cao, D.; Zhang, G.; Sun, R.; Wong, C.-P. Advancements in Copper Nanowires: Synthesis, Purification, Assemblies, Surface Modification, and Applications. Small 2018, 14, 1800047. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Yin, Z.X. Review of Wet Chemical Syntheses of Copper Nanowires and Their Recent Applications. Appl. Sci. Converg. Technol. 2019, 28, 186–193. [Google Scholar] [CrossRef]
- De, S.; King, P.J.; Lyons, P.E.; Khan, U.; Coleman, J.N. Size Effects and the Problem with Percolation in Nanostructured Transparent Conductors. ACS Nano 2010, 4, 7064–7072. [Google Scholar] [CrossRef]
- Forró, C.; Demkó, L.; Weydert, S.; Vörös, J.; Tybrandt, K. Predictive Model for the Electrical Transport within Nanowire Networks. ACS Nano 2018, 12, 11080–11087. [Google Scholar] [CrossRef]
- Nirmalraj, P.N.; Lyons, P.E.; De, S.; Coleman, J.N.; Boland, J.J. Electrical Connectivity in Single-Walled Carbon Nanotube Networks. Nano Lett. 2009, 9, 3890–3895. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Wang, R.R.; Wen, M.C.; Weng, D.; Cui, X.; Sun, J.; Li, H.X.; Lu, Y.F. Synthesis of Ultralong Copper Nanowires for High-Performance Transparent Electrodes. J. Am. Chem. Soc. 2012, 134, 14283–14286. [Google Scholar] [CrossRef]
- Guo, H.; Lin, N.; Chen, Y.; Wang, Z.; Xie, Q.; Zheng, T.; Gao, N.; Li, S.; Kang, J.; Cai, D.; et al. Copper Nanowires as Fully Transparent Conductive Electrodes. Sci. Rep. 2013, 3, 2323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, F.; Yu, Y.; Dou, L.T.; Sun, J.W.; Yang, Q.; Schildknecht, C.; Schierle-Arndt, K.; Yang, P.D. Synthesis of Ultrathin Copper Nanowires Using Tris(trimethylsilyl)silane for High-Performance and Low-Haze Transparent Conductors. Nano Lett. 2015, 15, 7610–7615. [Google Scholar] [CrossRef]
- Mayousse, C.; Celle, C.; Carella, A.; Simonato, J.-P. Synthesis and purification of long copper nanowires. Application to high performance flexible transparent electrodes with and without PEDOT:PSS. Nano Res. 2014, 7, 315–324. [Google Scholar] [CrossRef]
- Khanarian, G.; Joo, J.; Liu, X.Q.; Eastman, P.; Werner, D.; O’Connell, K.; Trefonas, P. The optical and electrical properties of silver nanowire mesh films. J. Appl. Phys. 2013, 114, 024302. [Google Scholar] [CrossRef]
- Kang, C.X.; Yang, S.J.; Tan, M.; Wei, C.H.N.; Liu, Q.M.; Fang, J.; Liu, G. Purification of Copper Nanowires To Prepare Flexible Transparent Conductive Films with High Performance. Acs Appl. Nano Mater. 2018, 1, 3155–3163. [Google Scholar] [CrossRef]
- Hashimi, A.S.; Ginting, R.T.; Chin, S.X.; Lau, K.S.; Nazhif Mohd Nohan, M.A.; Zakaria, S.; Yap, C.C.; Chia, C.H. Fast microwave-assisted synthesis of copper nanowires as reusable high-performance transparent conductive electrode. Curr. Appl. Phys. 2020, 20, 205–211. [Google Scholar] [CrossRef]
- Kim, D.; Kwon, J.; Jung, J.; Kim, K.; Lee, H.; Yeo, J.; Hong, S.; Han, S.; Ko, S.H. A Transparent and Flexible Capacitive-Force Touch Pad from High-Aspect-Ratio Copper Nanowires with Enhanced Oxidation Resistance for Applications in Wearable Electronics. Small Methods 2018, 2, 1800077. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, J.; Xu, D.; Sun, Y.; Yan, F. Synthesis of Ultralong Copper Nanowires for High-Performance Flexible Transparent Conductive Electrodes: The Effects of Polyhydric Alcohols. Langmuir 2018, 34, 3884–3893. [Google Scholar] [CrossRef]
- Wang, X.; Wang, R.; Shi, L.; Sun, J. Kinetically controlled synthesis of Cu nanowires with tunable diameters and their applications in transparent electrodes. J. Mater. Chem. C 2018, 6, 1048–1056. [Google Scholar] [CrossRef]
- Yin, Z.; Song, S.K.; You, D.-J.; Ko, Y.; Cho, S.; Yoo, J.; Park, S.Y.; Piao, Y.; Chang, S.T.; Kim, Y.S. Novel Synthesis, Coating, and Networking of Curved Copper Nanowires for Flexible Transparent Conductive Electrodes. Small 2015, 11, 4576–4583. [Google Scholar] [CrossRef]
- Yin, Z.; Song, S.K.; Cho, S.; You, D.-J.; Yoo, J.; Chang, S.T.; Kim, Y.S. Curved copper nanowires-based robust flexible transparent electrodes via all-solution approach. Nano Res. 2017, 10, 3077–3091. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, J.N.; Xu, D.; Sun, Y.; Yan, F. Synthesis of ultrathin semicircle-shaped copper nanowires in ethanol solution for low haze flexible transparent conductors. Nano Res. 2018, 11, 3899–3910. [Google Scholar] [CrossRef]
- Lonne, Q.; Endrino, J.; Huang, Z.R. UV Treatment of Flexible Copper Nanowire Mesh Films for Transparent Conductor Applications. Nanoscale Res. Lett. 2017, 12, 577. [Google Scholar] [CrossRef] [Green Version]
- Stewart, I.E.; Rathmell, A.R.; Yan, L.; Ye, S.; Flowers, P.F.; You, W.; Wiley, B.J. Solution-processed copper–nickel nanowire anodes for organic solar cells. Nanoscale 2014, 6, 5980–5988. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Song, J.; Zou, Y.; Huo, C.; Dong, Y.; Xu, L.; Li, J.; Zeng, H. Nickel concentration-dependent opto-electrical performances and stability of Cu@CuNi nanowire transparent conductors. RSC Adv. 2016, 6, 91394–91400. [Google Scholar] [CrossRef]
- Stewart, I.E.; Ye, S.; Chen, Z.; Flowers, P.F.; Wiley, B.J. Synthesis of Cu–Ag, Cu–Au, and Cu–Pt Core–Shell Nanowires and Their Use in Transparent Conducting Films. Chem. Mater. 2015, 27, 7788–7794. [Google Scholar] [CrossRef]
- Niu, Z.G.; Cui, F.; Yu, Y.; Becknell, N.; Sun, Y.C.; Khanarian, G.; Kim, D.; Dou, L.; Dehestani, A.; Schierle-Arndt, K.; et al. Ultrathin Epitaxial Cu@Au Core-Shell Nanowires for Stable Transparent Conductors. J. Am. Chem. Soc. 2017, 139, 7348–7354. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.F.; Ye, S.R.; Stewart, I.E.; Wiley, B.J. Copper Nanowire Networks with Transparent Oxide Shells That Prevent Oxidation without Reducing Transmittance. Acs Nano 2014, 8, 9673–9679. [Google Scholar] [CrossRef]
- Tigan, D.; Genlik, S.P.; Imer, B.; Unalan, H.E. Core/shell copper nanowire networks for transparent thin film heaters. Nanotechnology 2019, 30, 325202. [Google Scholar] [CrossRef]
- Won, Y.; Kim, A.; Lee, D.; Yang, W.; Woo, K.; Jeong, S.; Moon, J. Annealing-free fabrication of highly oxidation-resistive copper nanowire composite conductors for photovoltaics. NPG Asia Mater. 2014, 6, e105. [Google Scholar] [CrossRef] [Green Version]
- Kholmanov, I.N.; Domingues, S.H.; Chou, H.; Wang, X.; Tan, C.; Kim, J.-Y.; Li, H.; Piner, R.; Zarbin, A.J.G.; Ruoff, R.S. Reduced Graphene Oxide/Copper Nanowire Hybrid Films as High-Performance Transparent Electrodes. ACS Nano 2013, 7, 1811–1816. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.T.; Cui, F.; Yu, Y.; Khanarian, G.; Eaton, S.W.; Yang, Q.; Resasco, J.; Schildknecht, C.; Schierle-Arndt, K.; Yang, P.D. Solution-Processed Copper/Reduced-Graphene-Oxide Core/Shell Nanowire Transparent Conductors. Acs Nano 2016, 10, 2600–2606. [Google Scholar] [CrossRef]
- Ahn, Y.; Jeong, Y.; Lee, D.; Lee, Y. Copper Nanowire–Graphene Core–Shell Nanostructure for Highly Stable Transparent Conducting Electrodes. ACS Nano 2015, 9, 3125–3133. [Google Scholar] [CrossRef]
- Navik, R.; Xiao, D.; Gai, Y.; Tan, H.; Zhao, Y. One-pot synthesis of copper nanowire-graphene composite with excellent stability and electrical performance for flexible electrodes. Appl. Surf. Sci. 2020, 527, 146694. [Google Scholar] [CrossRef]
- Polat Genlik, S.; Tigan, D.; Kocak, Y.; Ercan, K.E.; Cicek, M.O.; Tunca, S.; Koylan, S.; Coskun, S.; Ozensoy, E.; Unalan, H.E. All-Solution-Processed, Oxidation-Resistant Copper Nanowire Networks for Optoelectronic Applications with Year-Long Stability. ACS Appl. Mater. Interfaces 2020, 12, 45136–45144. [Google Scholar] [CrossRef]
- Garnett, E.C.; Cai, W.; Cha, J.J.; Mahmood, F.; Connor, S.T.; Greyson Christoforo, M.; Cui, Y.; McGehee, M.D.; Brongersma, M.L. Self-limited plasmonic welding of silver nanowire junctions. Nat. Mater. 2012, 11, 241–249. [Google Scholar] [CrossRef]
- Hu, A.; Peng, P.; Alarifi, H.; Zhang, X.Y.; Guo, J.Y.; Zhou, Y.; Duley, W.W. Femtosecond laser welded nanostructures and plasmonic devices. J. Laser Appl. 2012, 24, 042001. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.; Jiu, J.; Tian, Y.; Sugahara, T.; Nagao, S.; Suganuma, K. Fast fabrication of copper nanowire transparent electrodes by a high intensity pulsed light sintering technique in air. Phys. Chem. Chem. Phys. 2015, 17, 31110–31116. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Jiu, J.; Gao, Y.; Tian, Y.; Araki, T.; Sugahara, T.; Nagao, S.; Nogi, M.; Koga, H.; Suganuma, K.; et al. One-Step Fabrication of Stretchable Copper Nanowire Conductors by a Fast Photonic Sintering Technique and Its Application in Wearable Devices. ACS Appl. Mater. Interfaces 2016, 8, 6190–6199. [Google Scholar] [CrossRef]
- Wang, R.R.; Zhai, H.T.; Wang, T.; Wang, X.; Cheng, Y.; Shi, L.J.; Sun, J. Plasma-induced nanowelding of a copper nanowire network and its application in transparent electrodes and stretchable conductors. Nano Res. 2016, 9, 2138–2148. [Google Scholar] [CrossRef]
- Park, J.H.; Han, S.; Kim, D.; You, B.K.; Joe, D.J.; Hong, S.; Seo, J.; Kwon, J.; Jeong, C.K.; Park, H.-J.; et al. Plasmonic-Tuned Flash Cu Nanowelding with Ultrafast Photochemical-Reducing and Interlocking on Flexible Plastics. Adv. Funct. Mater. 2017, 27, 1701138. [Google Scholar] [CrossRef]
- Han, S.; Hong, S.; Ham, J.; Yeo, J.; Lee, J.; Kang, B.; Lee, P.; Kwon, J.; Lee, S.S.; Yang, M.Y.; et al. Fast Plasmonic Laser Nanowelding for a Cu-Nanowire Percolation Network for Flexible Transparent Conductors and Stretchable Electronics. Adv. Mater. 2014, 26, 5808–5814. [Google Scholar] [CrossRef]
- Zhao, L.; Yu, S.H.; Li, X.P.; Wu, M.Y.; Li, L.X. High-performance flexible transparent conductive films based on copper nanowires with electroplating welded junctions. Sol. Energy Mater. Sol. Cells 2019, 201, 110067. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, Y.; Wang, S.; Huang, Y.; Wen, J.; Hang, C.; Zheng, Z.; Wang, C. Highly stable flexible transparent electrode via rapid electrodeposition coating of Ag-Au alloy on copper nanowires for bifunctional electrochromic and supercapacitor device. Chem. Eng. J. 2020, 399, 125075. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Tian, Y.; Liu, Y.; Wen, J.; Huang, Y.; Hang, C.; Zheng, Z.; Wang, C. Electrodeposition fabrication of Cu@Ni core shell nanowire network for highly stable transparent conductive films. Chem. Eng. J. 2020, 390, 124495. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Hang, C.; Tian, Y. Joining of copper nanowires by electrodepositing silver layer for high-performance transparent electrode. Weld. World 2021, 65, 1021–1030. [Google Scholar] [CrossRef]
- Hu, W.; Wang, R.; Lu, Y.; Pei, Q. An elastomeric transparent composite electrode based on copper nanowires and polyurethane. J. Mater. Chem. C 2014, 2, 1298–1305. [Google Scholar] [CrossRef]
- Aliprandi, A.; Moreira, T.; Anichini, C.; Stoeckel, M.A.; Eredia, M.; Sassi, U.; Bruna, M.; Pinheiro, C.; Laia, C.A.T.; Bonacchi, S.; et al. Hybrid Copper-Nanowire-Reduced-Graphene-Oxide Coatings: A “Green Solution” Toward Highly Transparent, Highly Conductive, and Flexible Electrodes for (Opto)Electronics. Adv. Mater. 2017, 29, 1703225. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.Y.; Tang, Z.; Tian, D.; Liu, K.Y.; Wu, W. A self-healing flexible transparent conductor made of copper nanowires and polyurethane. Mater. Res. Bull. 2017, 90, 175–181. [Google Scholar] [CrossRef]
- Ohiienko, O.; Oh, Y.-J. Preparation of narrow copper nanowires with less oxidized surface for flexible and transparent electrodes under octadecylamine. Mater. Chem. Phys. 2020, 246, 122783. [Google Scholar] [CrossRef]
- Yoon, H.; Shin, D.S.; Babu, B.; Kim, T.G.; Song, K.M.; Park, J. Control of copper nanowire network properties and application to transparent conducting layer in LED. Mater. Des. 2017, 132, 66–71. [Google Scholar] [CrossRef]
- Zhong, Z.; Lee, H.; Kang, D.; Kwon, S.; Choi, Y.-M.; Kim, I.; Kim, K.-Y.; Lee, Y.; Woo, K.; Moon, J. Continuous Patterning of Copper Nanowire-Based Transparent Conducting Electrodes for Use in Flexible Electronic Applications. ACS Nano 2016, 10, 7847–7854. [Google Scholar] [CrossRef]
- Yu, S.; Liu, Z.; Zhao, L.; Gong, B. High-performance flexible transparent conductive tape based on copper nanowires. Opt. Mater. 2021, 119, 111301. [Google Scholar] [CrossRef]
- Sachse, C.; Weiß, N.; Gaponik, N.; Müller-Meskamp, L.; Eychmüller, A.; Leo, K. ITO-Free, Small-Molecule Organic Solar Cells on Spray-Coated Copper-Nanowire-Based Transparent Electrodes. Adv. Energy Mater. 2014, 4, 1300737. [Google Scholar] [CrossRef]






| Test Type | Substrate/Matrix | Number of Cycles | R/R0 | Ref. |
|---|---|---|---|---|
| Bending | Filtration membrane | 1000 | 1 | [18] |
| Bending | PET | 1000 | 1.33 | [19] |
| Bending | PI | 100 | ~1 | [29] |
| Bending | PI | 20,000 | ~1 | [60] |
| Folding | PI | 6 | 1.4 | [60] |
| Stretching | Eco-flex | 850 | 1.1 | [60] |
| Stretching | PU | 200 | 1 | [65] |
| Bending | PET | 1280 | 1.5 | [48] |
| Bending | PET | 2000 | <1.1 | [38] |
| Stretching | PU | 1000 | 2 | [57] |
| Bending | PU | 100 | 1.5 | [57] |
| Bending | PET | 10,000 | <1.04 | [66] |
| Bending | PU | 1000 | <1.1 | [39] |
| Bending | PU | 500 | <1.1 | [67] |
| Bending | PU | 1500 | <1.05 | [35] |
| Bending | PET/PDMS | 1000 | ~1.04 | [40] |
| Stretching | PDMS | 1000 | 1.1 | [40] |
| Twisting | PDMS | 1000 | 1.05 | [40] |
| Bending | PET | 2000 | ~1.2 | [62] |
| Bending | PET | 2000 | <1.05 | [63] |
| Bending | PET | 1000 | <1.05 | [64] |
| Bending | PET | 1000 | 1.15 | [52] |
| Bending | PET | 500 | 1.33 | [68] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scardaci, V. Copper Nanowires for Transparent Electrodes: Properties, Challenges and Applications. Appl. Sci. 2021, 11, 8035. https://doi.org/10.3390/app11178035
Scardaci V. Copper Nanowires for Transparent Electrodes: Properties, Challenges and Applications. Applied Sciences. 2021; 11(17):8035. https://doi.org/10.3390/app11178035
Chicago/Turabian StyleScardaci, Vittorio. 2021. "Copper Nanowires for Transparent Electrodes: Properties, Challenges and Applications" Applied Sciences 11, no. 17: 8035. https://doi.org/10.3390/app11178035
APA StyleScardaci, V. (2021). Copper Nanowires for Transparent Electrodes: Properties, Challenges and Applications. Applied Sciences, 11(17), 8035. https://doi.org/10.3390/app11178035






