Analytical Methods for Exploring Nutraceuticals Based on Phenolic Acids and Polyphenols
Abstract
:Featured Application
Abstract
1. Introduction
2. Polyphenols and Related Compounds
- (i)
- Phenolic acids comprise hydroxybenzoic and hydroxycinnamic acids. They account for 30% of the total dietary phenolics, and, in general, cinnamic derivatives, such as caffeic, ferulic, and coumaric acids, are more abundant than the benzoic ones. Tartaric esters of these acids (e.g., caftaric and coutaric acids) are especially abundant in grape, vine, and wines. In addition, quinic acid derivatives such as chlorogenic acids are present in a broad range of products (e.g., coffee, tea, and pear). Phenolic acids also include hydrolyzable tannins, which consist of sugar residues esterified with gallic or ellagic acids. This type of tannin can be found at 10 to 100 mg kg−1 levels in coffee, fruits, nuts, tea, and wine.
- (ii)
- Flavonoids are the largest family of polyphenols both qualitatively and quantitatively as more than 5000 different molecules have been described, accounting for 60% of the total dietary polyphenols in humans [14]. The flavonoid backbone consists of two aromatic rings connected through a linking heterocycle with an oxygen and three carbon atoms (C6-C3-C6 skeleton). Flavonoids can be divided into six subclasses, namely: flavonols, flavones, isoflavones, flavanones, anthocyanidins, and flavanols. Additional details are given below.
- (iii)
- Stilbenes are characterized by a double-bond connecting two aromatic rings. Despite being found in low quantities in the human diet, the significance of compounds such as trans-resveratrol is outstanding. Apart from the more controversial antiaging effects, chemopreventive, chemotherapeutic, cardioprotective, and neuroprotective benefits have been attributed to resveratrol and its derivatives [29]. Curcuminoids are structurally related stilbenoids exhibiting a great range of nutritional and health beneficial properties [30]. Their structure has been used as the basis to design new drugs for the treatment of several types of cancers and microbial infections [30].
- (iv)
- Lignans are a minor class of polyphenols consisting of two phenylpropane units. The main food source of lignans is linseed, although they are also found at lower concentrations in cereals, fruits, and vegetables [31]. Recently, lignans have attracted the attention of researchers because of their potential anti-inflammatory, anti-neurodegenerative, antiviral, antimicrobial, and phytoestrogenic activities.
3. Nutraceuticals from Plant Extracts
4. Analytical Methods for the Determination of Active Compounds in Nutraceutical Products
4.1. Spectroscopic Methods
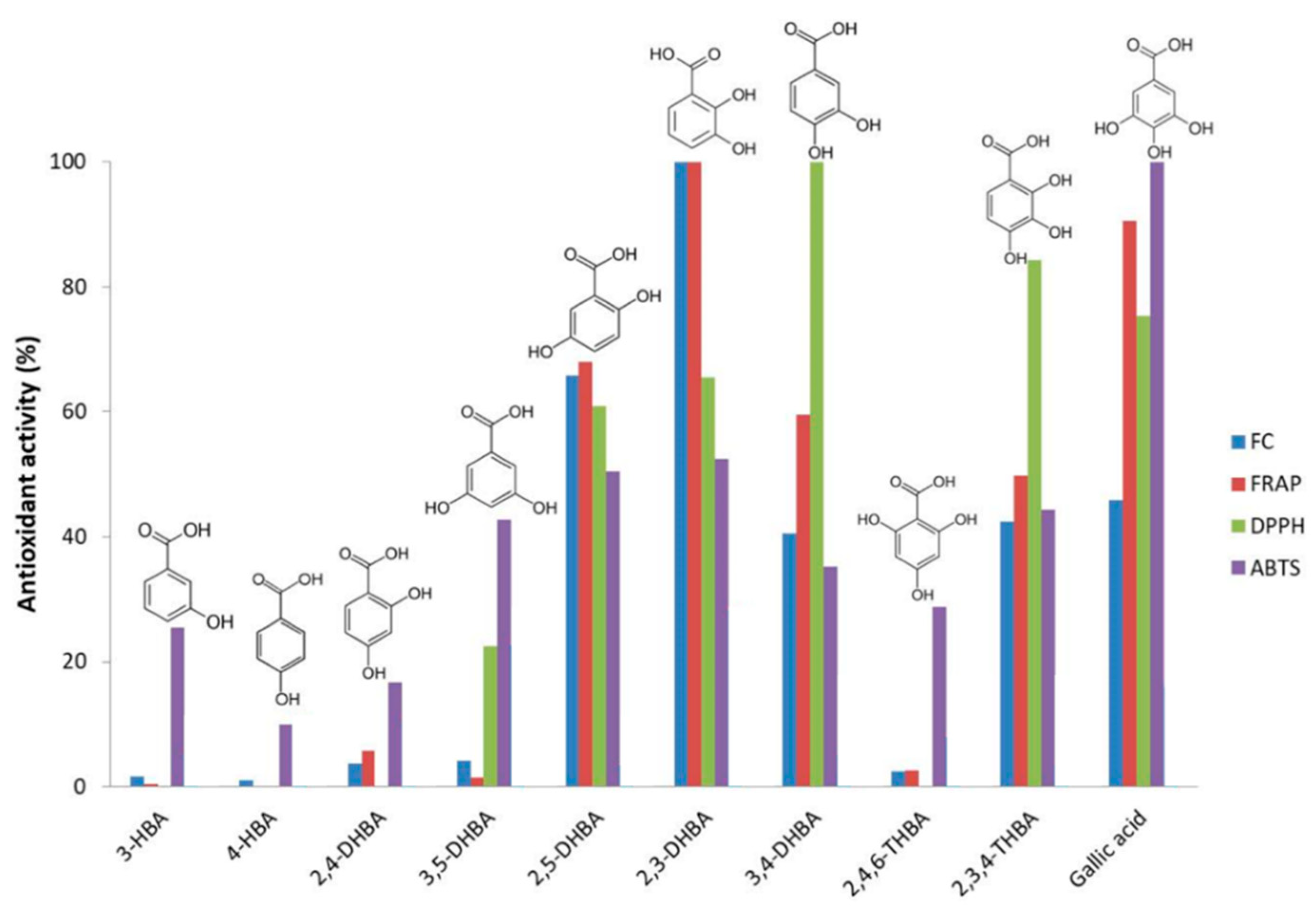
4.1.1. Antioxidant Indexes
4.1.2. Flavonoid Assays
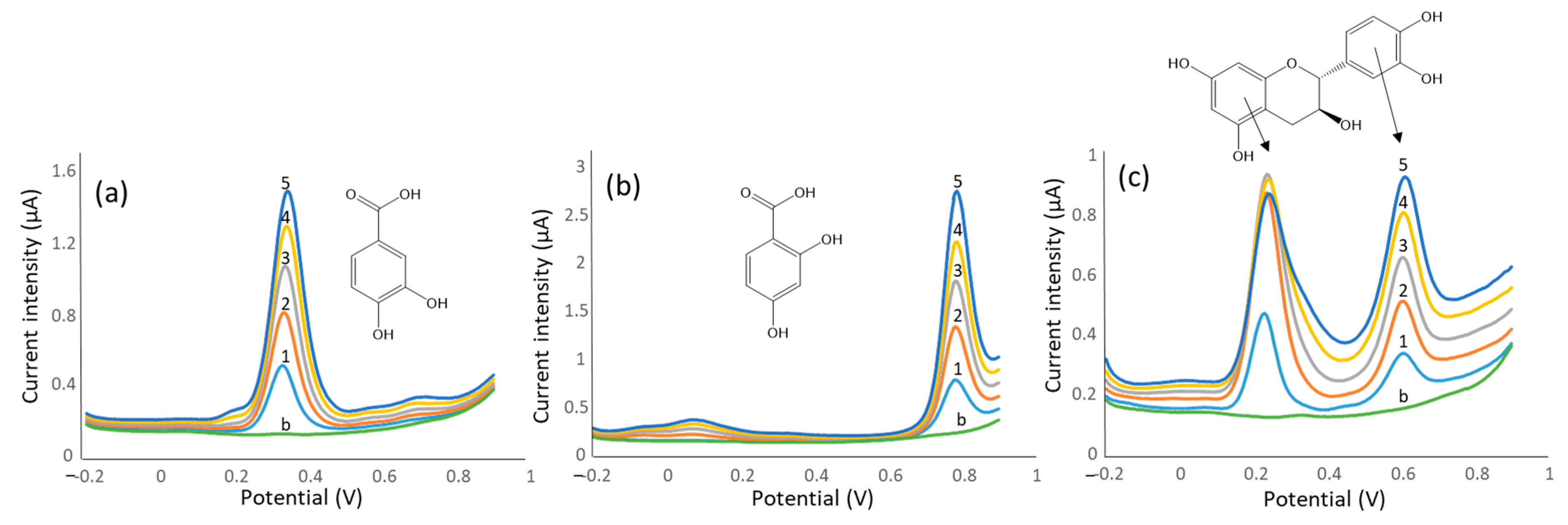
4.2. Voltammetric Methods
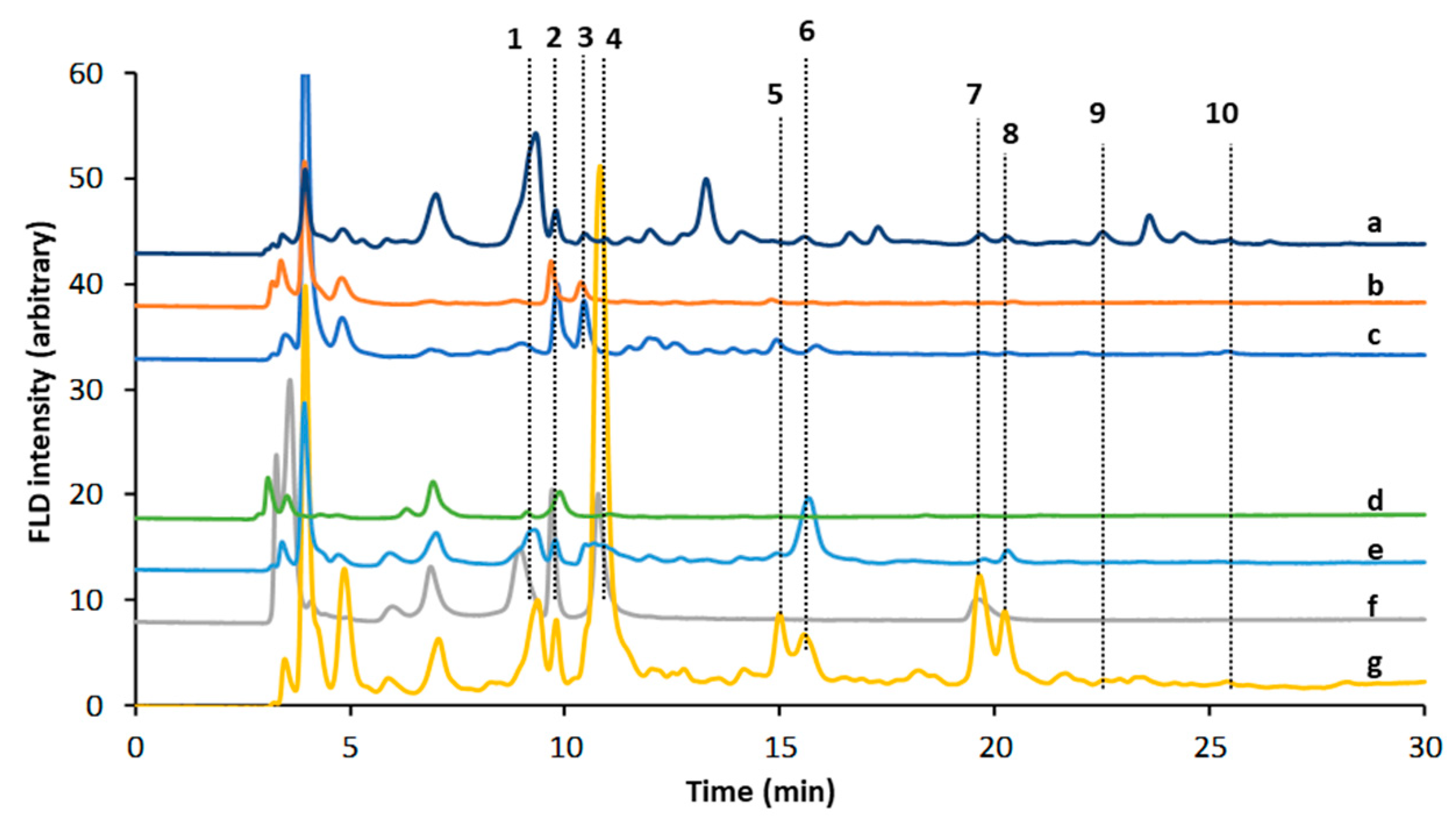
4.3. Chromatographic Methods
Liquid Chromatography Coupled to Mass Spectrometry
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy effects of plant polyphenols molecular mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef] [Green Version]
- Tomé-Carneiro, J.; Visioli, F. Polyphenol-based nutraceuticals for the prevention and treatment of cardiovascular disease: Review of human evidence. Phytomedicine 2016, 23, 1145–1174. [Google Scholar] [CrossRef]
- Abuajah, C.I.; Ogbonna, A.C.; Osuji, C.M. Functional components and medicinal properties of food: A review. J. Food Sci. Technol. 2015, 52, 2522–2529. [Google Scholar] [CrossRef] [Green Version]
- Lucci, P.; Saurina, J.; Núñez, O. Trends in LC-MS and LC-HRMS analysis and characterization of polyphenols in food. Trends Anal. Chem. 2017, 88, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Saurina, J. Characterization of wines using compositional profiles and chemometrics. Trends Anal. Chem. 2010, 29, 234–245. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Farhood, B.; Mortezaee, K.; Goradel, N.H.; Khanlarkhani, N.; Salehi, E.; Nashtaei, M.S.; Najafi, M.; Sahebkar, A. Curcumin as an anti-inflammatory agent: Implications to radiotherapy and chemotherapy. J. Cell. Physiol. 2019, 234, 5728–5740. [Google Scholar] [CrossRef]
- Tabrizi, R.; Vakili, S.; Akbari, M.; Mirhosseini, N.; Lankarani, K.B.; Rahimi, M.; Mobini, M.; Jafarnejad, S.; Vahedpoor, Z.; Asemi, Z. The effects of curcumin-containing supplements on biomarkers of inflammation and oxidative stress: A systematic review and meta-analysis of randomized controlled trials. Phyther. Res. 2019, 33, 253–262. [Google Scholar] [CrossRef]
- Tao, W.; Zhang, Y.; Shen, X.; Cao, Y.; Shi, J.; Ye, X.; Chen, S. Rethinking the Mechanism of the Health Benefits of Proanthocyanidins: Absorption, Metabolism, and Interaction with Gut Microbiota. Compr. Rev. Food Sci. Food Saf. 2019, 18, 971–985. [Google Scholar] [CrossRef] [Green Version]
- Coman, V.; Vodnar, D.C. Hydroxycinnamic acids and human health: Recent advances. J. Sci. Food Agric. 2020, 100, 483–499. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chemie Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and human health: The role of bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Flamini, R.; Mattivi, F.; De Rosso, M.; Arapitsas, P.; Bavaresco, L. Advanced knowledge of three important classes of grape phenolics: Anthocyanins, stilbenes and flavonols. Int. J. Mol. Sci. 2013, 14, 19651–19669. [Google Scholar] [CrossRef] [PubMed]
- Kubina, R.; Iriti, M.; Kabala-Dzik, A. Anticancer potential of selected flavonols: Fisetin, kaempferol, and quercetin on head and neck cancers. Nutrients 2021, 13, 845. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, H.G.; Sanlier, N. A minireview of quercetin: From its metabolism to possible mechanisms of its biological activities. Crit. Rev. Food Sci. Nutr. 2020, 60, 3290–3303. [Google Scholar] [CrossRef] [PubMed]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Kaur, M.; Silakari, O. Flavones: An important scaffold for medicinal chemistry. Eur. J. Med. Chem. 2014, 84, 206–239. [Google Scholar] [CrossRef] [PubMed]
- Ko, K. Isoflavones: Chemistry, Analysis, Functions and Effects on Health and Cancer. Asian Pac. J. Cancer Prev. 2014, 15, 7001–7010. [Google Scholar] [CrossRef] [Green Version]
- Messina, M. Soy foods, isoflavones, and the health of postmenopausal women. Am. J. Clin. Nutr. 2014, 100, 423S–430S. [Google Scholar] [CrossRef] [Green Version]
- Testai, L.; Calderone, V. Nutraceutical value of citrus flavanones and their implications in cardiovascular disease. Nutrients 2017, 9, 502. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, T. Dietary anthocyanin-rich plants: Biochemical basis and recent progress in health benefits studies. Mol. Nutr. Food Res. 2012, 56, 159–170. [Google Scholar] [CrossRef]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef] [Green Version]
- Martin, M.A.; Ramos, S. Impact of dietary flavanols on microbiota, immunity andinflammation in metabolic diseases. Nutrients 2021, 13, 850. [Google Scholar] [CrossRef]
- Sabaghi, M.; Hoseyni, S.Z.; Tavasoli, S.; Mozafari, M.R.; Katouzian, I. Strategies of confining green tea catechin compounds in nano-biopolymeric matrices: A review. Colloids Surfaces B Biointerfaces 2021, 204, 111781. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Krueger, C.G.; Reed, J.D.; Feliciano, R.P.; Howell, A.B. Quantifying and characterizing proanthocyanidins in cranberries in relation to urinary tract health. Anal. Bioanal. Chem. 2013, 405, 4385–4395. [Google Scholar] [CrossRef] [PubMed]
- Mallebrera, B.; Maietti, A.; Tedeschi, P.; Font, G.; Ruiz, M.J.; Brandolini, V. Antioxidant capacity of trans-resveratrol dietary supplements alone or combined with the mycotoxin beauvericin. Food Chem. Toxicol. 2017, 105, 315–318. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zálešák, F.; Bon, D.J.Y.D.; Pospíšil, J. Lignans and Neolignans: Plant secondary metabolites as a reservoir of biologically active substances. Pharmacol. Res. 2019, 146, 104284. [Google Scholar] [CrossRef]
- Vidal-Casanella, O.; Nuñez, O.; Saurina, J. Liquid chromatographic fingerprints for the characterization of flavanol-rich nutraceuticals based on 4-dimethylaminocinnamaldehyde precolumn derivatization. Sci. Pharm. 2021, 89, 18. [Google Scholar] [CrossRef]
- Gudzinskaite, I.; Stackeviciene, E.; Liaudanskas, M.; Zymone, K.; Zvikas, V.; Viskelis, J.; Urbstaite, R.; Janulis, V. Variability in the qualitative and quantitative composition and content of phenolic compounds in the fruit of introduced American cranberry (Vaccinium macrocarpon Aiton). Plants 2020, 9, 1379. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Liu, H.; Gu, L. American cranberries and health benefits—An evolving story of 25 years. J. Sci. Food Agric. 2020, 100, 5111–5116. [Google Scholar] [CrossRef]
- Côté, J.; Caillet, S.; Doyon, G.; Sylvain, J.F.; Lacroix, M. Analyzing cranberry bioactive compounds. Crit. Rev. Food Sci. Nutr. 2010, 50, 872–888. [Google Scholar] [CrossRef]
- Blumberg, J.B.; Camesano, T.A.; Cassidy, A.; Kris-Etherton, P.; Howell, A.; Manach, C.; Ostertag, L.M.; Sies, H.; Skulas-Ray, A.; Vita, J.A. Cranberries and their bioactive constituents in human health. Adv. Nutr. 2013, 4, 618–632. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.S.; Shen, C.L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ríos-Hoyo, A.; Gutiérrez-Salmeán, G. New Dietary Supplements for Obesity: What We Currently Know. Curr. Obes. Rep. 2016, 5, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T. Recent progress in anti-obesity and anti-diabetes effect of berries. Antioxidants 2016, 5, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgiev, V.; Ananga, A.; Tsolova, V. Recent advances and uses of grape flavonoids as nutraceuticals. Nutrients 2014, 6, 391–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Li, H. Bin Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Pereira, C.; Barros, L.; Ferreira, I.C. Extraction, identification, fractionation and isolation of phenolic compounds in plants with hepatoprotective effects. J. Sci. Food Agric. 2016, 96, 1068–1084. [Google Scholar] [CrossRef] [PubMed]
- Gostin, A.I.; Waisundara, V.Y. Edible flowers as functional food: A review on artichoke (Cynara cardunculus L.). Trends Food Sci. Technol. 2019, 86, 381–391. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brainina, K.; Stozhko, N.; Vidrevich, M. Antioxidants: Terminology, methods, and future considerations. Antioxidants 2019, 8, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Alcalde, B.; Granados, M.; Saurina, J. Exploring the Antioxidant Features of Polyphenols by Spectroscopic and Electrochemical Methods. Antioxidants 2019, 8, 523. [Google Scholar] [CrossRef] [Green Version]
- Pękal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef] [Green Version]
- Feliciano, R.P.; Shea, M.P.; Shanmuganayagam, D.; Krueger, C.G.; Howell, A.B.; Reed, J.D. Comparison of isolated cranberry (Vaccinium macrocarpon Ait.) proanthocyanidins to catechin and procyanidins A2 and B2 for use as standards in the 4-(dimethylamino)cinnamaldehyde assay. J. Agric. Food Chem. 2012, 60, 4578–4585. [Google Scholar] [CrossRef]
- Payne, M.J.; Hurst, W.J.; Stuart, D.A.; Ou, B.; Fan, E.; Ji, H.; Kou, Y. Determination of total procyanidins in selected chocolate and confectionery products using DMAC. J. AOAC Int. 2010, 93, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Singh, A.P.; Hurst, W.J.; Glinski, J.A.; Koo, H.; Vorsa, N. Influence of Degree-of-Polymerization and Linkage on the Quantification of Proanthocyanidins using 4-Dimethylaminocinnamaldehyde (DMAC) Assay. J. Agric. Food Chem. 2016, 64, 2190–2199. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Casanella, O.; Nuñez, O.; Hernández-Cassou, S.; Saurina, J. Assessment of Experimental Factors Affecting the Sensitivity and Selectivity of the Spectrophotometric Estimation of Proanthocyanidins in Foods and Nutraceuticals. Food Anal. Methods 2021, 14, 485–495. [Google Scholar] [CrossRef]
- Krueger, C.G.; Chesmore, N.; Chen, X.; Parker, J.; Khoo, C.; Marais, J.P.J.; Shanmuganayagam, D.; Crump, P.; Reed, J.D. Critical reevaluation of the 4-(dimethylamino)cinnamaldehyde assay: Cranberry proanthocyanidin standard is superior to procyanidin A2 dimer for accurate quantification of proanthocyanidins in cranberry products. J. Funct. Foods 2016, 22, 13–19. [Google Scholar] [CrossRef]
- Arribas, A.S.; Martinez-Fernandez, M.; Chicharro, M. The role of electroanalytical techniques in analysis of polyphenols in wine. TrAC Trends Anal. Chem. 2012, 34, 78–96. [Google Scholar] [CrossRef]
- Petrovic, S.C. Correlation of perceived wine astringency to cyclic voltammetric response. Am. J. Enol. Vitic. 2009, 60, 373–378. [Google Scholar]
- Makhotkina, O.; Kilmartin, P.A. The use of cyclic voltammetry for wine analysis: Determination of polyphenols and free sulfur dioxide. Anal. Chim. Acta 2010, 668, 155–165. [Google Scholar] [CrossRef]
- David, I.G.; Buleandră, M.; Popa, D.E.; Bîzgan, A.-M.C.; Moldovan, Z.; Badea, I.-A.; Iorgulescu, E.E.; Tekiner, T.A.; Basaga, H. Voltammetric determination of polyphenolic content as rosmarinic acid equivalent in tea samples using pencil graphite electrodes. J. Food Sci. Technol. 2016, 53, 2589–2596. [Google Scholar] [CrossRef] [Green Version]
- David, I.G.; Bizgan, A.-M.C.; Popa, D.E.; Buleandra, M.; Moldovan, Z.; Badea, I.A.; Tekiner, T.A.; Basaga, H.; Ciucu, A.A. Rapid determination of total polyphenolic content in tea samples based on caffeic acid voltammetric behaviour on a disposable graphite electrode. Food Chem. 2015, 173, 1059–1065. [Google Scholar] [CrossRef]
- Oliveira-Neto, J.R.; Rezende, S.G.; de Fátima Reis, C.; Benjamin, S.R.; Rocha, M.L.; de Souza Gil, E. Electrochemical behavior and determination of major phenolic antioxidants in selected coffee samples. Food Chem. 2016, 190, 506–512. [Google Scholar] [CrossRef]
- Memon, A.F.; Solangi, A.R.; Memon, S.Q.; Mallah, A.; Memon, N. Quantitative separation of hesperidin, chrysin, epicatechin, epigallocatechin gallate, and morin using ionic liquid as a buffer additive in capillary electrophoresis. Electrophoresis 2018, 39, 1606–1612. [Google Scholar] [CrossRef]
- Arries, W.J.; Tredoux, A.G.J.; de Beer, D.; Joubert, E.; de Villiers, A. Evaluation of capillary electrophoresis for the analysis of rooibos and honeybush tea phenolics. Electrophoresis 2017, 38, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Dresler, S.; Bogucka-Kocka, A.; Kováčik, J.; Kubrak, T.; Strzemski, M.; Wójciak-Kosior, M.; Rysiak, A.; Sowa, I. Separation and determination of coumarins including furanocoumarins using micellar electrokinetic capillary chromatography. Talanta 2018, 187, 120–124. [Google Scholar] [CrossRef]
- Navarro, M.; Núñez, O.; Saurina, J.; Hernández-Cassou, S.; Puignou, L. Characterization of fruit products by capillary zone electrophoresis and liquid chromatography using the compositional profiles of polyphenols: Application to authentication of natural extracts. J. Agric. Food Chem. 2014, 62, 1038–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Przybylska, A.; Gackowski, M.; Koba, M. Application of capillary electrophoresis to the analysis of bioactive compounds in herbal raw materials. Molecules 2021, 26, 2135. [Google Scholar] [CrossRef]
- Ahmad, N.; Zuo, Y.; Lu, X.; Anwar, F.; Hameed, S. Characterization of free and conjugated phenolic compounds in fruits of selected wild plants. Food Chem. 2016, 190, 80–89. [Google Scholar] [CrossRef]
- Osman, M.F.; Hassan, N.M.; Khatib, A.; Tolos, S.M. Antioxidant activities of Dialium indum L. Fruit and gas chromatography-mass spectrometry (GC-MS) of the active fractions. Antioxidants 2018, 7, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.H.; Morgan, A.M.A.; Park, B.B.; Lee, S.Y.; Lee, S.; Kim, J.K.; Park, S.U. Metabolic analysis of four cultivars of liriope platyphylla. Metabolites 2019, 9, 59. [Google Scholar] [CrossRef] [Green Version]
- Ifeanacho, M.O.; Ikewuchi, C.C.; Ikewuchi, J.C. Investigation of the profile of phenolic compounds in the leaves and stems of Pandiaka heudelotii using gas chromatography coupled with flame ionization detector. Food Sci. Nutr. 2017, 5, 646–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Narciso, V.; Tenore, G.C.; Novellino, E. Effects of grape pomace polyphenolic extract (Taurisolo®) in reducing tmao serum levels in humans: Preliminary results from a randomized, placebo-controlled, cross-over study. Nutrients 2019, 11, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Gutiérrez, N.; Romero-González, R.; Plaza-Bolaños, P.; Martínez Vidal, J.L.; Garrido Frenich, A. Identification and quantification of phytochemicals in nutraceutical products from green tea by UHPLC-Orbitrap-MS. Food Chem. 2015, 173, 607–618. [Google Scholar] [CrossRef]
- López-Gutiérrez, N.; Romero-González, R.; Martínez Vidal, J.L.; Frenich, A.G. Determination of polyphenols in grape-based nutraceutical products using high resolution mass spectrometry. LWT Food Sci. Technol. 2016, 71, 249–259. [Google Scholar] [CrossRef]
- Bakhytkyzy, I.; Nuñez, O.; Saurina, J. Size Exclusion Coupled to Reversed Phase Liquid Chromatography for the Characterization of Cranberry Products. Food Anal. Methods 2019, 12, 604–611. [Google Scholar] [CrossRef] [Green Version]
- Parets, L.; Alechaga, É.; Núñez, O.; Saurina, J.; Hernández-Cassou, S.; Puignou, L. Ultrahigh pressure liquid chromatography-atmospheric pressure photoionization-tandem mass spectrometry for the determination of polyphenolic profiles in the characterization and classification of cranberry-based pharmaceutical preparations and natural ext. Anal. Methods 2016, 8, 4363–4378. [Google Scholar] [CrossRef] [Green Version]
- Vidal-Casanella, O.; Arias-Alpizar, K.; Nuñez, O.; Saurina, J. Hydrophilic interaction liquid chromatography to characterize nutraceuticals and food supplements based on flavanols and related compounds. Separations 2021, 8, 17. [Google Scholar] [CrossRef]
- Cacciola, F.; Rigano, F.; Dugo, P.; Mondello, L. Comprehensive two-dimensional liquid chromatography as a powerful tool for the analysis of food and food products. TrAC Trends Anal. Chem. 2020, 127, 115894. [Google Scholar] [CrossRef]
- Cacciola, F.; Arena, K.; Mandolfino, F.; Donnarumma, D.; Dugo, P.; Mondello, L. Reversed phase versus hydrophilic interaction liquid chromatography as first dimension of comprehensive two-dimensional liquid chromatography systems for the elucidation of the polyphenolic content of food and natural products. J. Chromatogr. A 2021, 1645, 462129. [Google Scholar] [CrossRef] [PubMed]
- Arena, K.; Cacciola, F.; Mangraviti, D.; Zoccali, M.; Rigano, F.; Marino, N.; Dugo, P.; Mondello, L. Determination of the polyphenolic fraction of Pistacia vera L. kernel extracts by comprehensive two-dimensional liquid chromatography coupled to mass spectrometry detection. Anal. Bioanal. Chem. 2019, 411, 4819–4829. [Google Scholar] [CrossRef]
- Sommella, E.; Ismail, O.H.; Pagano, F.; Pepe, G.; Ostacolo, C.; Mazzoccanti, G.; Russo, M.; Novellino, E.; Gasparrini, F.; Campiglia, P. Development of an improved online comprehensive hydrophilic interaction chromatography × reversed-phase ultra-high-pressure liquid chromatography platform for complex multiclass polyphenolic sample analysis. J. Sep. Sci. 2017, 40, 2188–2197. [Google Scholar] [CrossRef]
- Tamasi, G.; Pardini, A.; Bonechi, C.; Donati, A.; Pessina, F.; Marcolongo, P.; Gamberucci, A.; Leone, G.; Consumi, M.; Magnani, A.; et al. Characterization of nutraceutical components in tomato pulp, skin and locular gel. Eur. Food Res. Technol. 2019, 245, 907–918. [Google Scholar] [CrossRef]
- Simeoni, M.C.; Pellegrini, M.; Sergi, M.; Pittia, P.; Ricci, A.; Compagnone, D. Analysis of Polyphenols in the Lamiaceae Family by Matrix Solid-Phase Dispersion Extraction Followed by Ultra-High-Performance Liquid Chromatography-Tandem Mass Spectrometry Determination. ACS Omega 2018, 3, 17610–17616. [Google Scholar] [CrossRef]
- Núñez, N.; Vidal-Casanella, O.; Sentellas, S.; Saurina, J.; Núñez, O. Characterization, classification and authentication of turmeric and curry samples by targeted LC-HRMS polyphenolic and curcuminoid profiling and chemometrics. Molecules 2020, 25, 2942. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Mena, G.; Sánchez-González, M.; Parra, A.; Juan, M.E.; Planas, J.M. Identification of gut-derived metabolites of maslinic acid, a bioactive compound from Olea europaea L. Mol. Nutr. Food Res. 2016, 60, 2053–2064. [Google Scholar] [CrossRef]
- Garzón, G.A.; Narváez-Cuenca, C.E.; Kopec, R.E.; Barry, A.M.; Riedl, K.M.; Schwartz, S.J. Determination of carotenoids, total phenolic content, and antioxidant activity of Arazá (Eugenia stipitata McVaugh), an amazonian fruit. J. Agric. Food Chem. 2012, 60, 4709–4717. [Google Scholar] [CrossRef] [PubMed]
- Sut, S.; Zengin, G.; Maggi, F.; Malagoli, M.; Dall’Acqua, S. Triterpene acid and phenolics from ancient apples of Friuli Venezia Giulia as nutraceutical ingredients: LC-MS study and in vitro activities. Molecules 2019, 24, 1109. [Google Scholar] [CrossRef] [Green Version]
- Sut, S.; Poloniato, G.; Malagoli, M.; Dall’acqua, S. Fragmentation of the main triterpene acids of apple by LC- APCI-MSn. J. Mass Spectrom. 2018, 53, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Arrizabalaga-Larrañaga, A.; Campmajó, G.; Saurina, J.; Núñez, O.; Santos, F.J.; Moyano, E. Determination of capsaicinoids and carotenoids for the characterization and geographical origin authentication of paprika by UHPLC–APCI–HRMS. LWT- Food Sci. Technol. 2021, 139, 110533. [Google Scholar] [CrossRef]
- Giuffrida, D.; Pintea, A.; Dugo, P.; Torre, G.; Pop, R.M.; Mondello, L. Determination of carotenoids and their esters in fruits of sea buckthorn (Hippophae rhamnoides L.) by HPLC-DAD-APCI-MS. Phytochem. Anal. 2012, 23, 267–273. [Google Scholar] [CrossRef]
- Bettaiah, A.; Prabhushankar, H.B. Screening of Novel Source for Genistein by Rapid and Sensitive UPLC-APCI-TOF Mass Spectrometry. Int. J. Food Sci. 2021, 2021, 5537917. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, X.Y.; Gan, R.Y.; Zheng, J.; Li, Y.; Zhang, J.J.; Xu, D.P.; Li, H. Bin Optimization of ultrasound-assisted extraction of antioxidant polyphenols from the seed coats of red sword bean (Canavalia gladiate (Jacq.) DC.). Antioxidants 2019, 8, 200. [Google Scholar] [CrossRef] [Green Version]
- Sut, S.; Boschiero, I.; Solana, M.; Malagoli, M.; Bertucco, A.; Dall’Acqua, S. Supercritical CO2 extraction of eruca sativa using cosolvents: Phytochemical composition by LC-MS analysis. Molecules 2018, 23, 3240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Majdoub, Y.O.; Alibrando, F.; Cacciola, F.; Arena, K.; Pagnotta, E.; Matteo, R.; Micalizzi, G.; Dugo, L.; Dugo, P.; Mondello, L. Chemical Characterization of Three Accessions of Brassica juncea L. Extracts from Different Plant Tissues. Molecules 2020, 25, 5421. [Google Scholar] [CrossRef]
- Nkuimi Wandjou, J.G.; Lancioni, L.; Barbalace, M.C.; Hrelia, S.; Papa, F.; Sagratini, G.; Vittori, S.; Dall’Acqua, S.; Caprioli, G.; Beghelli, D.; et al. Comprehensive characterization of phytochemicals and biological activities of the Italian ancient apple ‘Mela Rosa dei Monti Sibillini. Food Res. Int. 2020, 137, 109422. [Google Scholar] [CrossRef] [PubMed]
- Nzekoue, F.K.; Angeloni, S.; Navarini, L.; Angeloni, C.; Freschi, M.; Hrelia, S.; Vitali, L.A.; Sagratini, G.; Vittori, S.; Caprioli, G. Coffee silverskin extracts: Quantification of 30 bioactive compounds by a new HPLC-MS/MS method and evaluation of their antioxidant and antibacterial activities. Food Res. Int. 2020, 133, 109128. [Google Scholar] [CrossRef] [PubMed]
- Amat-Ur-rasool, H.; Symes, F.; Tooth, D.; Schaffert, L.N.; Elmorsy, E.; Ahmed, M.; Hasnain, S.; Carter, W.G. Potential nutraceutical properties of leaves from several commonly cultivated plants. Biomolecules 2020, 10, 1556. [Google Scholar] [CrossRef]
- Reddy, M.N.; Adnan, M.; Alreshidi, M.M.; Saeed, M.; Patel, M. Evaluation of Anticancer, Antibacterial and Antioxidant Properties of a Medicinally Treasured Fern Tectaria coadunata with its Phytoconstituents Analysis by HR-LCMS. Anticancer. Agents Med. Chem. 2020, 20, 1845–1856. [Google Scholar] [CrossRef]
- Rocchetti, G.; Senizza, B.; Zengin, G.; Mahomodally, M.F.; Senkardes, I.; Lobine, D.; Lucini, L. Untargeted metabolomic profiling of three Crataegus species (hawthorn) and their in vitro biological activities. J. Sci. Food Agric. 2020, 100, 1998–2006. [Google Scholar] [CrossRef] [PubMed]
- Arshad, A.; Ahemad, S.; Saleem, H.; Saleem, M.; Zengin, G.; Abdallah, H.H.; Tousif, M.I.; Ahemad, N.; Mahomoodally, M.F. RP-UHPLC-MS chemical profiling, biological and in silico docking studies to unravel the therapeutic potential of heliotropium crispum desf. As a novel source of neuroprotective bioactive compounds. Biomolecules 2021, 11, 53. [Google Scholar] [CrossRef]
- Rouphael, Y.; Bernardi, J.; Cardarelli, M.; Bernardo, L.; Kane, D.; Colla, G.; Lucini, L. Phenolic Compounds and Sesquiterpene Lactones Profile in Leaves of Nineteen Artichoke Cultivars. J. Agric. Food Chem. 2016, 64, 8540–8548. [Google Scholar] [CrossRef]
- Lachowicz, S.; Oszmiański, J. Profile of Bioactive Compounds in the Morphological Parts of Wild Fallopia japonica (Houtt) and Fallopia sachalinensis (F. Schmidt) and Their Antioxidative Activity. Molecules 2019, 24, 1436. [Google Scholar] [CrossRef] [Green Version]
- Pepe, G.; Salviati, E.; Rapa, S.F.; Ostacolo, C.; Cascioferro, S.; Manfra, M.; Autore, G.; Marzocco, S.; Campiglia, P. Citrus sinensis and vitis vinifera protect cardiomyocytes from doxorubicin-induced oxidative stress: Evaluation of onconutraceutical potential of vegetable smoothies. Antioxidants 2020, 9, 378. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, L.; Izzo, L.; De Pascale, S.; Narváez, A.; Rodriguez-Carrasco, Y.; Ritieni, A. Chemical composition, in vitro bioaccessibility and antioxidant activity of polyphenolic compounds from nutraceutical fennel waste extract. Molecules 2021, 26, 1968. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zheng, J.; Zhang, L.; Yan, S.; Huang, W.; He, J. Phytochemical Profiling and Fingerprint Analysis of Chinese Jujube (Ziziphus jujuba Mill.) Leaves of 66 Cultivars from Xinjiang Province. Molecules 2019, 24, 4528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maia, M.; Ferreira, A.E.N.; Laureano, G.; Marques, A.P.; Torres, V.M.; Silva, A.B.; Matos, A.R.; Cordeiro, C.; Figueiredo, A.; Silva, M.S. Vitis vinifera “Pinot noir” leaves as a source of bioactive nutraceutical compounds. Food Funct. 2019, 10, 3822–3827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giffen, J.E.; Lesiak, A.D.; Dane, A.J.; Cody, R.B.; Musah, R.A. Rapid Species-level Identification of Salvias by Chemometric Processing of Ambient Ionisation Mass Spectrometry-derived Chemical Profiles. Phytochem. Anal. 2017, 28, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Riya, M.P.; Antu, K.A.; Vinu, T.; Chandrakanth, K.C.; Anilkumar, K.S.; Raghu, K.G. An in vitro study reveals nutraceutical properties of Ananas comosus (L.) Merr. var. Mauritius fruit residue beneficial to diabetes. J. Sci. Food Agric. 2014, 94, 943–950. [Google Scholar] [CrossRef]
- Perez, C.J.; Conceição, R.S.; Ifa, D.R. Chemical profiling and separation of bioactive secondary metabolites in Maca (Lepidium peruvianum) by normal and reverse phase thin layer chromatography coupled to desorption electrospray ionization-mass spectrometry. J. Mass Spectrom. 2021, 56, 1–14. [Google Scholar] [CrossRef]
- Monagas, M.; Quintanilla-López, J.E.; Gómez-Cordovés, C.; Bartolomé, B.; Lebrón-Aguilar, R. MALDI-TOF MS analysis of plant proanthocyanidins. J. Pharm. Biomed. Anal. 2010, 51, 358–372. [Google Scholar] [CrossRef]
- Pinto, G.; De Pascale, S.; Aponte, M.; Scaloni, A.; Addeo, F.; Caira, S. Polyphenol profiling of chestnut pericarp, integument and curing water extracts to qualify these food by-products as a source of antioxidants. Molecules 2021, 26, 2335. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidal-Casanella, O.; Núñez, O.; Granados, M.; Saurina, J.; Sentellas, S. Analytical Methods for Exploring Nutraceuticals Based on Phenolic Acids and Polyphenols. Appl. Sci. 2021, 11, 8276. https://doi.org/10.3390/app11188276
Vidal-Casanella O, Núñez O, Granados M, Saurina J, Sentellas S. Analytical Methods for Exploring Nutraceuticals Based on Phenolic Acids and Polyphenols. Applied Sciences. 2021; 11(18):8276. https://doi.org/10.3390/app11188276
Chicago/Turabian StyleVidal-Casanella, Oscar, Oscar Núñez, Mercè Granados, Javier Saurina, and Sonia Sentellas. 2021. "Analytical Methods for Exploring Nutraceuticals Based on Phenolic Acids and Polyphenols" Applied Sciences 11, no. 18: 8276. https://doi.org/10.3390/app11188276
APA StyleVidal-Casanella, O., Núñez, O., Granados, M., Saurina, J., & Sentellas, S. (2021). Analytical Methods for Exploring Nutraceuticals Based on Phenolic Acids and Polyphenols. Applied Sciences, 11(18), 8276. https://doi.org/10.3390/app11188276








