Role of Seasonality and Fire in Regulating the Enzymatic Activities in Soils Covered by Different Vegetation in a Mediterranean Area
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Soil Sampling
2.2. Soil Analyses
2.3. Statistical Analyses
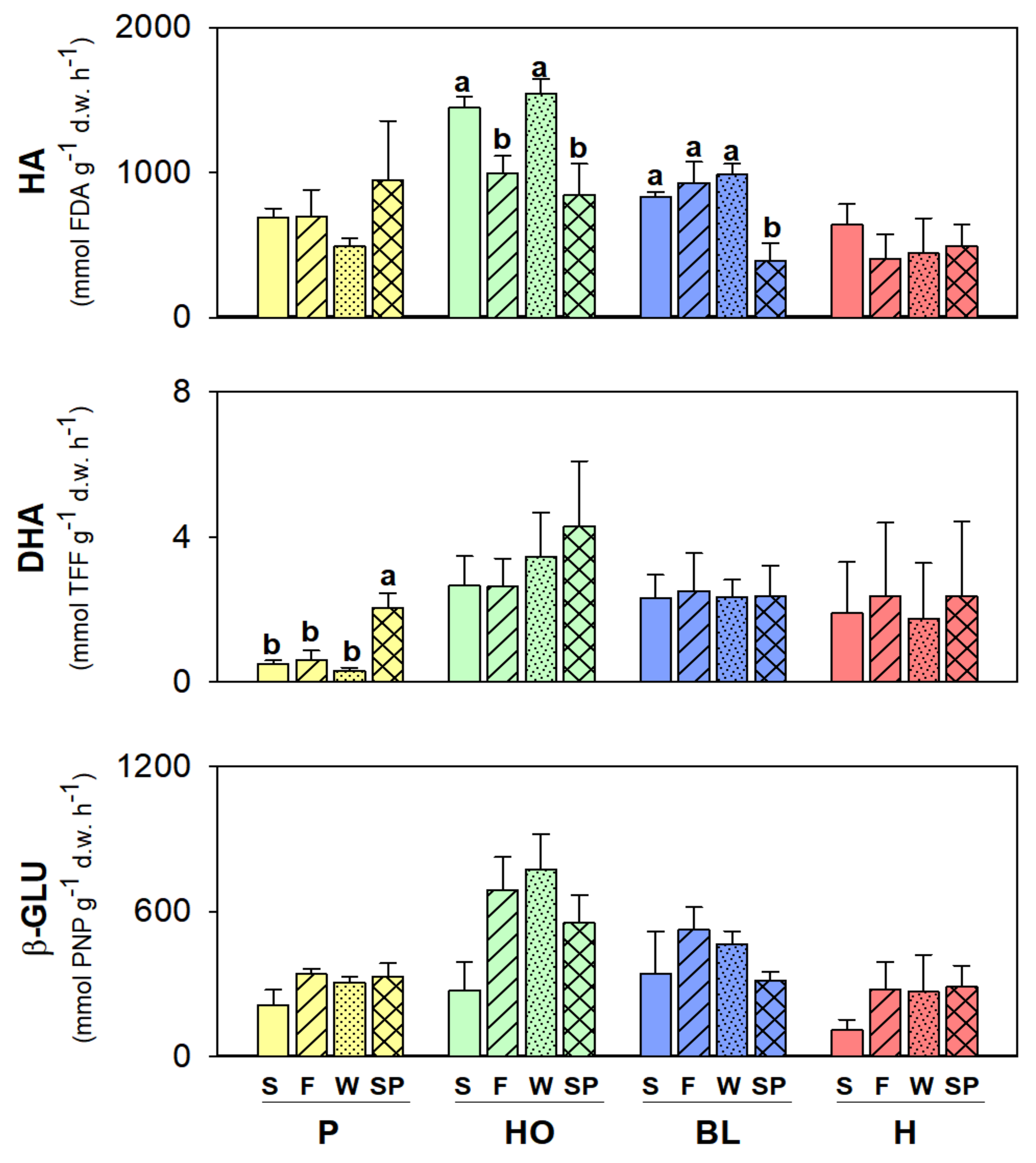
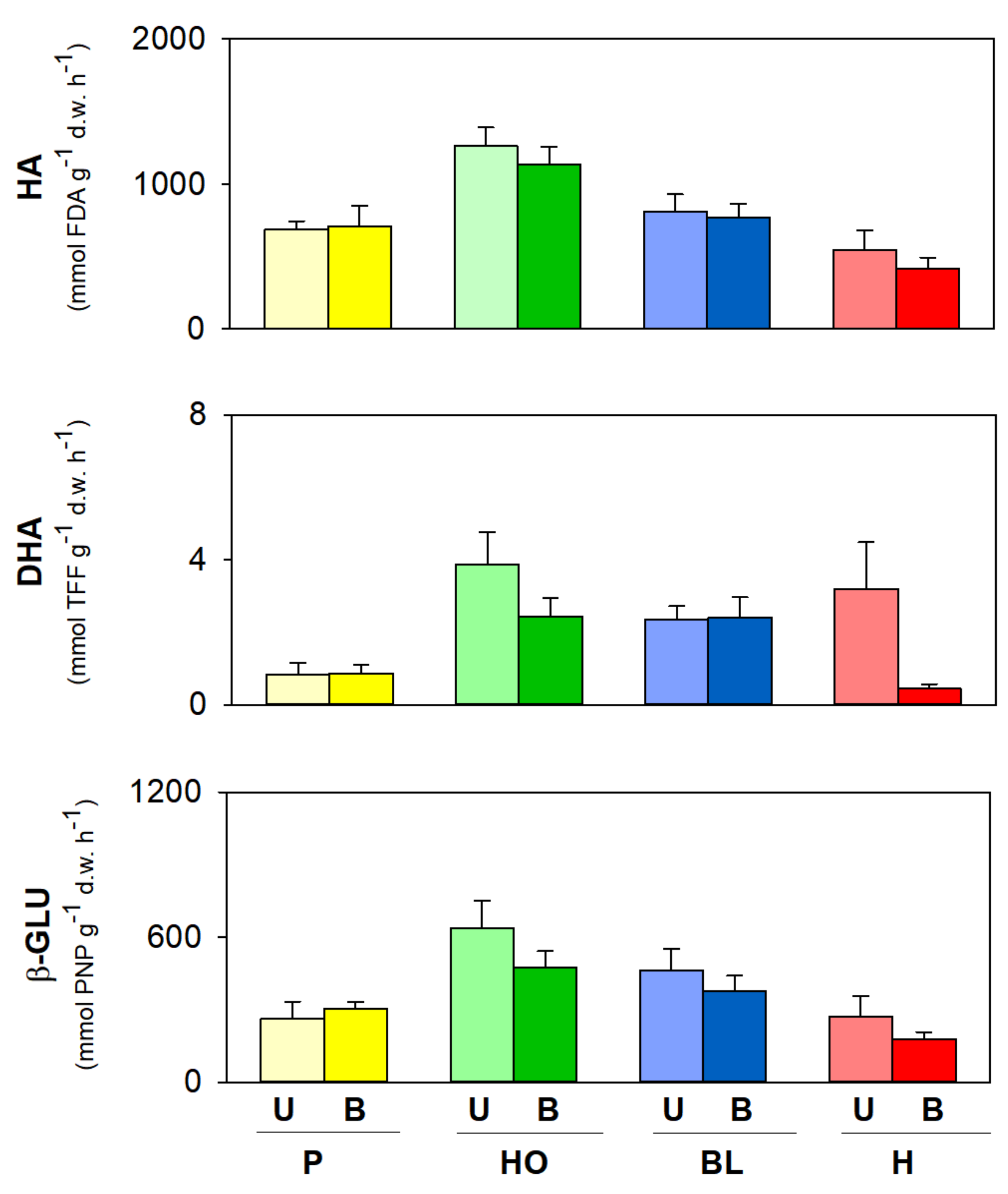
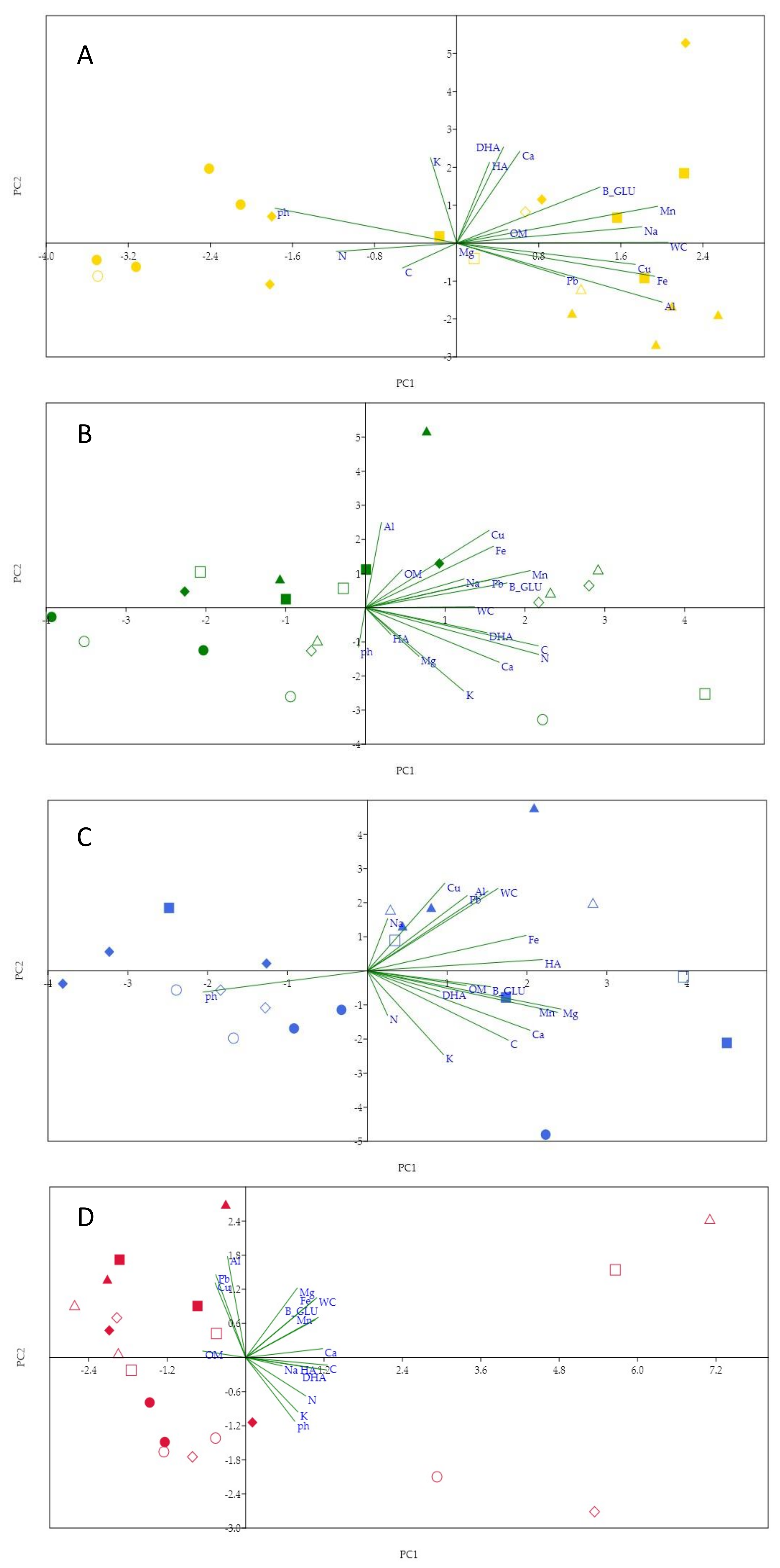
3. Results
Soil Abiotic and Biotic Properties within the Same Vegetation Cover
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karaca, A.; Cetin, S.C.; Turgay, O.C.; Kizilkaya, R. Soil enzymes as indication of soil quality. In Soil Enzymology; Shukla, G., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 119–148. [Google Scholar]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Kivlin, S.N.; Treseder, K.K. Soil extracellular enzyme activities correspond with abiotic factors more than fungal community composition. Biogeochemistry 2014, 117, 23–37. [Google Scholar] [CrossRef] [Green Version]
- Henry, H.A. Soil extracellular enzyme dynamics in a changing climate. Soil Biol. Biochem. 2012, 47, 53–59. [Google Scholar] [CrossRef]
- Cao, R.; Yang, W.; Chang, C.; Wang, Z.; Wang, Q.; Li, H.; Tan, B. Differential seasonal changes in soil enzyme activity along an altitudinal gradient in an alpine-gorge region. Appl. Soil Ecol. 2021, 166, 104078. [Google Scholar] [CrossRef]
- Kivlin, S.N.; Hawkes, C.V. Spatial and temporal turnover of soil microbial communities is not linked to function in a primary tropical forest. Ecology 2020, 101, e02985. [Google Scholar] [CrossRef]
- Midgley, M.G.; Phillips, R.P. Spatio-temporal heterogeneity in extracellular enzyme activities tracks variation in saprotrophic fungal biomass in a temperate hardwood forest. Soil Biol. Biochem. 2019, 138, 107600. [Google Scholar] [CrossRef]
- Li, Z.; Yang, W.; Yue, K.; Justine, M.F.; He, R.; Yang, K.; Zhuang, L.; Wu, F.; Tan, B.; Zhang, L.; et al. Effects of snow absence on winter soil nitrogen dynamics in a subalpine spruce forest of southwestern China. Geoderma 2017, 307, 107–113. [Google Scholar] [CrossRef]
- Akinyemi, D.S.; Zhu, Y.; Zhao, M.; Zhang, P.; Shen, H.; Fang, J. Response of soil extracellular enzyme activity to experimental precipitation in a shrub-encroached grassland in Inner Mongolia. Glob. Ecol. Conserv. 2020, 23, e01175. [Google Scholar] [CrossRef]
- Ouyang, Y.; Li, X. Effect of repeated drying-rewetting cycles on soil extracellular enzyme activities and microbial community composition in arid and semi-arid ecosystems. Eur. J. Soil Biol. 2020, 98, 103187. [Google Scholar] [CrossRef]
- Loeppmann, S.; Blagodatskaya, E.; Pausch, J.; Kuzyakov, Y. Substrate quality affects kinetics and catalytic efficiency of exo-enzymes in rhizosphere and detritusphere. Soil Biol. Biochem. 2016, 92, 111–118. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, T.; Wang, S.; Wang, Z. Soil pH and C/N ratio determines spatial variations in soil microbial communities and enzymatic activities of the agricultural ecosystems in Northeast China: Jilin Province case. Appl. Soil Ecol. 2020, 155, 103629. [Google Scholar] [CrossRef]
- Santorufo, L.; Cortet, J.; Nahmani, J.; Pernin, C.; Salmon, S.; Pernot, A.; Morel, J.L.; Maisto, G. Responses of functional and taxonomic collembolan community structure to site management in Mediterranean urban and surrounding areas. Eur. J. Soil Biol. 2015, 70, 46–57. [Google Scholar] [CrossRef]
- Santorufo, L.; Memoli, V.; Panico, S.C.; Santini, G.; Barile, R.; Di Natale, G.; Trifuoggi, M.; De Marco, A.; Maisto, G. Early post-fire changes in properties of Andosols within a Mediterranean area. Geoderma 2021, 394, 115016. [Google Scholar] [CrossRef]
- Memoli, V.; Eymar, E.; García-Delgado, C.; Esposito, F.; Panico, S.C.; De Marco, A.; Barile, R.; Maisto, G. Soil element fractions affect phytotoxicity, microbial biomass and activity in volcanic areas. Sci. Total Environ. 2018, 636, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Memoli, V.; Panico, S.C.; Santorufo, L.; Barile, R.; Di Natale, G.; Di Nunzio, A.; Toscanesi, M.; Trifuoggi, M.; De Marco, A.; Maisto, G. Do wildfires cause changes in soil quality in short term? Int. J. Environ. Res. Public Health 2020, 17, 5343. [Google Scholar] [CrossRef] [PubMed]
- Saá, A.; Trasar-Cepeda, M.C.; Gil-Sotres, F.; Carballas, T. Changes in soil phosphorus and acid phosphatase activity immediately following forest fires. Soil Biol. Biochem. 1993, 25, 1223–1230. [Google Scholar] [CrossRef]
- Boerner, R.E.J.; Giai, C.; Huang, J.; Miesel, J.R. Initial effects of fire and mechanical thinning on soil enzyme activity and nitrogen transformations in eight North American forest ecosystems. Soil Biol. Biochem. 2008, 40, 3076–3085. [Google Scholar] [CrossRef]
- Rutigliano, F.A.; D’ascoli, R.; Virzo De Santo, A. Soil microbial metabolism and nutrient status in a Mediterranean area as affected by plant cover. Soil Biol. Biochem. 2004, 436, 1719–1729. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Drought decreases soil enzyme activity in a Mediterranean Quercus ilex L. forest. Soil Biol. Biochem. 2005, 37, 455–461. [Google Scholar] [CrossRef]
- Guénon, R.; Gros, R. Frequent-wildfires with shortened time-since-fire affect soil microbial functional stability to drying and rewetting events. Soil Biol. Biochem 2013, 57, 663–674. [Google Scholar] [CrossRef]
- Bárcenas-Moreno, G.; García-Orenes, F.; Mataix-Beneyto, J.; Bååth, E. Plant species influence on soil microbial short-term response after fire simulation. Plant Soil 2014, 374, 701–713. [Google Scholar] [CrossRef]
- Stott, D.E.; Andrews, S.S.; Liebig, M.A.; Wienhold, B.J.; Karlen, D.L. Evaluation of β-glucosidase activity as a soil quality indicator for the soil management assessment framework. Soil Sci. Soc. Am. J. 2010, 74, 107–119. [Google Scholar] [CrossRef] [Green Version]
- Dotaniya, M.L.; Aparna, K.; Dotaniya, C.K.; Singh, M.; Regar, K.L. Role of Soil Enzymes in Sustainable Crop Production. Enzym. Food Biotechnol. Prod. Appl. Future Prospect. 2019, 569–589. [Google Scholar] [CrossRef]
- Adam, G.; Duncan, H. Development of a sensitive and rapid method for the measurement of total microbial activity using Fluorescein Diacetate (FDA) in a range of soils. Soil Biol. Biochem. 2001, 33, 943–951. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Xu, Z.; Chen, C.; Zhou, G.; Liu, J.; Abdullah, K.M.; Reverchon, F.; Liu, X. Short-term effects of prescribed burning on phosphorus availability in a suburban native forest of subtropical Australia. J. Soils Sediments 2013, 13, 869–876. [Google Scholar] [CrossRef]
- Holden, S.R.; Gutierrez, A.; Treseder, K.K. Changes in soil fungal communities, extracellular enzyme activities, and litter decomposition across a fire chronosequence in Alaskan boreal forests. Ecosystems 2013, 16, 34–46. [Google Scholar] [CrossRef] [Green Version]
- Wolińska, A.; Stępniewska, Z. Dehydrogenase activity in the soil environment. Dehydrogenases 2012, 10, 183–210. [Google Scholar]
- Henriksson, G.; Sild, V.; Szabó, I.J.; Pettersson, G.; Johansson, G. Substrate specificity of cellobiose dehydrogenase from Phanerochaete chrysosporium. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1998, 1383, 48–54. [Google Scholar] [CrossRef]
- Vega, J.A.; Fontùrbel, T.; Merino, A.; Fernàndez, C.; Ferreiro, A.; Jiménez, E. Testing the suitability of visual indicators of soil burn severity to reflect changes in soil chemical and microbial properties in pine stands and shrublands. Plant Soil 2013, 369, 73–91. [Google Scholar] [CrossRef]
- Saulino, L.; Rita, A.; Migliozzi, A.; Maffei, C.; Allevato, E.; Garonna, A.P.; Saracino, A. Detecting burn severity across Mediterranean forest types by coupling medium-spatial resolution satellite imagery and field data. Remote Sens. 2020, 12, 741. [Google Scholar] [CrossRef] [Green Version]
- Panico, S.C.; Memoli, V.; Santorufo, L.; Esposito, F.; De Marco, A.; Barile, R.; Maisto, G. Linkage between site features and soil characteristics within a Mediterranean volcanic area. Front. For. Glob. Chang. 2021, 3, 621231. [Google Scholar] [CrossRef]
- Lindsay, W.N.; Norwell, W.A. Development of a DTPA micronutrient soil test. Agron. Abstr. 1969, 84, 69–87. [Google Scholar]
- Tabatabai, A. Soil Enzymes. In Methods of Soil Analysis; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; E-Publishing Inc.: Madison, WI, USA, 1982; pp. 903–947. [Google Scholar]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, G.; Roeland, K.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package. R Package 2014, 280. [Google Scholar]
- Certini, G. Effects of fire on properties of forest soils: A review. Oecologia 2005, 143, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Brando, P.M.; Oliveria-Santos, C.; Rocha, W.; Cury, R.; Coe, M.T. Effects of experimental fuel additions on fire intensity and severity: Unexpected carbon resilience of a neotropical forest. Glob. Chang. Biol. 2016, 2516–2525. [Google Scholar] [CrossRef] [PubMed]
- Panico, S.C.; Ceccherini, M.T.; Memoli, V.; Maisto, G.; Pietramellara, G.; Barile, R.; De Marco, A. Effects of different vegetation types on burnt soil properties and microbial communities. Int. J. Wildland Fire 2020, 29, 628–636. [Google Scholar] [CrossRef]
- Moya, D.; Madrigal, J.; Fontúrbel, T.; Marino, E.; Hernando, C.; Guijarro, M.; Fernández, C.; Jiménez, E.; Vega, J.A.; de las Heras, J. Fire severity assessments in both the laboratory and the field. In Fire Effects on Soil Properties; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Oxfordshire, UK, 2018; pp. 241–265. [Google Scholar]
- Memoli, V.; Panico, S.C.; Barile, R.; Di Natale, G.; Trifuoggi, M.; De Marco, A.; Maisto, G. Stability of mediterranean burnt soils under different plant covers. Catena 2021, 206, 105581. [Google Scholar] [CrossRef]
- Omer, M.; Idowu, O.J.; Ulery, A.L.; VanLeeuwen, D.; Guldan, S.J. Seasonal changes of soil quality indicators in selected arid cropping systems. Agriculture 2018, 8, 124. [Google Scholar] [CrossRef] [Green Version]
- Zavala, L.; de Celis, R.; Jordán, A. How wildfires affect soil properties. A brief review. Cuad. Investig. Geográfica/Geogr. Res. Lett. 2014, 40, 311–332. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Póma, R.; Bautista, S. Plant regeneration functional groups modulate the response to fire of soil enzyme activities in a Mediterranean shrubland. Soil Biol. Biochem. 2014, 79, 5–13. [Google Scholar] [CrossRef]
- Helmisaari, H. Nutrient retranslocation within the foliage of Pinus sylvestris. Tree Physiol. 1990, 10, 45–58. [Google Scholar] [CrossRef]
- Memoli, V.; Esposito, F.; Panico, S.C.; De Marco, A.; Barile, R.; Maisto, G. Evaluation of tourism impact on soil metal accumulation through single and integrated indices. Sci. Total Environ. 2019, 682, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Baležentiené, L. Hydrolases related to C and N cycles and soil fertility amendment: Responses to different management styles and agro-ecosystems. Pol. J. Environ. Stud. 2012, 21, 1153–1159. [Google Scholar]
- Bueis, T.; Turrión, M.B.; Bravo, F.; Pando, V.; Muscolo, A. Factors determining enzyme activities in soils under Pinus halepensis and Pinus sylvestris plantations in Spain: A basis for establishing sustainable forest management strategies. Ann. For. Sci. 2018, 75, 34. [Google Scholar] [CrossRef] [Green Version]
- Adak, T.; Singh, A.; Kumar, K.; Shukla, S.K.; Singh, A.; Kumar Singh, V. Soil organic carbon, dehydrogenase activity, nutrient availability and leaf nutrient content as affected by organic and inorganic source of nutrient in mango orchard soil. J. Soil Sci. Plant. Nutr. 2014, 2, 394–406. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Dong, Y.; Xie, X.; Li, X.; Zhang, X.; Shen, X. Effect of annual variation in soil pH on available soil nutrients in pear orchards. Acta Ecol. Sin. 2011, 31, 212–216. [Google Scholar] [CrossRef]
- Zhang, W.; Calvo-Polanco, M.; Chen, Z.C.; Zwiazek, J.J. Growth and physiological responses of trembling aspen (Populus tremuloides), white spruce (Picea glauca) and tamarack (Larix laricina) seedlings to root zone pH. Plant Soil 2013, 373, 775–786. [Google Scholar] [CrossRef]
- Terzano, R.; Rascio, I.; Allegretta, I.; Porfido, C.; Spagnuolo, M.; Khanghahi, M.Y.; Crecchio, C.; Sakellariadou, F.; Gattullo, C.E. Fire effects on the distribution and bioavailability of potentially toxic elements (PTEs) in agricultural soils. Chemosphere 2021, 281, 130752. [Google Scholar] [CrossRef] [PubMed]
| Sampling Site | Vegetation cover | Fire | Coordinates |
|---|---|---|---|
| HO1 | Holm Oak | Burnt | 40°80′72″ 14°43′46″ |
| HO2 | Holm Oak | Unburnt | 40°80′88″ 14°43′92″ |
| HO3 | Holm Oak | Burnt | 40°80′23″ 14°44′15″ |
| HO4 | Holm Oak | Unburnt | 40°81′03″ 14°40′86″ |
| HO5 | Holm Oak | Burnt | 40°81′67″ 14°40′86″ |
| HO6 | Holm Oak | Unburnt | 40°81′99″ 14°39′96″ |
| P1 | Pine | Burnt | 40°79′71″ 14°43′87″ |
| P2 | Pine | Unburnt | 40°80′19″ 14°43′85″ |
| P3 | Pine | Burnt | 40°80′19″ 14°26′13″ |
| P4 | Pine | Burnt | 40°81′31″ 14°43′80″ |
| P5 | Pine | Unburnt | 40°83′10″ 14°25′02″ |
| P6 | Pine | Unburnt | 40°82′41″ 14°39′18″ |
| BL1 | Black Locust | Unburnt | 40°81′20″ 14°44′07″ |
| BL2 | Black Locust | Burnt | 40°80′88″ 14°43′92″ |
| BL3 | Black Locust | Unburnt | 40°82′26″ 14°43′55 |
| BL4 | Black Locust | Burnt | 40°82′86″ 14°43′04″ |
| BL5 | Black Locust | Burnt | 40°82′36″ 14°43′53″ |
| BL6 | Black Locust | Unburnt | 40°82′13″ 14°43′62″ |
| H1 | Herbs | Burnt | 40°81′31″ 14°43′66″ |
| H2 | Herbs | Unburnt | 40°82′30″ 14°39′96″ |
| H3 | Herbs | Burnt | 40°83′07″ 14°25′28″ |
| H4 | Herbs | Unburnt | 40°81′81″ 14°43′50″ |
| H5 | Herbs | Unburnt | 40°82′17″ 14°43′57″ |
| H6 | Herbs | Burnt | 40°82′65″ 14°43′41″ |
| P | HO | BL | H | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | F | W | Sp | S | F | W | Sp | S | F | W | Sp | S | F | W | Sp | |
| pH | 7.8 a | 7.36 b | 7.06 b | 7.54 ab | 7.35 | 7.13 | 7.34 | 7.55 | 7.10 ab | 6.91 ab | 6.91 b | 7.29 a | 7.46 | 7.53 | 7.27 | 7.56 |
| ±0.05 | ±0.09 | ±0.30 | ±0.17 | ±0.28 | ±0.25 | ±0.25 | ±0.13 | ±0.11 | ±0.12 | ±0.05 | ±0.08 | ±0.11 | ±0.04 | ±0.09 | ±0.15 | |
| WC | 6.14 b | 12.9 ab | 16.9 a | 8.94 ab | 27.7 ab | 26.3 b | 46.5 a | 23.1 b | 3.54 b | 11.7 a | 17.0 a | 5.34 b | 5.60 | 13.6 | 13.7 | 4.20 |
| ±1.28 | ±2.61 | ±0.35 | ±3.01 | ±8.27 | ±3.07 | ±4.34 | ±3.91 | ±0.96 | ±1.64 | ±0.85 | ±0.98 | ±3.48 | ±5.22 | ±6.95 | ±1.09 | |
| C | 2.71 | 3.24 | 2.87 | 3.15 | 7.68 | 6.94 | 8.96 | 8.27 | 7.35 | 5.69 | 3.54 | 1.82 | 2.32 | 1.70 | 2.18 | 2.48 |
| ±0.43 | ±0.25 | ±0.11 | ±0.75 | ±3.23 | ±1.94 | ±1.39 | ±1.22 | ±1.88 | ±2.33 | ±0.41 | ±0.74 | ±0.95 | ±1.16 | ±1.92 | ±1.23 | |
| N | 0.23 | 0.20 | 0.18 | 0.18 | 0.51 | 0.54 | 0.57 | 0.49 | 1.09 | 0.62 | 0.36 | 0.76 | 0.29 | 0.27 | 0.10 | 0.17 |
| ±0.02 | ±0.05 | ±0.03 | ±0.05 | ±0.16 | ±0.19 | ±0.10 | ±0.07 | ±0.53 | ±0.25 | ±0.03 | ±0.57 | ±0.13 | ±0.09 | ±0.05 | ±0.08 | |
| OM | 4.82 | 4.27 | 4.46 | 4.46 | 5.37 | 5.79 | 6.40 | 5.50 | 10.9 | 12.6 | 11.3 | 8.68 | 8.32 | 8.07 | 7.98 | 6.29 |
| ±2.26 | ±1.75 | ±2.10 | ±2.73 | ±0.78 | ±1.37 | ±1.45 | ±0.47 | ±3.54 | ±3.63 | ±3.01 | ±1.99 | ±2.52 | ±2.26 | ±1.50 | ±1.89 | |
| Al | 5.15 c | 17.2 b | 25.6 a | 10.1 c | 7.09 b | 25.2 ab | 29.9 a | 11.3 ab | 6.76 c | 14.4 ac | 19.9 a | 8.41 b | 2.67 b | 7.53 a | 9.89 a | 4.33 ab |
| ±1.08 | ±1.41 | ±2.22 | ±2.20 | ±1.10 | ±7.77 | ±15.5 | ±2.98 | ±2.19 | ±1.74 | ±1.58 | ±1.28 | ±0.59 | ±1.19 | ±1.75 | ±1.23 | |
| Ca | 3730 b | 5983 a | 2078 b | 4541 ab | 8353 | 13,550 | 8275 | 12,413 | 5013 | 8083 | 3581 | 4748 | 3021 | 5432 | 6551 | 6864 |
| ±650 | ±705 | ±201 | ±971 | ±1844 | ±3428 | ±1749 | ±2165 | ±1501 | ±2123 | ±765 | ±882 | ±1218 | ±3555 | ±3598 | ±4558 | |
| Cu | 0.72 b | 2.02 ab | 2.18 a | 1.49 b | 0.64 b | 1.50 a | 1.99 a | 1.85 a | 0.72 b | 2.44 a | 3.32 a | 1.23 ab | 0.57 b | 2.58 a | 1.97 a | 1.60 ab |
| ±0.20 | ±0.69 | ±0.09 | ±0.25 | ±0.10 | ±0.18 | ±0.46 | ±0.30 | ±0.14 | ±0.45 | ±1.15 | ±0.19 | ±0.14 | ±0.44 | ±0.50 | ±0.50 | |
| Fe | 8.75 c | 35.6 ab | 38.8 a | 18.8 bc | 25.6 c | 68.5 ab | 95.1 a | 42.9 bc | 12.4 b | 24.1 ab | 38.3 a | 13.2 ab | 5.98 | 22.6 | 20.1 | 7.29 |
| ±0.85 | ±3.88 | ±3.48 | ±3.93 | ±5.88 | ±7.71 | ±12.4 | ±5.97 | ±2.59 | ±5.06 | ±9.57 | ±2.79 | ±1.57 | ±8.75 | ±12.6 | ±2.75 | |
| K | 410 ab | 414 a | 225 b | 376 ab | 718 | 688 | 526 | 740 | 394 | 370 | 256 | 350 | 545 | 438 | 394 | 668 |
| ±72.7 | ±44.2 | ±14.7 | ±69.7 | ±98.4 | ±102 | ±45.4 | ±54.2 | ±72.1 | ±42.3 | ±20.4 | ±39.9 | ±206 | ±126 | ±131 | ±327 | |
| Mg | 293 b | 449 a | 195 c | 40.5 d | 608 a | 836 a | 447 a | 48.4 b | 360 a | 517 a | 273 a | 38.9 b | 188 a | 290 a | 299 a | 33.8 ab |
| ±89.4 | ±32.5 | ±13.2 | ±2.11 | ±92.1 | ±173 | ±57.8 | ±2.74 | ±78.8 | ±99.4 | ±41.0 | ±2.40 | ±44.2 | ±122 | ±123 | ±33.5 | |
| Mn | 1.03 b | 3.77 a | 3.58 a | 3.98 a | 4.98 | 14.3 | 17.6 | 24.3 | 5.66 | 7.09 | 6.49 | 3.37 | 0.44 | 1.42 | 3.16 | 1.31 |
| ±0.11 | ±0.61 | ±0.38 | ±0.66 | ±2.26 | ±3.19 | ±2.86 | ±7.09 | ±2.44 | ±2.64 | ±0.96 | ±0.99 | ±0.09 | ±1.05 | ±2.39 | ±0.92 | |
| Na | 514 b | 874 a | 848 a | 833 ab | 585 c | 922 ab | 946 b | 1098 a | 482 c | 917 b | 958 ab | 1078 a | 514 b | 908 a | 937 a | 1284 a |
| ±49.1 | ±46.1 | ±23.5 | ±155 | ±75.4 | ±48.0 | ±23.2 | ±50.4 | ±40.4 | ±44.8 | ±28.0 | ±26.2 | ±58.9 | ±47.7 | ±44.9 | ±198 | |
| Pb | 0.81 b | 2.41 a | 2.26 a | 2.43 a | 2.00 b | 2.50 b | 2.75 b | 4.61 a | 0.53 b | 3.80 a | 6.65 a | 0.53 b | 0.48 | 2.69 | 2.31 | 2.15 |
| ±0.12 | ±0.65 | ±0.74 | ±0.58 | ±0.54 | ±1.04 | ±1.26 | ±0.55 | ±0.17 | ±1.46 | ±3.21 | ±0.15 | ±0.28 | ±1.13 | ±0.80 | ±1.33 | |
| P | HO | BL | H | |||||
|---|---|---|---|---|---|---|---|---|
| UB | B | UB | B | UB | B | UB | B | |
| pH | 7.48 | 7.43 | 7.43 | 7.21 | 7.08 | 7.03 | 7.58 a | 7.27 b |
| ±0.17 | ±0.12 | ±0.15 | ±0.14 | ±0.05 | ±0.08 | ±0.06 | ±0.05 | |
| WC | 11.5 | 11.2 | 34.7 | 25.1 | 10 | 8.96 | 11.9 | 5.34 |
| ±2.19 | ±1.61 | ±4.36 | ±4.12 | ±2.23 | ±1.73 | ±3.75 | ±1.36 | |
| C | 3.03 | 2.98 | 9.47 | 5.69 | 3.35 | 5.43 | 2.79 | 1.25 |
| ±0.32 | ±0.26 | ±1.32 | ±1.13 | ±0.65 | ±1.34 | ±0.98 | ±0.49 | |
| N | 0.15 | 0.20 | 0.61 | 0.39 | 0.66 | 0.74 | 0.25 | 0.14 |
| ±0.03 | ±0.02 | ±0.09 | ±0.04 | ±0.34 | ±0.24 | ±0.07 | ±0.02 | |
| OM | 3.49 | 4.75 | 5.04 | 6.86 | 10.4 | 11.2 | 8.18 | 6.88 |
| ±0.58 | ±1.28 | ±0.74 | ±0.45 | ±2.11 | ±2.06 | ±1.52 | ±0.93 | |
| Al | 14.6 | 14.5 | 11.8 b | 28.2 a | 11.2 | 13.2 | 4.75 | 8.13 |
| ±3.81 | ±2.30 | ±3.29 | ±9.69 | ±2.19 | ±1.95 | ±0.76 | ±1.65 | |
| Ca | 3832 | 4146 | 13,090 a | 6985 b | 5937 | 4969 | 6831 | 3420 |
| ±772 | ±542 | ±1553 | ±1139 | ±1095 | ±1052 | ±2485 | ±1533 | |
| Cu | 1.49 | 1.63 | 1.41 | 1.63 | 1.98 | 1.89 | 1.73 | 1.62 |
| ±0.33 | ±0.26 | ±0.20 | ±0.35 | ±0.44 | ±0.56 | ±0.34 | ±0.42 | |
| Fe | 18.4 | 27.3 | 59.9 | 55.2 | 25.5 | 19.7 | 18.3 | 7.52 |
| ±4.76 | ±3.75 | ±9.05 | ±12.4 | ±7.70 | ±3.19 | ±6.37 | ±1.38 | |
| K | 248 | 383 | 726 | 582 | 361 | 331 | 666 a | 278 b |
| ±23.6 | ±35.4 | ±59.5 | ±37.4 | ±23.9 | ±38.7 | ±150 | ±56.0 | |
| Mg | 206 | 254 | 546 | 393 | 297 | 298 | 221 | 175 |
| ±80.3 | ±40.9 | ±120 | ±93.5 | ±81.4 | ±66.5 | ±65.1 | ±72.8 | |
| Mn | 2.29 | 3.29 | 16.8 | 13.1 | 5.31 | 5.89 | 2.31 | 0.49 |
| ±0.56 | ±0.41 | ±3.64 | ±3.36 | ±1.4 | ±1.31 | ±1.14 | ±0.09 | |
| Na | 789 | 762 | 939 | 880 | 874 | 849 | 977 | 811 |
| ±119 | ±59.3 | ±55.9 | ±90.7 | ±97.1 | ±66.6 | ±119 | ±87.7 | |
| Pb | 1.69 | 2.05 | 3.35 | 2.39 | 2.91 | 2.86 | 1.37 | 2.72 |
| ±0.37 | ±0.37 | ±0.61 | ±0.74 | ±1.31 | ±1.46 | ±0.59 | ±0.79 | |
| P | HO | BL | H | |||||
|---|---|---|---|---|---|---|---|---|
| Seasonality | Fire | Seasonality | Fire | Seasonality | Fire | Seasonality | Fire | |
| HA | 0.52 | 0.45 | 0.11 | 0.06 | 0.14 | 0.02 | 0.21 | 0.38 |
| DHA | 0.40 | 0.25 | 0.29 | 0.32 | 0.23 | 0.25 | 0.35 | 1.58 |
| β-GLU | 0.17 | 0.09 | 0.05 | 0.24 | 0.34 | 0.04 | 0.44 | 0.67 |
| Vegetation Cover | Seasons | Multiple Regression Model |
|---|---|---|
| P | S | HA = 574.298 − (2456.318 × Mn) + (160.561 × Fe) − (2.893 × Mg) + (0.564 × Ca) R2 = 0.62 |
| DHA = 1.673 + (0.962 × Mn) − (0.115 × Fe) − (0.00652 × Mg) + (0.000201 × Ca) R2 = 0.59 | ||
| F | HA = 711.364 − (0.0279 × Mg) R2 = 0.58 | |
| DHA = 0.460 + (0.000337 × Mg) R2 = 0.70 | ||
| β-GLU = 406.772 − (0.147 × Mg) R2 = 0.72 | ||
| W | HA = 528.337 + (90.745 × Mn) + (0.827 × Fe) − (4.064 × Mg) + (0.193 × Ca) R2 = 0.80 | |
| DHA = 2.809 − (0.0349 × Mn) − (0.0291 × Fe) + (0.0000110 × Mg) − (0.000597 × Ca) R2 = 0.78 | ||
| β-GLU = −165.723 + (84.836 × Mn) + (1.887 × Fe) − (0.892 × Mg) + (0.129 × Ca) R2 = 0.63 | ||
| Sp | HA = 863.050 + (109.434 × Fe) − (82.788 × Mg) + (0.302 × Ca) R2 = 0.75 | |
| DHA = −0.0664 − (0.328 × Mn) + (0.0971 × Fe) − (0.0190 × Mg) + (0.000516 × Ca) R2 = 0.82 | ||
| β-GLU = 802.267 + (16.795 × Fe) − (24.072 × Mg) + (0.0404 × Ca) R2 = 0.84 | ||
| HO | S | HA = 622.891 − (298.233 × Mn) + (163.414 × Fe) − (1.045 × Mg) − (0.149 × Ca) R2 = 0.81 |
| DHA = 2.674 + (3.072 × Mn) − (0.895 × Fe) + (0.00618 × Mg) + (0.000847 × Ca) R2 = 0.72 | ||
| β-GLU = 1113.367 + (461.335 × Mn) − (281.902 × Fe) + (2.613 × Mg) + (0.299 × Ca) R2 = 0.84 | ||
| F | β-GLU = 124.246 + (17.697 × Mn) − (0.846 × Fe) + (0.0343 × Mg) + (0.0251 × Ca) R2 = 0.81 | |
| W | HA = 1674.910 − (7.305 × Mn) R2 = 0.67 | |
| DHA = −0.429 + (0.220 × Mn) R2 = 0.64 | ||
| β-GLU = 363.752 + (23.337 × Mn) R2 = 0.63 | ||
| Sp | HA = 1181.300 − (1.422 × Mn) + (0.0471 × Ca) R2 = 0.59 | |
| DHA = −5.839 + (0.285 × Mn) + (0.000516 × Ca) R2 = 0.60 | ||
| β-GLU = −379.443 + (32.196 × Mn) + (0.0710 × Ca) R2 = 0.77 | ||
| BL | S | HA = 883.630 − (3.860 × Fe) R2 = 0.67 |
| DHA = 0.630 + (0.136 × Fe) R2 = 0.64 | ||
| β-GLU = 559.202 − (17.491 × Fe) R2 = 0.68 | ||
| F | HA = −15233.898 + (4.175 × Mn) − (284.969 × Fe) + (10.395 × Mg) + (19.215 × Na) R2 = 0.79 | |
| DHA = 208.279 − (0.680 × Mn) + (3.825 × Fe) − (0.105 × Mg) − (0.261 × Na) R2 = 0.83 | ||
| β-GLU = 7134.575 − (34.826 × Mn) + (130.277 × Fe) − (2.506 × Mg) − (8.946 × Na) R2 = 0.88 | ||
| W | HA = 1399.255 − (84.113 × Mn) + (0.0368 × Ca) R2 = 0.67 | |
| DHA = −0.0275 − (0.179 × Mn) − (0.0283 × Fe) + (0.0110 × Mg) + (0.000451 × Ca) R2 = 0.82 | ||
| Sp | HA = 11274.414 + (453.431 × Mn) + (73.326 × Fe) − (395.247 × Mg) + (0.427 × Ca) R2 = 0.73 | |
| DHA = 39.340 + (2.090 × Mn) − (0.537 × Fe) − (1.436 × Mg) + (0.00402 × Ca) R2 = 0.84 | ||
| H | S | HA = 108.537 + (919.366 × Mn) + (0.229 × Na) R2 = 0.65 |
| DHA = −3.254 + (4.369 × Mn) + (0.00622 × Na) R2 = 0.67 | ||
| β-GLU = 492.480 − (120.333 × Mn) − (0.643 × Na | ||
| F | HA = −552.003 − (498.522 × Mn) − (12.495 × Fe) + (4.447 × Mg) + (0.522 × Na) R2 = 0.62 | |
| DHA = −31.416 + (0.0372 × Na) R2 = 0.89 | ||
| β-GLU = 9.838 − (194.965 × Mn) − (8.247 × Fe) + (2.389 × Mg) − (0.0366 × Na) R2 = 0.88 | ||
| W | HA = −1819.093 − (443.852 × Mn) + (96.649 × Fe) − (0.00256 × Mg) + (1.839 × Na) R2 = 0.79 | |
| DHA = −0.357 + (0.548 × Mn) + (0.0151 × Fe) + (0.000637 × Mg) − (0.000122 × Na) R2 = 0.78 | ||
| β-GLU = −2466.230 + (0.290 × Mg) + (2.824 × Na) R2 = 0.67 | ||
| Sp | HA = −3337.011 + (2000.693 × Mn) − (561.684 × Fe) + (537.311 × Mg) − (10.017 × Na) R2 = 0.68 | |
| β-GLU = −685.425 + (843.654 × Mn) − (226.863 × Fe) + (170.056 × Mg) − (3.292 × Na) R2 = 0.77 |
| Vegetation Cover | Fire | Multiple Regression Model |
|---|---|---|
| P | UB | HA = 1136.464 − (232.733 × Mn) + (19.395 × Fe) − (1.311 × Mg) R2 = 0.62 |
| DHA = 0.407 + (0.980 × Mn) − (0.126 × Fe) + (0.00244 × Mg) R2 = 0.58 | ||
| β-GLU = −192.955 + (263.969 × Mn) − (17.263 × Fe) + (0.816 × Mg) R2 = 0.84 | ||
| B | HA = −1647.699 + (280.039 × Cu) − (124.302 × Pb) + (380.605 × pH) − (115.994 × C) − (767.327 × N) − (35.016 × OM) R2 = 0.63 | |
| DHA = −3.280 − (0.0869 × Cu) + (0.0158 × Pb) + (0.660 × pH) − (0.0281 × C) − (3.368 × N) + (0.0288 × OM) R2 = 0.73 | ||
| β-GLU = 123.032 + (39.383 × Cu) − (15.053 × Pb) + (47.597 × pH) − (14.792 × C) − (510.130 × N) − (11.591 × OM) R2 = 0.85 | ||
| HO | UB | HA = 2276.187 − (3.268 × Mn) + (16.144 × Fe) − (0.527 × Mg) − (0.0698 × Ca) − (2.135 × Na) + (3.443 × K) − (37.494 × Al) + (1094.257 × Cu) − (387.797 × Pb) − (23.830 × WC) − (26.092 × pH) R2 = 0.79 |
| DHA = 34.487 + (0.298 × Mn) + (0.0114 × K) − (0.0277 × Al) + (0.0130 × WC) − (5.568 × pH) R2 = 0.71 | ||
| β-GLU = 1851.453 + (18.433 × Mn) + (20.033 × Fe) + (0.171 × Mg) − (0.00604 × Ca) − (0.252 × Na) + (0.167 × K) − (21.172 × Al) − (42.703 × Cu) − (62.309 × Pb) − (27.947 × WC) − (152.040 × pH) R2 = 0.81 | ||
| B | HA = 5403.332 − (54.698 × Mn) + (60.722 × Fe) − (3.431 × Mg) + (0.224 × Ca) − (4.760 × Na) − (2.999 × K) − (41.834 × Al)R2 = 0.69 | |
| DHA = 12.864 + (0.156 × Mn) + (0.0182 × Fe) − (0.00526 × Mg) + (0.000709 × Ca) − (0.0106 × Na) − (0.0106 × K) − (0.0295 × Al) R2 = 0.64 | ||
| β-GLU = −877.077 + (177.894 × Mn) − (65.043 × Fe) + (6.652 × Mg) − (0.355 × Ca) + (5.526 × Na) − (4.029 × K) − (1.771 × Al) R2 = 0.71 | ||
| BL | UB | HA = 1388.988 + (2.103 × Fe) − (1.760 × K) R2 = 0.58 |
| DHA = 0.570 + (0.0345 × Fe) + (0.00250 × K) R2 = 0.64 | ||
| β-GLU = 851.993 + (0.0343 × Fe) − (1.080 × K) R2 = 0.71 | ||
| B | HA = −19257.673 − (187.103 × Mn) + (48.790 × Fe) − (2.244 × Mg) + (0.234 × Ca) − (2.767 × Na) + (5.980 × K) + (126.847 × Al) + (717.584 × Cu) − (323.015 × Pb) + (70.248 × WC) + (2462.431 × pH) R2 = 0.75 | |
| H | UB | HA = 1032.227 − (0.109 × Na) + (0.138 × K) − (42.703 × Al) − (158.552 × Cu) + (2.663 × Pb) R2 = 0.81 |
| DHA = 1.559 + (0.00207 × Na) + (0.00273 × K) − (0.274 × Al) − (0.192 × Cu) − (0.412 × Pb) R2 = 0.73 | ||
| β-GLU = −116.330 + (0.305 × Na) + (0.0830 × K) + (3.845 × Al) − (4.984 × Cu) + (18.675 × Pb) R2 = 0.87 | ||
| B | HA = 363.289 + (65.104 × Mn) − (10.143 × Fe) + (0.171 × Na) R2 = 0.75 | |
| DHA = 0.488 + (0.603 × Mn) − (0.0359 × Fe) − (0.000124 × Na) R2 = 0.79 | ||
| β-GLU = 213.791 + (187.946 × Mn) + (12.950 × Fe) − (0.277 × Na) R2 = 0.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Memoli, V.; Santorufo, L.; Santini, G.; Musella, P.; Barile, R.; De Marco, A.; Di Natale, G.; Trifuoggi, M.; Maisto, G. Role of Seasonality and Fire in Regulating the Enzymatic Activities in Soils Covered by Different Vegetation in a Mediterranean Area. Appl. Sci. 2021, 11, 8342. https://doi.org/10.3390/app11188342
Memoli V, Santorufo L, Santini G, Musella P, Barile R, De Marco A, Di Natale G, Trifuoggi M, Maisto G. Role of Seasonality and Fire in Regulating the Enzymatic Activities in Soils Covered by Different Vegetation in a Mediterranean Area. Applied Sciences. 2021; 11(18):8342. https://doi.org/10.3390/app11188342
Chicago/Turabian StyleMemoli, Valeria, Lucia Santorufo, Giorgia Santini, Paola Musella, Rossella Barile, Anna De Marco, Gabriella Di Natale, Marco Trifuoggi, and Giulia Maisto. 2021. "Role of Seasonality and Fire in Regulating the Enzymatic Activities in Soils Covered by Different Vegetation in a Mediterranean Area" Applied Sciences 11, no. 18: 8342. https://doi.org/10.3390/app11188342
APA StyleMemoli, V., Santorufo, L., Santini, G., Musella, P., Barile, R., De Marco, A., Di Natale, G., Trifuoggi, M., & Maisto, G. (2021). Role of Seasonality and Fire in Regulating the Enzymatic Activities in Soils Covered by Different Vegetation in a Mediterranean Area. Applied Sciences, 11(18), 8342. https://doi.org/10.3390/app11188342









