First Report and 3D Reconstruction of a Presumptive Microscopic Liver Lipoma in a Black Barbel (Barbus balcanicus) from the River Bregalnica in the Republic of North Macedonia
Abstract
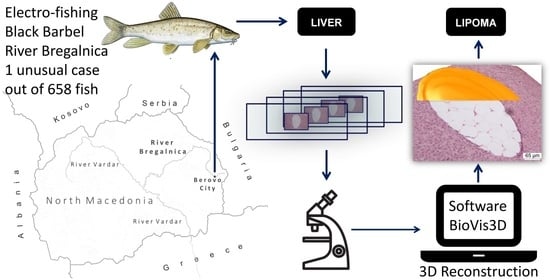
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schlumberger, H.H.; Lucké, B. Tumors of fishes, amphibians and reptiles. Cancer Res. 1948, 8, 657–754. [Google Scholar] [PubMed]
- Johnson, C.N.; Ha, A.S.; Chen, E.; Davidson, D. Lipomatous soft-tissue tumors. J. Am. Acad. Orthop. Surg. 2018, 26, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Charifa, A.; Azmat, C.E.; Badri, T. Lipoma Pathology. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Stolk, A. Tumours of fishes 29. Lipoma in the eel Anguilla anguilla. Beaufortia 1959, 7, 193–198. [Google Scholar]
- Tubiash, H.D.; Hendricks, J.D. A possible lipoma in the weakfish, Cynoscion regalis (Bloch and Schneider). Chesap. Sci. 1973, 14, 145–146. [Google Scholar] [CrossRef]
- Bruno, D.W.; McVicar, A.H.; Fraser, C.O. Multiple lipoma in the common dab, Limanda limanda L. J. Appl. Ichthyol. 1991, 7, 238–243. [Google Scholar] [CrossRef]
- Marino, F.; Monaco, S.; Salvaggio, A.; Macrì, B. Lipoma in a farmed northern bluefin tuna, Thunnus thynnus (L.). J. Fish Dis. 2006, 29, 697–699. [Google Scholar] [CrossRef] [PubMed]
- Gómez, S. Multiple dermal lipomas in farmed striped seabream Lithognathus mormyrus on the Spanish Mediterranean coast. Dis. Aquat. Organ. 2009, 85, 77–79. [Google Scholar] [CrossRef] [Green Version]
- McCoy, C.P.; Bowser, P.R.; Steeby, J.; Bleau, M.; Schwedler, T.E. Lipoma in channel catfish (Ictalurus punctatus Rafinesque). J. Wild. Dis. 1985, 21, 74–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Easa, M.L.S.; Easa, M.; Harshbarger, J.C.; Hetrick, F.M. Hypodermal lipoma in a striped (grey) mullet Mugil cephalus. Dis. Aquat. Organ. 1989, 6, 157–160. [Google Scholar] [CrossRef]
- Szewc, M.; Gawlik, P.; Żebrowski, R.; Sitarz, R. Giant lipoma in the fronto-temporo-parietal region in an adult man: Case report and literature review. Clin. Cosmet. Investig. Dermatol. 2020, 13, 1015–1020. [Google Scholar] [CrossRef]
- Schwartz, F.J.; Màrquez, R. A lipoma in the striped mojarm, Diapterus plumieri (Pisces: Gerridae) from Mexico. J. Elisha Mitchell Sci. Soc. 1971, 87, 87–90. [Google Scholar]
- Hard, G.C.; Williams, R.; Lee, J. Survey of Demersal Fish in Port Phillip Bay for Incidence of Neoplasia. Aust. J. Mar. Freshw. Res. 1979, 30, 73–79. [Google Scholar] [CrossRef]
- Chen, H.C.; Pan, I.J.; Tu, W.J.; Lin, W.H.; Hong, C.C.; Brittelli, M.R. Neoplastic Response in Japanese Medaka and Channel Catfish Exposed to N-Methyl-N′-Nitro-N-Nitrosoguanidine. Toxicol. Pathol. 1996, 24, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Abowei, J.F.N.; Briyai, O.F.; Bassey, S.E. A Review of Some Viral, Neoplastic, Environmental and Nutritional Diseases of African Fish. Br. J. Pharmacol. Toxicol. 2011, 2, 227–235. [Google Scholar]
- De Stefano, C.; Bonfiglio, R.; Montalbano, G.; Giorgianni, P.; Lanteri, G. Multicentric lipoma in a molly (Poecilia velifera). Bull. Eur. Assoc. Fish Pathol. 2012, 32, 220–224. [Google Scholar]
- Sood, N.; Swaminathan, T.R.; Yadav, M.K.; Pradhan, P.K.; Kumar, R.; Sood, N.K. First report of cutaneous infiltrative lipoma in goldfish Carassius auratus. Dis. Aquat. Org. 2017, 125, 243–247. [Google Scholar] [CrossRef]
- Johnston, C.J.; Deveney, M.R.; Bayly, T.; Nowak, B.F. Gross and histopathological characteristics of two lipomas and a neurofibrosarcoma detected in aquacultured southern bluefin tuna, Thunnus maccoyii (Castelnau), in South Australia. J. Fish Dis. 2008, 31, 241–247. [Google Scholar] [CrossRef]
- Marino, F.; Chiofalo, B.; Mazzullo, G.; Panebianco, A. Multicentric infiltrative lipoma in a farmed Mediterranean seabass Dicentrarchus labrax: A pathological and biochemical case study. Dis. Aquat. Organ. 2011, 96, 259–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lester, R.J.G.; Kelly, W.R. Tumour-like growths from southern Australian marine fish. Tasmanian Fish Res. 1983, 25, 27–32. [Google Scholar]
- Ramos, P.; Faisca, P.; Carneiro, M.; Rosa, F. Dermal Melanoma and Mesenteric Lipoma in a Senegal Seabream, Diplodus bellottii (Steindachner, 1882) from the Portuguese Coast. Exp. Pathol. Health Sci. 2016, 8, 67–68. [Google Scholar]
- Singaravel, V.; Gopalakrishnan, A.; Vijayakumar, R.; Raja, K. Prevalence and pathology of gastric tumours in Indian oil sardine (Sardinella longiceps) from Parangipettai coastal waters, southeast coast of India. J. Coast. Life Med. 2015, 3, 592–595. [Google Scholar] [CrossRef]
- Hinck, J.E.; Blazer, V.S.; Denslow, N.D.; Echols, K.R.; Gale, R.W.; Wieser, C.; May, T.W.; Ellersieck, M.; Coyle, J.J.; Tillitt, D.E. Chemical contaminants, health indicators, and reproductive biomarker responses in fish from rivers in the Southeastern United States. Sci. Total Environ. 2008, 390, 538–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stejskal, V.; Kouřil, J.; Policar, T.; Svobodová, Z. Splenic lipidosis in intensively cultured perch, Perca fluviatilis L. J. Fish Dis. 2015, 39, 87–93. [Google Scholar] [CrossRef]
- Thomas, L. Sur un lipome abdominal chez un colin. Bull. Ass. Fr. Etude Cancer 1933, 22, 419–435. [Google Scholar]
- Figueiredo-Fernandes, A.M.; Fontaínhas-Fernandes, A.A.; Monteiro, R.A.F.; Reis-Henriques, M.A.; Rocha, E. Spatial relationships of the intrahepatic vascular-biliary tracts and associated pancreatic acini of Nile tilapia, Oreochromis niloticus (Teleostei, Cichlidae): A serial section study by light microscopy. Ann. Anat. 2007, 189, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Imrie, D.; Sadler, K.C. White adipose tissue development in zebrafish is regulated by both developmental time and fish size. Dev. Dyn. 2010, 239, 3013–3023. [Google Scholar] [CrossRef] [Green Version]
- Salmerón, C. Adipogenesis in fish. J. Exp. Biol. 2018, 221, jeb161588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa, S.; Rocha, M.J.; Rocha, E. Characterization and spatial relationships of the hepatic vascular–biliary tracts, and their associated pancreocytes and macrophages, in the model fish guppy (Poecilia reticulata): A study of serial sections by light microscopy. Tissue Cell 2018, 50, 104–113. [Google Scholar] [CrossRef]
- Jordanova, M.; Rebok, K.; Naskovska, M.; Kostov, V.; Rocha, E. Splenic pigmented macrophage aggregates in barbel (Barbus peloponnesius, Valenciennes, 1844) from River Bregalnica—Influences of age, sex and season on a pollution biomarker. Turkish J. Fish Aquat. Sci. 2016, 16, 881–890. [Google Scholar] [CrossRef]
- Rebok, K.; Jordanova, M.; Slavevska-Stamenković, V.; Ivanova, L.; Kostov, V.; Stafilov, T.; Rocha, E. Frequencies of erythrocyte nuclear abnormalities and of leucocytes in the fish Barbus peloponnesius correlate with a pollution gradient in the River Bregalnica (Macedonia). Environ. Sci. Pollut. Res. 2017, 24, 10493–10509. [Google Scholar] [CrossRef]
- McTighe, S.; Chernev, I. Intramuscular lipoma: A review of the literature. Orthop. Rev. Pavia 2014, 6, 5618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Church, C.; Horowitz, M.; Rodeheffer, M. WAT is a functional adipocyte? Adipocyte 2012, 1, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Snel, M.; Jonker, J.T.; Schoones, J.; Lamb, H.; de Roos, A.; Pijl, H.; Smit, J.W.A.; Meinders, A.E.; Jazet, I.M. Ectopic fat and insulin resistance: Pathophysiology and effect of diet and lifestyle interventions. Int. J. Endocrinol. 2012, 2012, 983814. [Google Scholar] [CrossRef]
- De Munck, T.J.I.; Soeters, P.B.; Koek, G.H. The role of ectopic adipose tissue: Benefit or deleterious overflow? Eur. J. Clin. Nutr. 2021, 75, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Kosztyuova, T.; Shim, T.N. Rapidly enlarging lipoma. BMJ Case Rep. 2017, 2017, bcr2017221272. [Google Scholar] [CrossRef] [PubMed]
- Caliskan, A.; Kohlmann, W.; Affolter, K.; Downs-Kelly, E.; Kanth, P.; Bronner, M.P. Intramucosal lipomas of the colon implicate Cowden syndrome. Mod. Pathol. 2018, 31, 643–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO Classification of Tumours Editorial Board. WHO Classification of Tumours of Soft Tissue and Bone, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2013; p. 427. [Google Scholar]
- Moulton, J.E. (Ed.) Tumors in Domestic Animals; University of California Press: Berkeley, CA, USA, 1990. [Google Scholar]
- Kolb, L.; Yarrarapu, S.N.S.; Ameer, M.A.; Rosario-Collazo, J.A. Lipoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507906/ (accessed on 6 October 2020).
- Bertoli, M.; Pizzul, E.; Devescovi, V.; Franz, F.; Pastorino, P.; Giulianini, P.G.; Ferrari, C.; Nonnis Marzano, F. Biology and distribution of Danube barbel (Barbus balcanicus) (Osteichthyes: Cyprinidae) at the Northwestern limit of its range. Eur. Zool. J. 2019, 86, 280–293. [Google Scholar] [CrossRef] [Green Version]
- Žutinić, P.; Jelić, D.; Jelić, M.; Buj, I. A contribution to understanding the ecology of the large spot barbel-sexual dimorphism, growth and population structure of Barbus balcanicus (Actinopterygii; Cyprinidae) in Central Croatia. N.-West. J. Zool. 2013, 10, 158–166. [Google Scholar]
- Amat Trigo, F.; Roberts, C.G.; Britton, J.R. Spatial variability in the growth of invasive European barbel Barbus barbus in the River Severn basin, revealed using anglers as citizen scientists. Knowl. Manag. Aquat. Ecosyst. 2017, 418, 17. [Google Scholar] [CrossRef]
- Aust, M.C.; Spies, M.; Kall, S.; Jokuszies, A.; Gohritz, A.; Vogt, P. Posttraumatic lipoma: Fact or fiction? Skinmed 2007, 6, 266–270. [Google Scholar] [CrossRef]
- Chu, C.-Y.; Chen, C.-F.; Rajendran, R.S.; Shen, C.-N.; Chen, T.-H.; Yen, C.-C.; Chuang, C.-K.; Lin, D.-S.; Hsiao, C.-D. Overexpression of Akt1 enhances adipogenesis and leads to lipoma formation in zebrafish. PLoS ONE 2012, 7, e36474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, D.; Yoshitomi, K.; Boorman, G.A.; Maronpot, R.R. “Lipomatous” lesions of unknown cellular origin in the liver of B6C3F1 mice. Vet. Pathol. 1994, 31, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Puljiz, Ž.; Petričević, M.; Bratanić, A.; Barišić, I.; Puljiz, M.; Karin, Ž. An unusually large liver lipoma. Case report. Med. Glas. Zenica 2012, 9, 411–414. [Google Scholar]
- Martin-Benitez, G.; Marti-Bonmati, L.; Barber, C.; Vila, R. Hepatic lipomas and steatosis: An association beyond chance. Eur. J. Radiol. 2011, 81, 491–494. [Google Scholar] [CrossRef]
- Farahani, N.; Braun, A.; Jutt, D.; Huffman, T.; Reder, N.; Liu, Z.; Yagi, Y.; Pantanowitz, L. Three-dimensional imaging and scanning: Current and future applications for pathology. J. Pathol. Inform. 2017, 8, 36. [Google Scholar] [CrossRef]
- Manenti, G.; Picchi, E.; Castrignanò, A.; Muto, M.; Nezzo, M.; Floris, R. Liver lipoma: A case report. BJR Case Rep. 2016, 3, 20150467. [Google Scholar] [CrossRef] [PubMed]
- Reddy, O.J.; Gafoor, J.A.; Suresh, B.; Prasad, P.O. Lipoma in liver: A rare presentation. J. Dr. NTR Univ. Health Sci. 2015, 4, 185–187. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rebok, K.; Jordanova, M.; Azevedo, J.; Rocha, E. First Report and 3D Reconstruction of a Presumptive Microscopic Liver Lipoma in a Black Barbel (Barbus balcanicus) from the River Bregalnica in the Republic of North Macedonia. Appl. Sci. 2021, 11, 8392. https://doi.org/10.3390/app11188392
Rebok K, Jordanova M, Azevedo J, Rocha E. First Report and 3D Reconstruction of a Presumptive Microscopic Liver Lipoma in a Black Barbel (Barbus balcanicus) from the River Bregalnica in the Republic of North Macedonia. Applied Sciences. 2021; 11(18):8392. https://doi.org/10.3390/app11188392
Chicago/Turabian StyleRebok, Katerina, Maja Jordanova, Júlia Azevedo, and Eduardo Rocha. 2021. "First Report and 3D Reconstruction of a Presumptive Microscopic Liver Lipoma in a Black Barbel (Barbus balcanicus) from the River Bregalnica in the Republic of North Macedonia" Applied Sciences 11, no. 18: 8392. https://doi.org/10.3390/app11188392