Abstract
Calcium sulfate/calcium phosphate (CS-CP)-based bone substitutes have been developed in premixed putty for usage in clinical applications. However, it is difficult to completely stop the bleeding during an operation because premixed putty can come into contact with blood or body fluids leading to disintegration. Under certain conditions depending on particle size and morphology, collapsed (washed) particles can cause inflammation and delay bone healing. In this context, anti-washout premixed putty CS-CP was prepared by mixing glycerin with 1, 2, and 4 wt% of hydroxypropyl methylcellulose (HPMC), and the resultant anti-washout properties were evaluated. The results showed that more than 70% of the premixed putty without HPMC was disintegrated after being immersed into simulated body fluid (SBF) for 15 min. The results demonstrated that the more HPMC was contained in the premixed putty, the less disintegration occurred. We conclude that CS-CP pre-mixed putty with glycerin and HPMC is a potential bone substitute that has good anti-washout properties for clinical applications.
1. Introduction
Calcium sulfate/calcium phosphate (CS-CP) composites are commonly used as bone substitute materials in clinical applications such as symptomatic osteonecrosis of the femoral head, bone tumors, critical size bone defects, spine and dental surgery, hemostatic agent in orthodontic treatment, and increased angiogenesis [1,2,3,4,5]. CS-CP combines the advantages of the two materials with the properties of biocompatibility, osteo-conduction, and osteo-integration [6,7,8]. CS-CP is available in different forms, such as granules, injectable kits, and premixed putties [9,10,11,12]. Compared with other forms, premixed putty is simpler to use and is cohesive and moldable. Moreover, it does not require intraoperative mixing, nor is restricted by defect forms [10]. There is a higher risk of infection in the mixing process of preparing the injectable kits [13]; moreover, different brands of bone substitutes use various different mixing methods, rules, and surgical instruments, making their use more complicated. Moreover, some critical issues arise. For example, it is difficult to completely stop the bleeding during an operation because bone substitutes can come into contact with blood or body fluids that can change the anti-washout properties of the premixed putties [14] and disintegrate the implant [15,16]. Washed particles can cause slight inflammation and severe foreign-body responses [15]. The inflammation reaction depends on the size, morphology, and concentration of the particles determined predominantly by the washout rate. It has been shown that particles smaller than 10 μm and concentrations more than 0.1% reduced osteoblast viability and proliferation activity [17]. Compared with spherical-shaped or irregular particles, needle-shaped particles generate a prolonged inflammatory response in macrophages [18]. Macrophages are directly internalized and can digest small particles (<10 μm). In addition, they secret inflammatory cytokines (IL-1, IL-6, MCP-1, and TNF-α) to produce an inflammatory response [19]. In addition, the implantation of commercial CS-CP in premixed putty produces an inflammatory response, and particles were found in the inflammatory area in case reports and clinical trials [14,15].
Therefore, anti-washout properties are important in the clinic to prevent disintegration. Anti-washout property can be improved by cohesion promoters such as sodium alginate [16], chitosan [18], sodium polyacrylate [19], collagen [20], hyaluronic acid [21], hydroxypropyl methylcellulose (HPMC) [22], gelatin [23], carrageenan [24], and thermosensitive hydrogel [25].
HPMC and glycerin are used as plasticizers in injectable or putty forms in commercially available bone substitutes [26,27,28,29]. Because of its biocompatibility [30], degradability [31], and non-toxic properties, HPMC has been used as a scaffold material in bone tissue engineering [32,33]. In addition, HPMC increases osteogenesis [34,35] and does not affect the degradation time [36]. GLY greatly improves injectability and increases the setting time [27,37]. Apart from this, it can be stored at room temperature without drying out and has been frequently used in clinical applications [28,29]. Although research on using plasticizers to improve cohesion in bone substitute materials has been quite extensive, two different plasticizers, which are washed particles, produced by plasticizers, have not been fully explored.
In this work, we evaluated CS-CP in premixed putty using a HPMC and glycerol carrier, and the anti-wash out properties of CS-CP powder loaded in HPMC at various ratios were characterized by microstructure analysis and particle morphology.
2. Materials and Methods
2.1. Preparation of Premixed Putty
SH (CaSO4·0.5H2O) was prepared using a wet hot method. CSD (CaSO4·2H2O; J.T. Baker, Phillipsburg, NJ, USA) was mixed in a 30% CaCl2 (SHOWA Corporation, Osaka, Japan) solution at 121 °C for 30 min. Afterward, boiling water was added to remove CaCl2 and the mixture was incubated at 121 °C for 30 min [38].
In this study, the liquid–solid ratio of premixed putty was selected to be 0.35 mL/g. The liquid ratio was a mixture of 99% glycerin (Sigma-Aldrich, St. Louis, MO, USA) and 15% deionized water. Powders were mixed in the ratios of CSH, β-tri-calcium phosphate (β-TCP) (Sigma-Aldrich, St. Louis, MO, USA), and HPMC (viscosity 2600–5600 cP, Sigma-Aldrich, St. Louis, MO, USA), shown in Table 1.

Table 1.
Composition and powder content of premixed putty.
2.2. Washout Resistance Test
The premixed putty was placed in a 304 stainless steel mold, 6 mm in diameter and 3 mm in height, to make a cylindrical form (Figure 1a). Premixed putty was then immersed in simulated body fluid (SBF) contained in a 20 mm × 20 mm cylinder at 37 °C and placed at the center (Figure 1b). The ratio of sample to SBF was 0.2 g/mL. The ion concentration of the SBF solution was similar to that of human plasma [39]. After immersion for 15 min, 1 h, and 4 h, the washout resistance of premixed putty was observed through visual inspection. Figure 1c shows the main body with a diameter of 6 mm and the remaining particles. The SBF solution was then removed, 1 mL of 99.5% ethanol was added, and the mixture was allowed to stand for 30 s. The ethanol was removed, and the premixed putty of the main body and the particles were dried at 50 °C for 3 days. The weight of the main body was measured, and disintegration was estimated by subtracting the final dry weight from the initial powder weight.
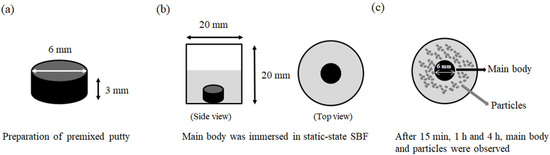
Figure 1.
Schematic diagram of washout resistance test. (a) Premixed putty mold, 6 mm in diameter and 3 mm in height, to make a cylindrical shape. (b) Premixed putty main body immersed in static-state SBF (gray area) at 37 °C (top view). (c) After 15 min, 1 h, and 4 h, the main body and particles were observed, and the main body was identified as 6 mm in diameter, and particles (top view).
2.3. Analysis of the Particles
The particles were examined using a scanning electron microscope. Micrographs of the particles were then analyzed using image analyzer software (Image J, National Institutes of Health, Bethesda, MD, USA, version 1.8) to measure their dimensions. Measurements were made for at least 250 particles in each condition.
2.4. Microstructure and Phase Composition
The microstructure of surfaces of dried samples or disintegrated particles was assessed by SEM (S-3000H, Hitachi, Tokyo, Japan) with an accelerating voltage of 15 kV. Specimens were sputtered with a thin Au coating for 90 s. Dry premixed putty specimens from washout resistance tests ground into a fine powder or disintegrated particles were analyzed by XRD. The patterns were recorded with a powder XRD (X’Pert3 powder, PANalytical B.V., Almelo, The Netherlands) using Cu-Ka irradiation generated at 45 kV and 40 mA, in a theta-theta setup with scanning diffraction angles (2θ) of 10° to 60° in steps of 0.1°.
2.5. Statistical Analysis
The results are shown as the mean ± standard deviation or median ± lower/upper quartile, and a Shapiro–Wilk test is carried out to check the normality of the variables. The one-way analysis of variance (ANOVA) statistical analysis was used to evaluate significant differences between the means. Levene’s test was used to test for homogeneity of variance between the samples. When equal variances could be assumed, Scheffe’s multiple comparisons post hoc test was performed. In the case of normal distribution and variance of the data, we used parametric tests such as the washout resistance test. In the case of significant differences between the variances of the normal distribution, non-parametric tests were performed using the method of Kruskal–Wallis, and the Mann–Whitney test was used to identify significant differences. In case of non-normal distribution or variance of the date, we used parametric tests such as analysis of the particles. Statistical analysis was performed with SPSS Statistics (IBM Company, Chicago, IL, USA, version 22). A value of p < 0.05 was considered significant.
3. Results
3.1. Washout Resistance of Premixed Putty at Different Ratios
The qualitative and quantitative evaluation of premixed putty before and after being soaked in simulated body fluid (SBF) for 15 min, 1 h, and 4 h are shown in Figure 2A,B. None of the initial premixed putty groups disintegrated until immersion in SBF. The control group without HPMC disintegrated faster than those with HPMC after immersion in SBF for 15 min (p < 0.001). The premixed putty group with 1 wt% HPMC was almost 70% particles after 1 h. It disintegrated significantly faster than the premixed putty with 2 wt% and 4 wt% (p < 0.001). Further, the premixed putty group with 2 wt% HPMC was a better anti-washout than that with 1 wt% HPMC (p < 0.001). The premixed putty group with 4 wt% HPMC disintegrated less than 40% after 4 h and decayed significantly less than the other groups (p < 0.001). We conclude that the less HPMC in the premixed putty, the more quickly it decayed during prolonged soaking.
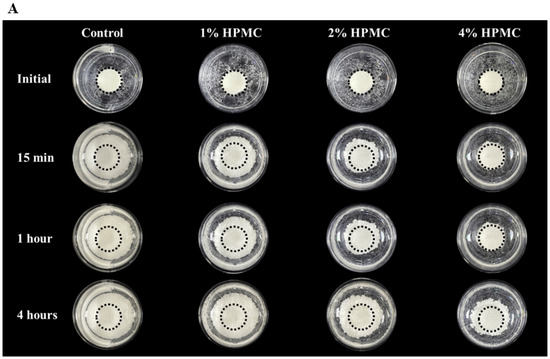
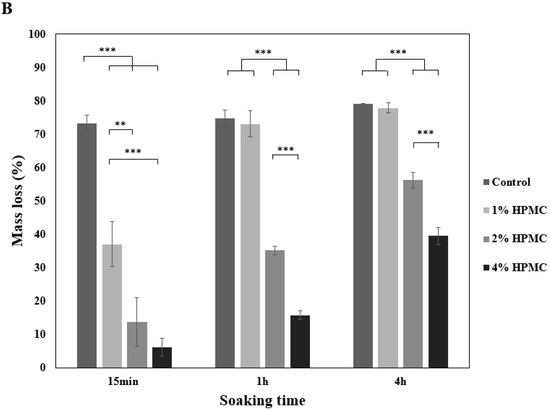
Figure 2.
Washout resistance test. (A) Anti-washout optical images of premixed putty with different HPMC mass ratios in SBF after being soaked for different periods. (B) Mass loss of premixed putty containing HPMC with different mass ratios in SBF after being soaked for 15 min, 1 h, and 4 h. Dotted circles indicate the 6 mm diameter of the main body premixed putty, and outer circles indicate the disintegrated particles. Error bars denote the mean ± SD for n = 3. ** and *** indicate p < 0.01 and p < 0.001, respectively.
3.2. Surface Morphology Analysis
Scanning electron microscopy (SEM) analysis was conducted for the main bodies with different ratios of premixed putty after being soaked in SBF at 37 °C for 4 h. As shown in Figure 3A, no significant structural difference was observed between the premixed putty without HPMC and that with the other ratios. In each group, the micropore structures seen in SEM micrographs had surfaces and pores with diameters of 10 to 30 μm. Figure 3B shows that particles were rod-shaped and irregular, and the premix putty group with 4 wt% HPMC had larger shedding particles than other groups.
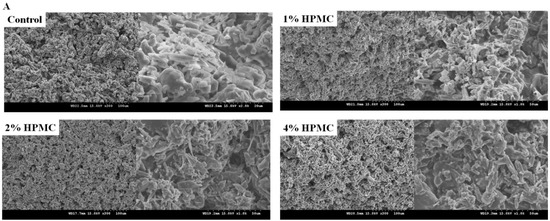
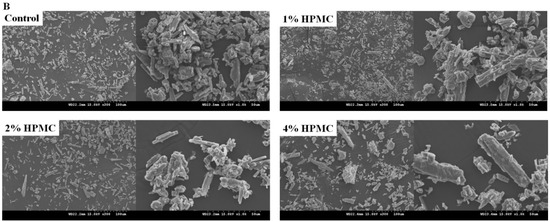
Figure 3.
SEM micrographs (300× and 1000×) of premixed putty containing HPMC with different mass ratios after being soaked for 4 h. (A) Micrographs of the main body. (B) Micrographs of particles.
3.3. Morphology of Premixed Putty with Collapsed Particle Structures
Particle size was estimated based on SEM images using the ImageJ software, and in each picture more than 250 particles were counted. Table 2 shows that the premixed putty group of 4 wt% HPMC had a longer particle than other groups, and that the control group, 1 wt%, and 2 wt% HPMC groups were similar. The particle size distribution of the control group and the premixed putty group with 1 wt% HPMC was similar, with a particle size smaller than 10 μm making up the majority (Table 3). The particle size distribution of the 2 wt% and 4 wt% HPMC groups was mainly between 11 and 30 μm. The premixed putty group with 4 wt% HPMC had a larger particle size distribution than other groups.

Table 2.
Particle length and aspect ratio of the particle in a collapsed process.

Table 3.
Particle size distribution of the collapsed particles.
3.4. X-ray Diffractometer Analysis
The X-ray diffractometry (XRD) patterns of premixed putty after being soaked in SBF for 4 h are shown in Figure 4A,B. The diffracted peaks of calcium sulfate hemihydrate (CSH), calcium sulfate dihydrate (CSD), and β-TCP were indexed by JCPDS card numbers 41-0224, 33-0311, and 09-0169, respectively. The high intensity of diffracted peaks of CSH was observed at 14.7°, 25.6°, 29.7°, and 31.9°; CSD at 11.5°, 20.7°, 23.6°, 29.1°, 31.1°, and 33.3°; and β-TCP at 25.8°, 27.7°, 29.5°, 31.0°, 34.3°, 46.9°, and 52.9°. In the main body of all groups, a hydration conversion of CSH to CSD was observed (Figure 4A). Interestingly, in the particle group, as the proportion of HPMC increased, a lower amount of CSH converted to CSD (Figure 4B).
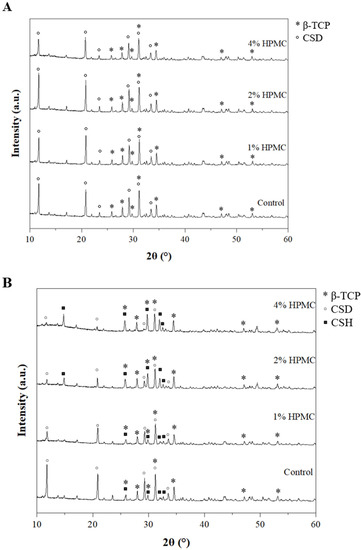
Figure 4.
X-ray diffractometry patterns of premixed putty containing HPMC with different mass ratios after being soaked for 4 h (*: β-TCP; ◦: calcium sulfate dihydrate (CSD), ▪: CSH). (A) Patterns of the main body. (B) Patterns of particles.
4. Discussion
Although CS-CP-based premixed putty has good biocompatibility, osteo-conduction, and osteo-integration, the poor anti-washout ability limits the clinical utility of premixed putty. HPMC and glycerin could increase the cohesion and maintain stability before use [27,33]. Our results indicated the addition of HPMC could effectively improve the anti-washout properties of premixed putty; as HPMC increased, premixed putty was less washed out. The possible mechanism of HPMC action can be summarized into two aspects. On the one hand, it has been shown that HPMC can provide 3D networking for materials such as mixing with collagen [23,40], and without HPMC the material structure was looser so it falls apart easily. It is possible that HPMC provided stronger structure for premixed putty, thus we observed that the anti-washout property was better when there was more HPMC added into the premixed putty (Figure 2A).
On the other hand, HPMC has a hemostatic effect by water adsorption capacity [40], the water competition between CSH and HPMC. Addition of HPMC was predicted to have a negative effect on CSH hydration, and there was an effect on the hydration reaction of the washed particle. For 2 wt% and 4 wt% HPMC groups, peaks of CSH still existed after soaking in SBF, which can be seen from XRD (Figure 4B). The possible reason for this is that the gel network prevented particles from washing out, but it also makes CSH inaccessible to water [41].
In situ bone substitute containing CS and β-TCP has been clinically studied with good outcomes, and used in osteotomies, vertebral bodies, fracture surgery, and bone tumors [1,2,3]. However, the bone substitute is often in direct contact with the muscle and sometimes leaking out from the bone defect area, which may lead to a local inflammatory process at the implantation site [14,15]. The degree of inflammatory response is related to the number, size and morphology of collapsed particles [42,43]. Our results indicated that when HPMC percentage was increased, the disintegration of premixed putty can be improved from more than 70% to less than 40% (Figure 2B), resulting in a reduction in the particle numbers (Figure 2B). The inflammatory response is related to the size and shape of the particle; when its size is less than 10 µm or in a needle-like morphology, it produces a relatively strong inflammatory response [42,43]. For the putty group with 4 wt% HPMC, it was affected by the proportion of particles whose sizes were lower than 10 µm, but it had no significant effect on the other groups (Table 3). The possible reason for this is that the particles were coated with and hydrated between each other with HPMC because it absorbed water molecules on the surface and formed a monomolecular layer, resulting in increased Van der Waals forces and reduced inter-particle separation [41]. However, our results indicated that the morphology of washed particles had no significant effect on the aspect ratio of the particles (Table 3) with irregular and rod-like shapes (Figure 3B). When the size of the particles was smaller than 10 µm [44], the macrophage tended to respond and the particles were phagocytosed, resulting in inflammation. Our results showed that when the premixed putty contained 4% HPMC, only 6.4% of particles were smaller than 10 µm, suggesting that less inflammation could occur. When the premixed putty contained 1% and 2% HPMC, the percentage of particles smaller than 10 µm was similar to the control, thus it may result in inflammation. However, further investigation should be conducted in order to verify this possibility.
In one particular craniectomy, a small portion of the skull was temporarily removed so that a surgeon could establish a passage for surgical instruments in order to remove a brain tumor. The bone at the craniotomy was replaced by premixed putty for healing. However, the disintegrated particles led to cysts after an inflammatory reaction and compressed the patient’s brain, resulting in the need for a second operation in order to remove the cysts [15]. The inflammation caused by the particle was also reported in the artificial joint system, and the size of particles was related to the inflammation response [45]. It is possible that if the premixed putty from this study was applied in the craniectomy, the inflammation might not occur due to fewer collapsed particles. Therefore, patients would recover well without requiring a second operation.
The present study has some limitations. We did not perform the dynamic washout resistance test, which would be closer to the surgical process. Furthermore, it is still unclear whether the inflammation response is related to the size of collapse particles, and cellular or animal experiments should be investigated further. Our study provided a bone substitute with good anti-washout property that may cause less clinical complications after being implanted.
5. Conclusions
In this study, we evaluated the anti-washout properties of CS-CP and HPMC/glycerin premixed putty. Increased proportion of HPMC in premixed putty led to less decay and larger disintegrated particles. Our proposed premixed putty may help reduce inflammation and bone repair in clinical applications, which should be confirmed by further cell or animal experiments in the future.
Author Contributions
Conceptualization, H.-H.C. and H.-W.F.; methodology, H.-H.C., L.-H.H. and M.-H.Y.; validation, H.-H.C., L.-H.H. and M.-H.Y.; formal analysis, H.-H.C., L.-H.H. and M.-H.Y.; investigation, H.-H.C.; data curation, H.-H.C. and C.-Y.S.; writing—original draft preparation, H.-H.C. and C.-Y.S.; writing—review and editing, H.-W.F.; supervision, H.-W.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ministry of Science and Technology (MOST), Taiwan, under grant number MOST 109-2622-8-027-001-TE4.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jia, P.; Tang, H.; Chen, H.; Bao, L.; Feng, F.; Yang, H.; Li, J. Prophylactic vertebroplasty procedure applied with a resorbable bone cement can decrease the fracture risk of sandwich vertebrae: Long-term evaluation of clinical outcomes. Regen. Biomater. 2017, 4, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.-C.; Fan, K.-F.; Keorochana, G.; Chen, W.-J.; Chen, L.-H. Transpedicular grafting after short-segment pedicle instrumentation for thoracolumbar burst fracture: Calcium sulfate cement versus autogenous iliac bone graft. Spine 2010, 35, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Sinjab, Y.H.; Sinjab, K.H.; Navarrete-Bedoya, C.; Gutmann, J.L. Calcium sulfate applications in dentistry: A literature review. Endodontology 2020, 32, 167. [Google Scholar]
- Scarano, A.; Carinci, F.; Cimorelli, E.; Quaranta, M.; Piattelli, A. Application of calcium sulfate in surgical-orthodontic treatment of impacted teeth: A new procedure to control hemostasis. J. Oral Maxillofac. Surg. 2010, 68, 964–968. [Google Scholar] [CrossRef]
- Strocchi, R.; Orsini, G.; Iezzi, G.; Scarano, A.; Rubini, C.; Pecora, G.; Piattelli, A. Bone regeneration with calcium sulfate: Evidence for increased angiogenesis in rabbits. J. Oral Implantol. 2002, 28, 273–278. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Ginebra, M.-P.; Espanol, M.; Montufar, E.B.; Perez, R.A.; Mestres, G. New processing approaches in calcium phosphate cements and their applications in regenerative medicine. Acta Biomater. 2010, 6, 2863–2873. [Google Scholar] [CrossRef]
- Urban, R.M.; Turner, T.M.; Hall, D.J.; Inoue, N.; Gitelis, S. Increased bone formation using calcium sulfate-calcium phosphate composite graft. Clin. Orthop. Relat. Res.® 2007, 459, 110–117. [Google Scholar] [CrossRef]
- Landgraeber, S.; Warwas, S.; Claßen, T.; Jäger, M. Modifications to advanced Core decompression for treatment of Avascular necrosis of the femoral head. BMC Musculoskelet. Disord. 2017, 18, 1–8. [Google Scholar] [CrossRef]
- Bohner, M. Resorbable biomaterials as bone graft substitutes. Mater. Today 2010, 13, 24–30. [Google Scholar] [CrossRef]
- Sandiford, N.A. Complication rates are low with the use of Stimulan calcium sulphate based antibiotic delivery system in the management of patients with hip-related PJI: Early results of a consecutive case series. HIP Int. 2020, 30, 3–6. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Cheng, C.-Y.; Chen, A.C.-Y.; Chan, Y.-S. Arthroscopy-assisted corrective osteotomy, reduction, internal fixation and strut allograft augmentation for tibial plateau malunion or nonunion. J. Clin. Med. 2020, 9, 973. [Google Scholar] [CrossRef]
- Peersman, G.; Laskin, R.; Davis, J.; Peterson, M.; Richart, T. Prolonged operative time correlates with increased infection rate after total knee arthroplasty. HSS J.® 2006, 2, 70–72. [Google Scholar] [CrossRef]
- Friesenbichler, J.; Maurer-Ertl, W.; Sadoghi, P.; Pirker-Fruehauf, U.; Bodo, K.; Leithner, A. Adverse reactions of artificial bone graft substitutes: Lessons learned from using tricalcium phosphate geneX®. Clin. Orthop. Relat. Res.® 2014, 472, 976–982. [Google Scholar] [CrossRef]
- Park, J.-H.; Suh, S.-J.; Lee, Y.-S.; Lee, J.-H.; Ryu, K.-Y.; Kang, D.-G. Lytic Complications after Skull Reconstruction Using GeneX®. Korean J. Neurotrauma 2015, 11, 135. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.; Xiang, H.; Ye, J. Influence of anti-washout agents on the rheological properties and injectability of a calcium phosphate cement. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 81, 410–418. [Google Scholar] [CrossRef]
- Sato, T.; Kikuchi, M.; Aizawa, M. Preparation of hydroxyapatite/collagen injectable bone paste with an anti-washout property utilizing sodium alginate. Part 1: Influences of excess supplementation of calcium compounds. J. Mater. Sci. Mater. Med. 2017, 28, 49. [Google Scholar] [CrossRef]
- Yokoyama, A.; Yamamoto, S.; Kawasaki, T.; Kohgo, T.; Nakasu, M. Development of calcium phosphate cement using chitosan and citric acid for bone substitute materials. Biomaterials 2002, 23, 1091–1101. [Google Scholar] [CrossRef]
- Li, X.; He, F.; Ye, J. Preparation, characterization and in vitro cell performance of anti-washout calcium phosphate cement modified by sodium polyacrylate. RSC Adv. 2017, 7, 32842–32849. [Google Scholar] [CrossRef]
- Tamimi, F.; Kumarasami, B.; Doillon, C.; Gbureck, U.; Le Nihouannen, D.; Cabarcos, E.L.; Barralet, J.E. Brushite–collagen composites for bone regeneration. Acta Biomater. 2008, 4, 1315–1321. [Google Scholar] [CrossRef]
- Alkhraisat, M.; Rueda, C.; Marino, F.; Torres, J.; Jerez, L.; Gbureck, U.; Cabarcos, E. The effect of hyaluronic acid on brushite cement cohesion. Acta Biomater. 2009, 5, 3150–3156. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Rethore, G.; Khairoun, K.; Pilet, P.; Tancret, F.; Bouler, J.-M.; Weiss, P. A novel injectable, cohesive and toughened Si-HPMC (silanized-hydroxypropyl methylcellulose) composite calcium phosphate cement for bone substitution. Acta Biomater. 2014, 10, 3335–3345. [Google Scholar] [CrossRef] [PubMed]
- Bigi, A.; Bracci, B.; Panzavolta, S. Effect of added gelatin on the properties of calcium phosphate cement. Biomaterials 2004, 25, 2893–2899. [Google Scholar] [CrossRef]
- Qian, G.; Li, X.; He, F.; Ye, J. Improving the anti-washout property of calcium phosphate cement by introducing konjac glucomannan/κ-carrageenan blend. J. Biomater. Appl. 2019, 33, 1094–1104. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, J.; Cai, S.; Bao, X.; Li, Q.; Xu, G. Setting Characteristics and High Compressive Strength of an Anti-washout, Injectable Calcium Phosphate Cement Combined with Thermosensitive Hydrogel. Materials 2020, 13, 5779. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M. Design of ceramic-based cements and putties for bone graft substitution. Eur. Cell Mater. 2010, 20, 3–10. [Google Scholar] [CrossRef]
- Takagi, S.; Chow, L.C.; Hirayama, S.; Sugawara, A. Premixed calcium–phosphate cement pastes. J. Biomed. Mater. Res. Part B Appl. Biomater. 2003, 67, 689–696. [Google Scholar] [CrossRef]
- Carey, L.E.; Xu, H.H.; Simon Jr, C.G.; Takagi, S.; Chow, L.C. Premixed rapid-setting calcium phosphate composites for bone repair. Biomaterials 2005, 26, 5002–5014. [Google Scholar] [CrossRef]
- Xu, H.H.; Carey, L.E.; Simon, C.G., Jr.; Takagi, S.; Chow, L.C. Premixed calcium phosphate cements: Synthesis, physical properties, and cell cytotoxicity. Dent. Mater. 2007, 23, 433–441. [Google Scholar] [CrossRef]
- Jyoti, M.A.; Min, Y.K.; Lee, B.-T.; Song, H.-Y. In vitro bioactivity and biocompatibility of calcium phosphate cements using Hydroxy-propyl-methyl-Cellulose (HPMC). Appl. Surf. Sci. 2010, 257, 1533–1539. [Google Scholar] [CrossRef]
- Yao, Q.; Ye, J.; Xu, Q.; Mo, A.; Gong, P. Composite scaffolds of dicalcium phosphate anhydrate/multi-(amino acid) copolymer: In vitro degradability and osteoblast biocompatibility. J. Biomater. Sci. Polymer Ed. 2015, 26, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Kuo, C.-L.; Liang, C.-J.; Chang, L.-Y.; Hsu, C.-Y.; Lee, S.-Y.; Teng, N.-C.; Yang, J.-C. 3D pore-interconnected calcium phosphate bone blocks for bone tissue engineering. Ceram. Int. 2020, 46, 16465–16471. [Google Scholar] [CrossRef]
- Iqbal, H.; Ali, M.; Zeeshan, R.; Mutahir, Z.; Iqbal, F.; Nawaz, M.A.H.; Shahzadi, L.; Chaudhry, A.A.; Yar, M.; Luan, S. Chitosan/hydroxyapatite (HA)/hydroxypropylmethyl cellulose (HPMC) spongy scaffolds-synthesis and evaluation as potential alveolar bone substitutes. Colloids Surf. B Biointerfaces 2017, 160, 553–563. [Google Scholar] [CrossRef]
- Struillou, X.; Rakic, M.; Badran, Z.; Macquigneau, L.; Colombeix, C.; Pilet, P.; Verner, C.; Gauthier, O.; Weiss, P.; Soueidan, A. The association of hydrogel and biphasic calcium phosphate in the treatment of dehiscence-type peri-implant defects: An experimental study in dogs. J. Mater. Sci. Mater. Med. 2013, 24, 2749–2760. [Google Scholar] [CrossRef]
- Woo, K.M.; Yu, B.; Jung, H.M.; Lee, Y.K. Comparative evaluation of different crystal-structured calcium sulfates as bone-filling materials. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91, 545–554. [Google Scholar] [CrossRef]
- Urban, R.M.; Turner, T.M.; Hall, D.J.; Infanger, S.I.; Cheema, N.; Lim, T.H.; Richelsoph, K. An injectable calcium sulfate-based bone graft putty using hydroxypropylmethylcellulose as the plasticizer. Orthopedics 2004, 27, S155–S159. [Google Scholar] [CrossRef]
- Wang, X.; Ye, J.; Wang, H. Effects of additives on the rheological properties and injectability of a calcium phosphate bone substitute material. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 78, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lee, E.J.; Park, C.S.; Yoon, B.H.; Shin, D.S.; Kim, H.E.; Koh, Y.H.; Park, S.H. Calcium sulfate hemihydrate powders with a controlled morphology for use as bone cement. J. Am. Ceram. Soc. 2008, 91, 2039–2042. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yang, M.; Ni, X.; Yang, L.; Kutty, M.G. Using calcium sulfate cement—Hydroxypropyl methyl cellulose/sodium alginate composites as substitutes of bone wax. Int. J. Appl. Ceram. Technol. 2018, 15, 903–909. [Google Scholar] [CrossRef]
- Nokhodchi, A.; Ford, J.L.; Rubinstein, M.H. Studies on the interaction between water and (hydroxypropyl) methylcellulose. J. Pharm. Sci. 1997, 86, 608–615. [Google Scholar] [CrossRef]
- Pioletti, D.P.; Takei, H.; Lin, T.; Van Landuyt, P.; Ma, Q.J.; Kwon, S.Y.; Sung, K.-L.P. The effects of calcium phosphate cement particles on osteoblast functions. Biomaterials 2000, 21, 1103–1114. [Google Scholar] [CrossRef]
- Lebre, F.; Sridharan, R.; Sawkins, M.J.; Kelly, D.J.; O’Brien, F.J.; Lavelle, E.C. The shape and size of hydroxyapatite particles dictate inflammatory responses following implantation. Sci. Rep. 2017, 7, 2922. [Google Scholar] [CrossRef] [PubMed]
- Gallo, J.; Slouf, M.; Goodman, S.B. The relationship of polyethylene wear to particle size, distribution, and number: A possible factor explaining the risk of osteolysis after hip arthroplasty. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 94, 171–177. [Google Scholar] [CrossRef]
- Goodman, S.B.; Gibon, E.; Yao, Z. The basic science of periprosthetic osteolysis. Instr. Course Lect. 2013, 62, 201. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).