Leaching Behavior of Cesium, Strontium, Cobalt, and Europium from Immobilized Cement Matrix
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cement and Nuclides
2.2. Cement Solidification
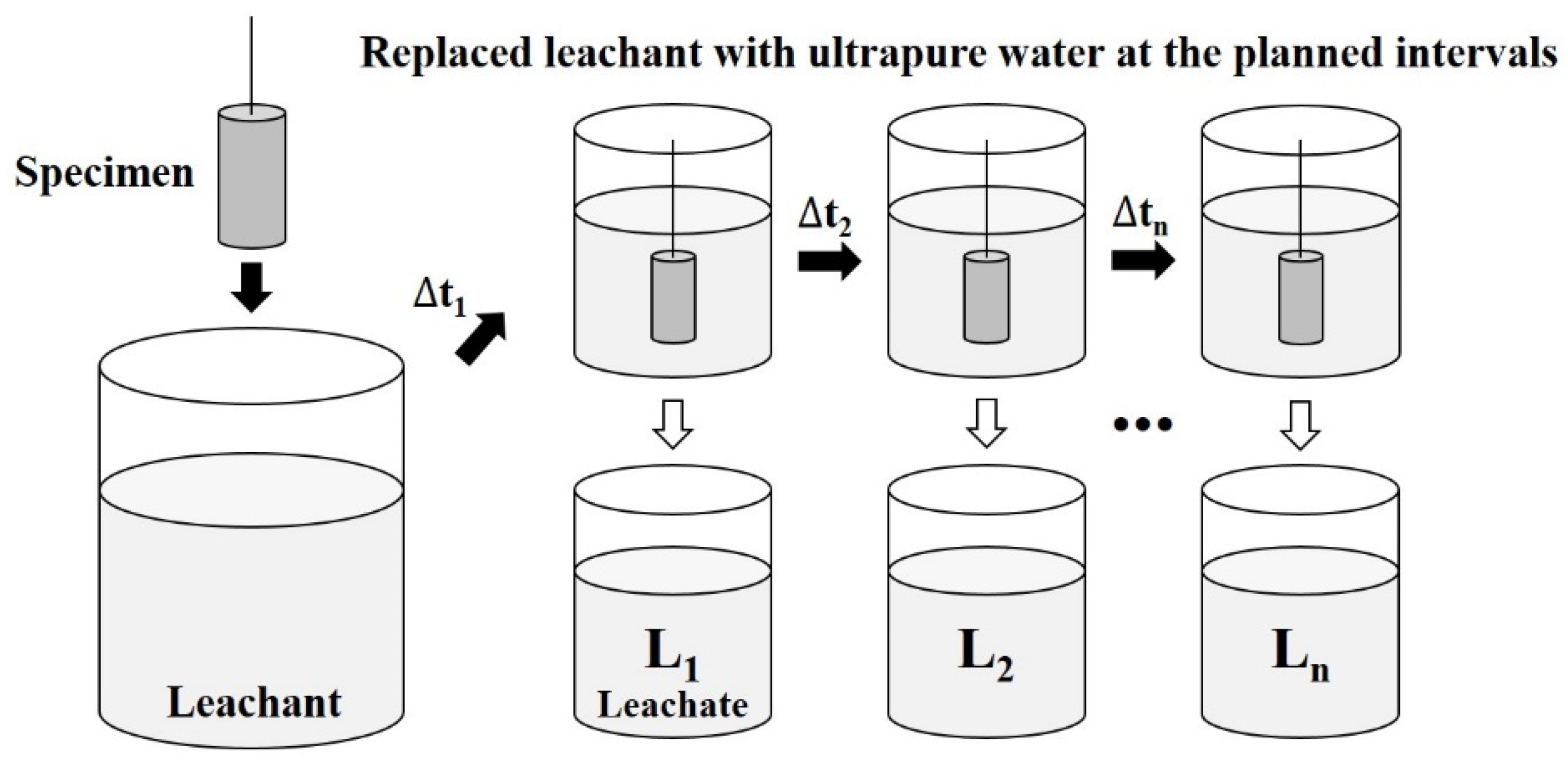
2.3. Leaching Test
2.4. Analysis
3. Results
3.1. Leaching of the Solidified Cement Specimen
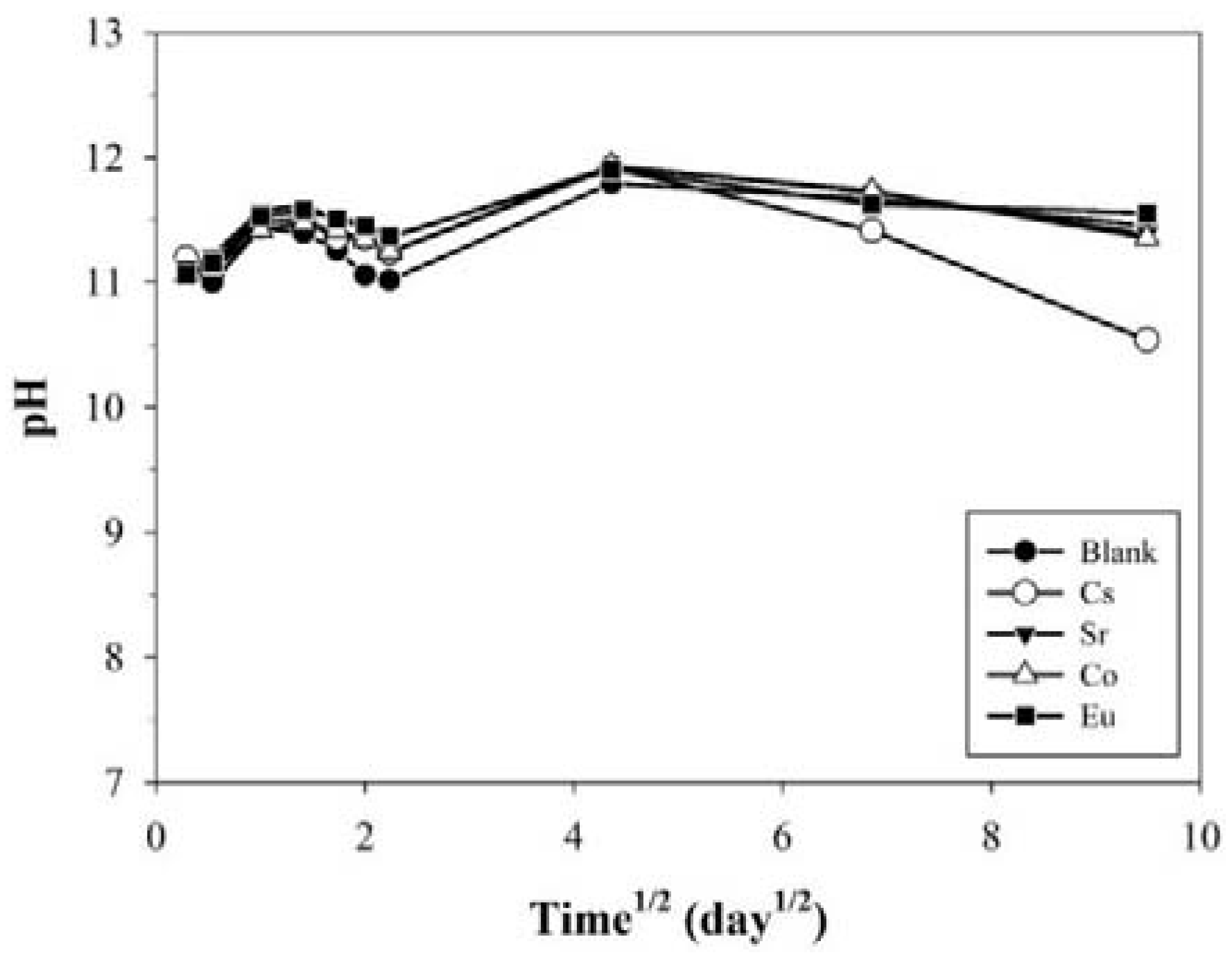
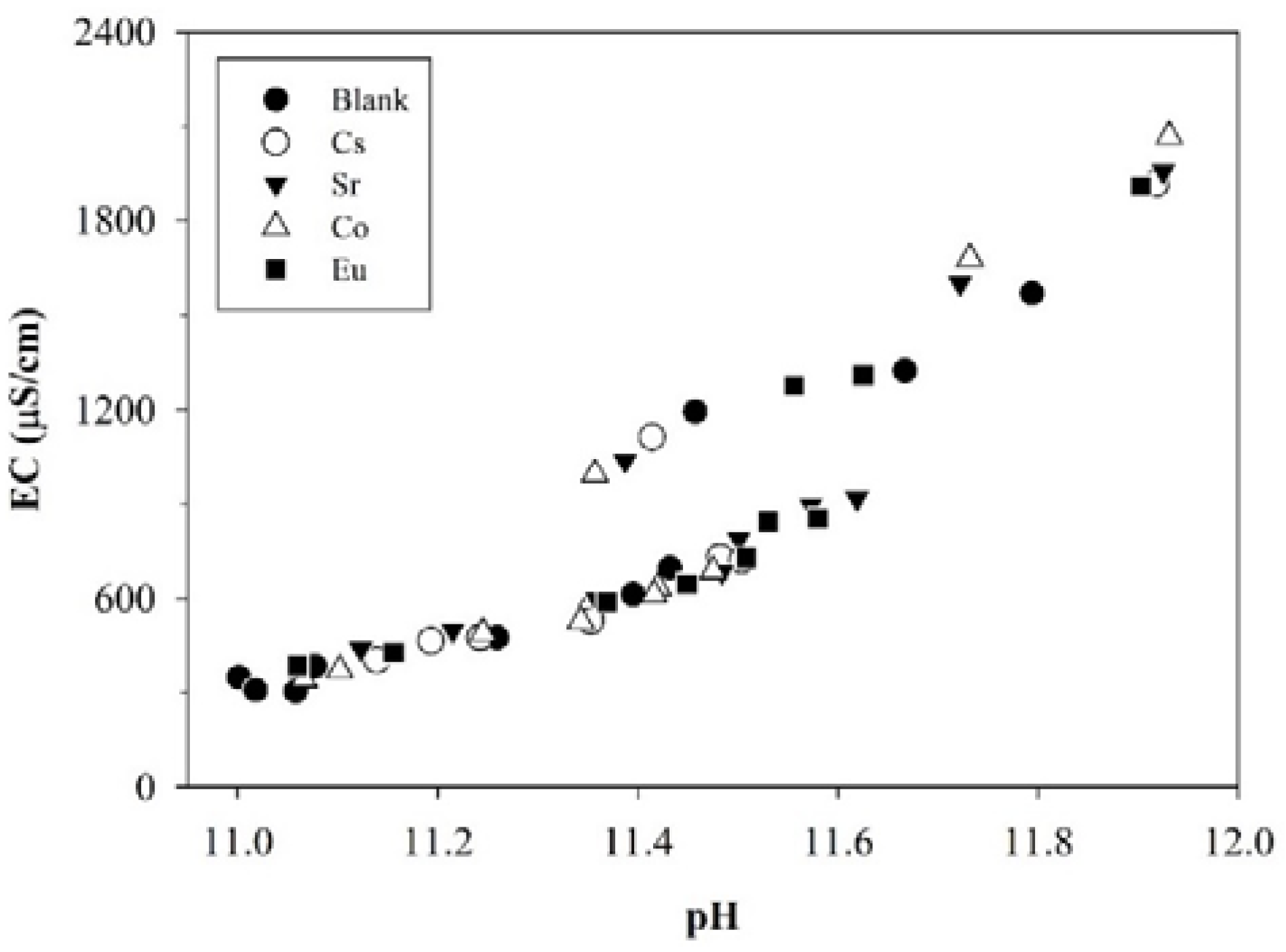
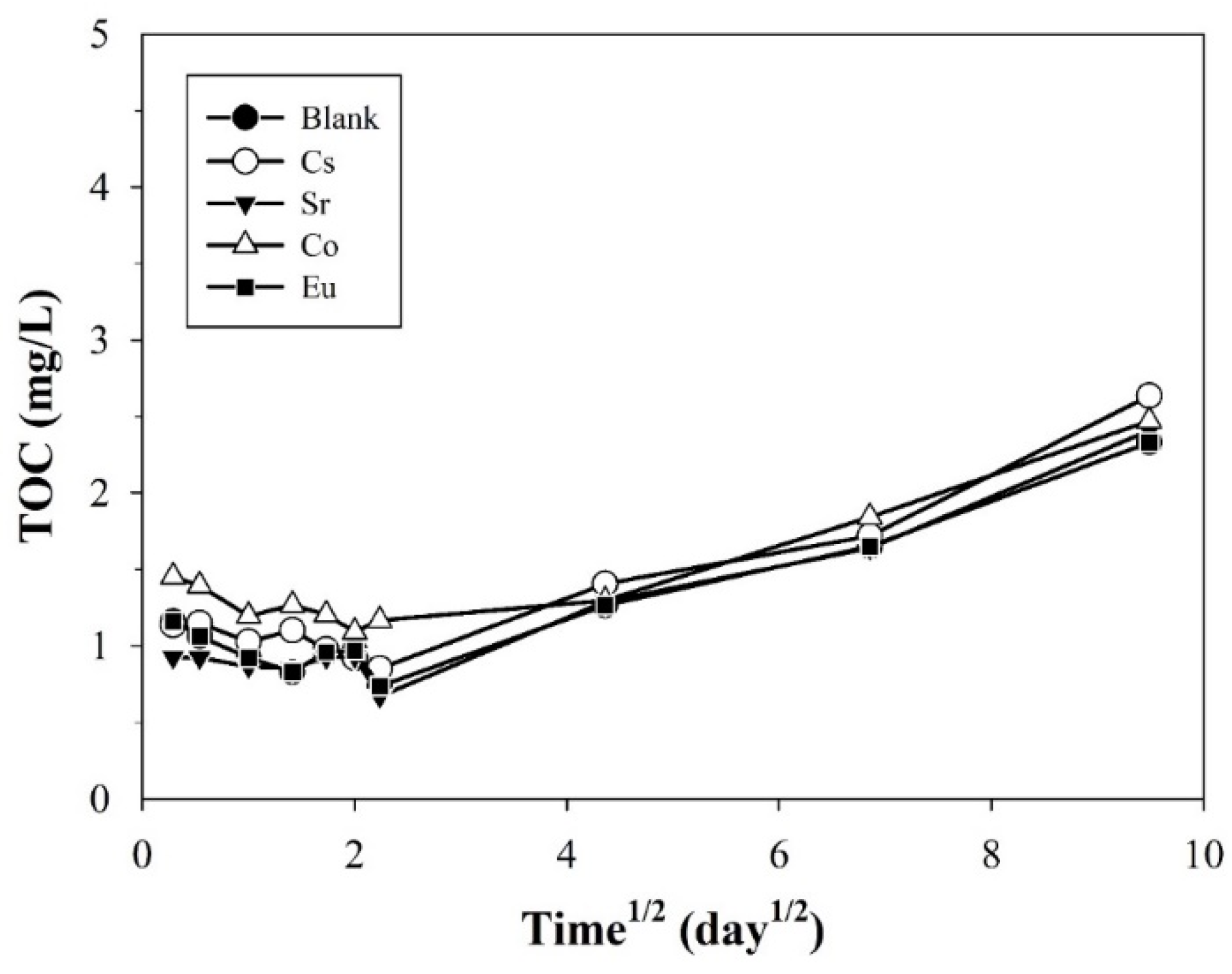
3.1.1. Temporal Variations in pH, EC, and TOC
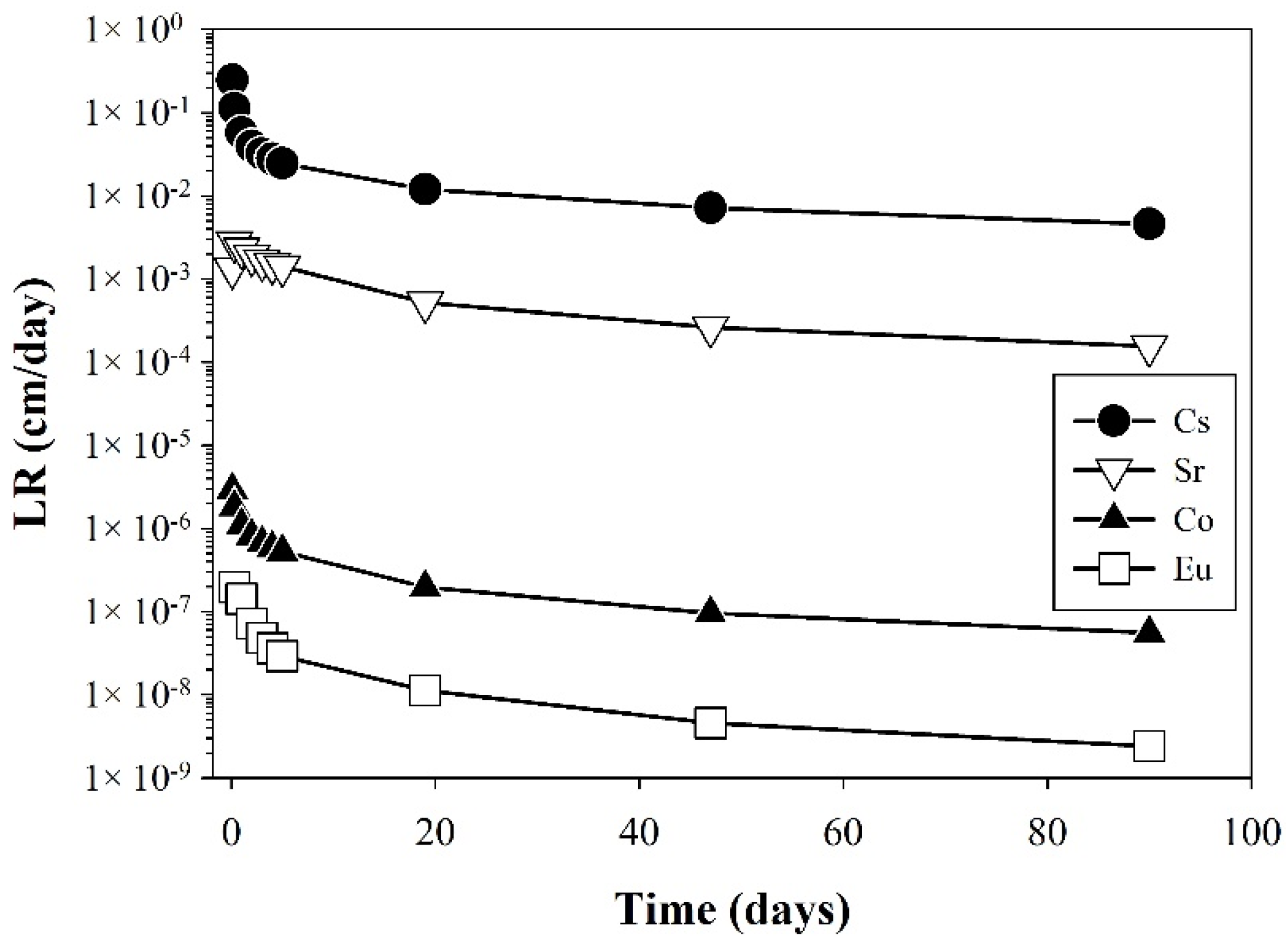
3.1.2. Leaching Rate (LR)
3.1.3. Cumulative Fraction Leached (CFL)
3.1.4. Effective Diffusion Coefficient (De) and Leachability Index (LI)
3.2. Precipitation in Leachate
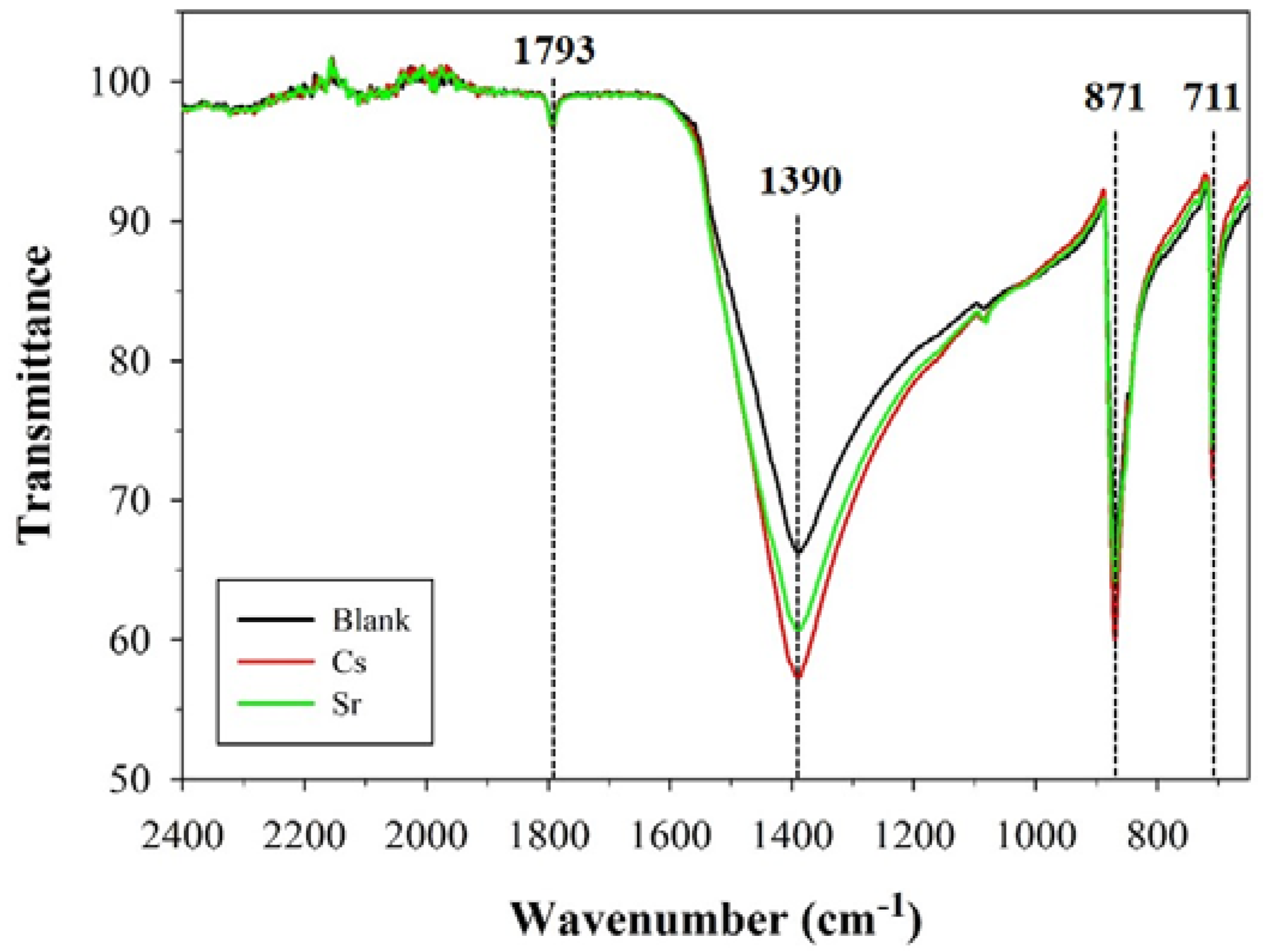
3.2.1. ATR–FTIR Analysis
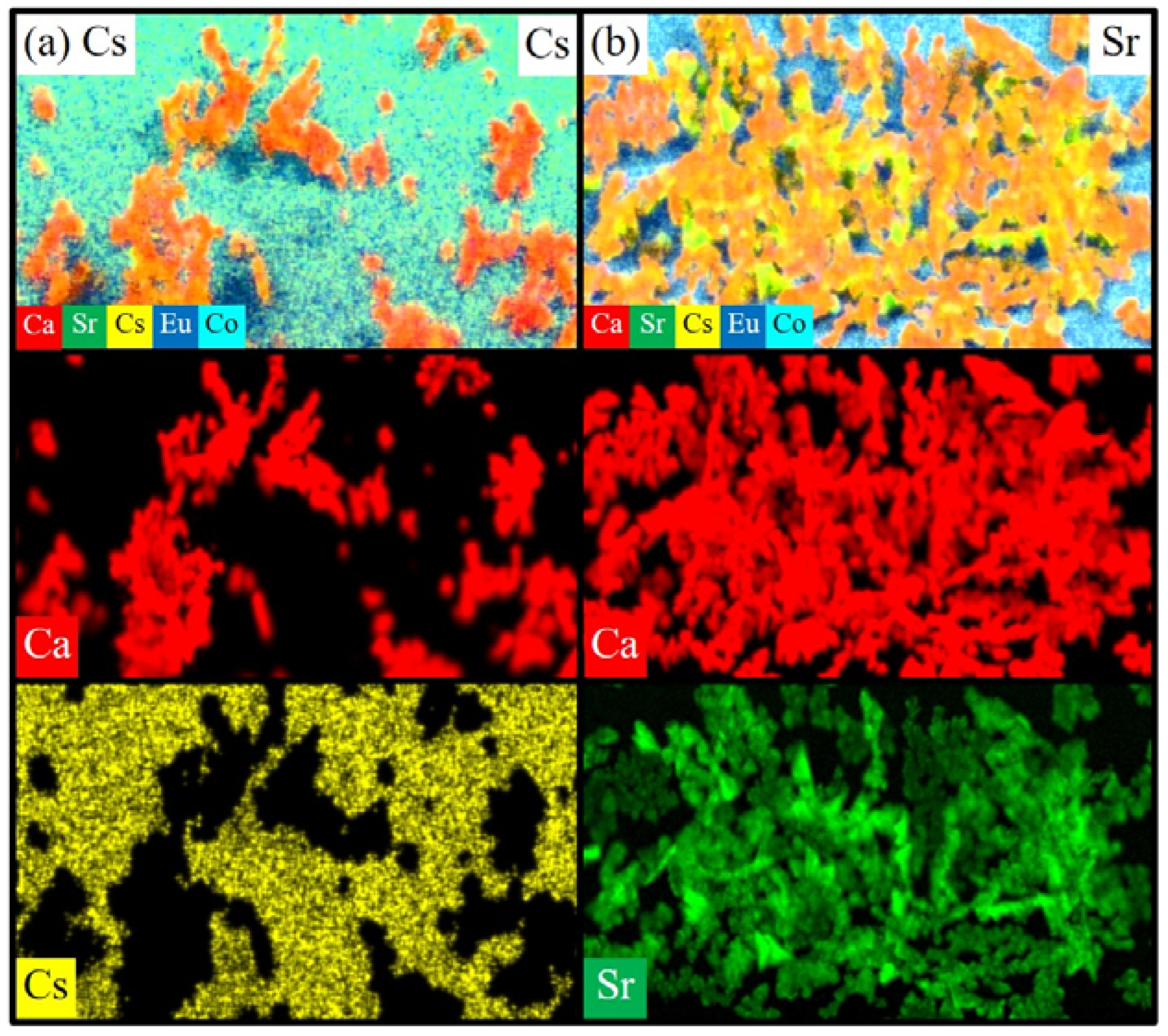
3.2.2. Micro-XRF Analysis
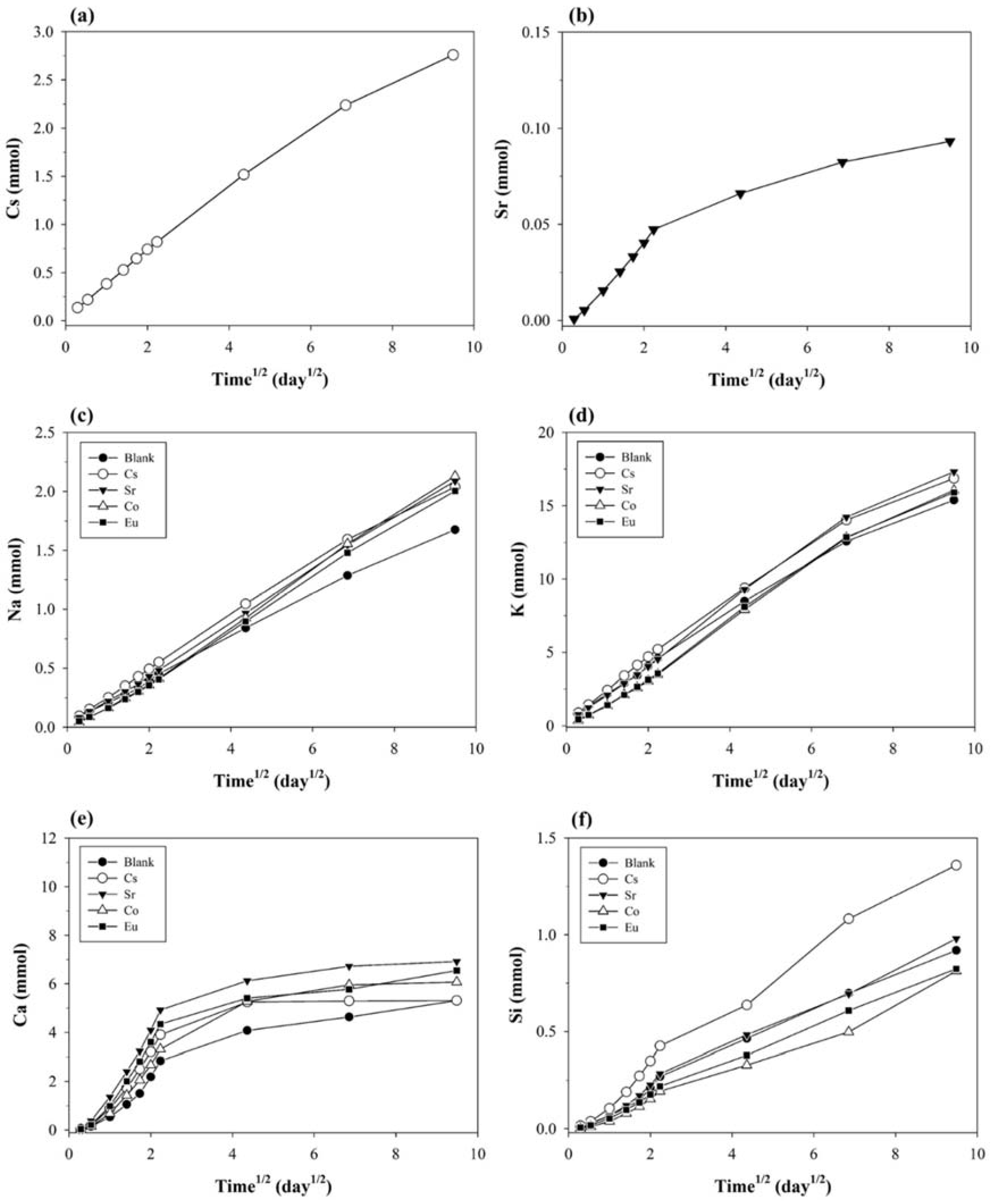
3.2.3. Water-Soluble Ions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IAEA. Decommissioning of Nuclear Power Plants, Research Reactors and Other Nuclear Fuel Cycle Facilities in Specific Safety Guide No. SSG-47, IAEA Safety Standards for Protecting People and the Environment; IAEA: Wienna, Austria, 2018. [Google Scholar]
- Lee, K.-Y.; Park, M.; Kim, J.; Oh, M.; Lee, E.-H.; Kim, K.-W.; Chung, D.-Y.; Moon, J.-K. Equilibrium, kinetic and thermodynamic study of cesium adsorption onto nanocrystalline mordenite from high-salt solution. Chemosphere 2016, 150, 765–771. [Google Scholar] [CrossRef]
- Kamei-Ishikawa, N.; Ito, A.; Tagami, K.; Umita, T. Fate of radiocesium in sewage treatment process released by the nuclear accident at Fukushima. Chemosphere 2013, 93, 689–694. [Google Scholar] [CrossRef]
- IAEA. Classification of Radioactive Waste: General Safety Guide GSG-1; IAEA: Wienna, Austria, 2009. [Google Scholar]
- Bae, S.-C.; Park, J. Status and Research Progress of Nuclear Decommissioning Concrete Waste Disposal. Mag. RCR 2020, 15, 45–51. [Google Scholar]
- Komljenović, M.; Tanasijević, G.; Džunuzović, N.; Provis, J. Immobilization of cesium with alkali-activated blast furnace slag. J. Hazard. Mater. 2020, 388, 121765. [Google Scholar] [CrossRef]
- Koťátková, J.; Zatloukal, J.; Reiterman, P.; Kolář, K. Concrete and cement composites used for radioactive waste deposition. J. Environ. Radioact. 2017, 178, 147–155. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, Y.; Zhang, Z.; Ma, X. The impacts of sodium nitrate on hydration and microstructure of Portland cement and the leaching behavior of Sr2+. J. Hazard. Mater. 2020, 388, 121805. [Google Scholar] [CrossRef]
- NSSC. Regulations on the Delivery of Low and Intermediate Level Radioactive Wastes; NSSC Notice No. 2013-29; Nuclear Safety and Security Commission: Seoul, Korea, 2013. [Google Scholar]
- IAEA. Performance of Engineered Barrier Materials in Near Surface Disposal Facilities for Radioactive Waste; International Atomic Energy Agency: Wienna, Austria, 2001. [Google Scholar]
- IAEA. The Behaviours of Cementitious Materials in Long Term Storage and Disposal of Radioactive Waste; IAEA: Wienna, Austria, 2013. [Google Scholar]
- Rahman, R.O.A.; El Abidin, D.H.A.Z.; Abou-Shady, H. Cesium binding and leaching from single and binary contaminant cement–bentonite matrices. Chem. Eng. J. 2014, 245, 276–287. [Google Scholar] [CrossRef]
- El-Kamash, A.M.; El-Naggar, M.R.; El-Dessouky, M.I. Immobilization of cesium and strontium radionuclides in zeolite-cement blends. J. Hazard. Mater. 2006, 136, 310–316. [Google Scholar] [CrossRef]
- Faiz, Z.; Fakhi, S.; Bouih, A.; Outayad, R.; Benkdad, A.; Hannache, H. Leaching study of cesium from spent ion-exchange resins and Portland cement package. Int. J. Environ. Sci. Technol. 2017, 14, 1019–1026. [Google Scholar] [CrossRef]
- Glasser, F.P. Progress in the immobilization of radioactive wastes in cement. Cem. Concr. Res. 1992, 22, 201–216. [Google Scholar] [CrossRef]
- Szajerski, P.; Bogobowicz, A.; Bem, H.; Gasiorowski, A. Quantitative evaluation and leaching behavior of cobalt immobilized in sulfur polymer concrete composites based on lignite fly ash, slag and phosphogypsum. J. Clean. Prod. 2019, 222, 90–102. [Google Scholar] [CrossRef]
- Plećaš, I.; Dimovic, S. Curing Time Effect on Compressive Strength and Incremental Leaching Rates of 137Cs and 60Co in Cement Immobilized Sludge. Prog. Nucl. Energy 2006, 48, 629–633. [Google Scholar] [CrossRef]
- Rahman, R.O.A.; Zaki, A.A.; El-Kamash, A.M. Modeling the long-term leaching behavior of 137Cs, 60Co, and 152,154 Eu radionuclides from cement–clay matrices. J. Hazard. Mater. 2007, 145, 372–380. [Google Scholar] [PubMed]
- Yoon, H.; Seo, J.; Kim, S.; Lee, H.-K.; Park, S. Characterization of blast furnace slag-blended Portland cement for immobilization of Co. Cem. Concr. Res. 2020, 134, 106089. [Google Scholar] [CrossRef]
- Yin, M.; Tsang, D.C.; Sun, J.; Wang, J.; Shang, J.; Fang, F.; Wu, Y.; Liu, J.; Song, G.; Xiao, T. Critical insight and indication on particle size effects towards uranium release from uranium mill tailings: Geochemical and mineralogical aspects. Chemosphere 2020, 250, 126315. [Google Scholar] [CrossRef] [PubMed]
- Ul Hassan, M.; Iqbal, S.; Yun, J.-I.; Ryu, H.J. Immobilization of radioactive corrosion products by cold sintering of pure hydroxyapatite. J. Hazard. Mater. 2019, 374, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Ofer-Rozovsky, E.; Haddad, M.A.; Bar-Nes, G.; Borojovich, E.; Binyamini, A.; Nikolski, A.; Katz, A. Cesium immobilization in nitrate-bearing metakaolin-based geopolymers. J. Nucl. Mater. 2019, 514, 247–254. [Google Scholar] [CrossRef]
- Kim, M.; Kim, H.G.; Kim, S.; Yoon, J.-H.; Sung, J.Y.; Jin, J.S.; Lee, M.-H.; Kim, C.-W.; Heo, J.; Hong, K.-S. Leaching behaviors and mechanisms of vitrified forms for the low-level radioactive solid wastes. J. Hazard. Mater. 2020, 384, 121296. [Google Scholar] [CrossRef]
- Jang, J.G.; Park, S.M.; Lee, H.-K. Physical barrier effect of geopolymeric waste form on diffusivity of cesium and strontium. J. Hazard. Mater. 2016, 318, 339–346. [Google Scholar] [CrossRef]
- El-Dessouky, M.; Sami, N.; El-Gammal, B.; Abd El-Rehim, S. Leaching Study in Immobilization of Cesium and Cobalt Radionuclides in Fly Ash-Zeolite Cement. Arab. J. Nucl. Sci. Appl. 2013, 46, 52–62. [Google Scholar]
- Wagh, A.S.; Sayenko, S.; Shkuropatenko, V.; Tarasov, R.; Dykiy, M.; Svitlychniy, Y.; Virych, V.; Ulybkina, E. Experimental study on cesium immobilization in struvite structures. J. Hazard. Mater. 2016, 302, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.; Peterson, J.A.; Riley, B.J.; Tabada, D.; Wall, D.; Corkhill, C.L.; McCloy, J.S. Glass-bonded iodosodalite waste form for immobilization of 129I. J. Nucl. Mater. 2018, 504, 109–121. [Google Scholar] [CrossRef]
- Luo, M.; Wen, M.; Wang, J.; Zhu, J. The study of cooperation solidification of Cs based on ZSM-5 zeolite. Energy Procedia 2013, 39, 434–442. [Google Scholar] [CrossRef] [Green Version]
- Ngwenya, N.; Chirwa, E. Improvement of metakaolin on radioactive Sr and Cs immobilization of alkali-activated slag matrix. J. Hazard. Mater. 2002, 92, 289–300. [Google Scholar]
- NRC. Waste Form. Technical Position, Revision 1; US Nuclear Regulatory Commission: Washington, DC, USA, 1991.
- Ngwenya, N.; Chirwa, E. Single and binary component sorption of the fission products Sr2+, Cs+ and Co2+ from aqueous solutions onto sulphate reducing bacteria. Miner. Eng. 2010, 23, 463–470. [Google Scholar] [CrossRef]
- Ma, B.; Oh, S.; Shin, W.S.; Choi, S.-J. Removal of Co2+, Sr2+ and Cs+ from aqueous solution by phosphate-modified montmorillonite (PMM). Desalination 2011, 276, 336–346. [Google Scholar] [CrossRef]
- Fang, X.-H.; Fang, F.; Lu, C.-H.; Zheng, L. Removal of Cs+, Sr2+, and Co2+ ions from the mixture of organics and suspended solids aqueous solutions by zeolites. Nucl. Eng. Technol. 2017, 49, 556–561. [Google Scholar] [CrossRef]
- Turkington, G.; Gamage, K.A.; Graham, J. Beta detection of strontium-90 and the potential for direct in situ beta detection for nuclear decommissioning applications. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2018, 911, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Dubourg, M. Review of advanced methods for treating radioactive contaminated water; Examen des methodes avancees de traitement de l’eau radioactive contaminee a la suite d’accidents nucleaires. Radioprotection 1998, 33, 35–46. [Google Scholar] [CrossRef]
- Rahman, R.A.; Moamen, O.A.; Abdelmonem, N.; Ismail, I. Optimizing the removal of strontium and cesium ions from binary solutions on magnetic nano-zeolite using response surface methodology (RSM) and artificial neural network (ANN). Environ. Res. 2019, 173, 397–410. [Google Scholar] [CrossRef]
- Saleh, H.; Bayoumi, T.; Mahmoud, H.; Aglan, R. Uptake of cesium and cobalt radionuclides from simulated radioactive wastewater by Ludwigia stolonifera aquatic plant. Nucl. Eng. Des. 2017, 315, 194–199. [Google Scholar] [CrossRef]
- Shao, D.; Fan, Q.; Li, J.; Niu, Z.; Wu, W.; Chen, Y.; Wang, X. Removal of Eu (III) from aqueous solution using ZSM-5 zeolite. Microporous Mesoporous Mater. 2009, 123, 1–9. [Google Scholar] [CrossRef]
- Saleh, H. Water hyacinth for phytoremediation of radioactive waste simulate contaminated with cesium and cobalt radionuclides. Nucl. Eng. Des. 2012, 242, 425–432. [Google Scholar] [CrossRef]
- Rahman, M.; Chand, M.; Koddus, A.; Zaman, M.; Voigt, G. Transfer of radiocobalt from soil to selected plant species in tropical environments. J. Environ. Radioact. 2008, 99, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.M.; Attallah, M.F.; Metwally, S.S. Simultaneous solid phase extraction of cobalt, strontium and cesium from liquid radioactive waste using microcrystalline naphthalene. Radiochim. Acta 2014, 102, 1017–1024. [Google Scholar] [CrossRef]
- Shin, J.; Kwak, J.; Lee, Y.-G.; Kim, S.; Son, C.; Cho, K.H.; Lee, S.-H.; Park, Y.; Ren, X.; Chon, K. Changes in adsorption mechanisms of radioactive barium, cobalt, and strontium ions using spent coffee waste biochars via alkaline chemical activation: Enrichment effects of O-containing functional groups. Environ. Res. 2021, 199, 111346. [Google Scholar] [CrossRef]
- Yoshida, G.; Nishikawa, K.; Nakamura, H.; Yashima, H.; Sekimoto, S.; Miura, T.; Masumoto, K.; Toyoda, A.; Matsumura, H. Investigation of variations in cobalt and europium concentrations in concrete to prepare for accelerator decommissioning. J. Radioanal. Nucl. Chem. 2020, 325, 801–806. [Google Scholar] [CrossRef]
- Burnham, S.; Moffitt, G.; Jevremovic, T. Methodology for detection of trace amounts of cobalt and europium in neutron shielding structural concrete. In Proceedings of the 23rd International Conference on Nuclear Engineering (ICONE-23), Chiba, Japan, 17–21 May 2015. [Google Scholar]
- ANSI. ANSI/ANS-16.1–2003 (R2017) Measurement of the Leachability of Solidified Low-Level Radioactive Wastes by a Short-Term Test Procedure; American National Standards Institute: Washington, DC, USA, 2017. [Google Scholar]
- McIsaac, C.V. Leachability of chelated ion-exchange resins solidified in cement or cement and fly ash. Waste Manag. 1993, 13, 41–54. [Google Scholar] [CrossRef]
- Means, J.L.; Crerar, D.A.; Duguid, J.O. Migration of radioactive wastes: Radionuclide mobilization by complexing agents. Science 1978, 200, 1477–1481. [Google Scholar] [CrossRef]
- Glasser, F. Fundamental aspects of cement solidification and stabilisation. J. Hazard. Mater. 1997, 52, 151–170. [Google Scholar] [CrossRef]
- Crawford, R.; McCulloch, C.; Angus, M.; Glasser, F. Instrinsic sorption potential of cement components for 134Cs. Cem. Concr. Res. 1984, 14, 595–599. [Google Scholar] [CrossRef]
- Bagosi, S.; Csetenyi, L. Caesium immobilisation in hydrated calcium-silicate-aluminate systems. Cem. Concr. Res. 1998, 28, 1753–1759. [Google Scholar] [CrossRef]
- Matsuzuru, H.; Moriyama, N.; Wadachi, Y.; Ito, A. Leaching behavior of cesium-137 in cement-waste composites. Health Phys. 1977, 32, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Soler, J.M. Reactive transport modeling of the interaction between a high-pH plume and a fractured marl: The case of Wellenberg. Appl. Geochem. 2003, 18, 1555–1571. [Google Scholar] [CrossRef]
- Bagosi, S.; Csetenyi, L.J. Immobilization of caesium-loaded ion exchange resins in zeolite-cement blends. Cem. Concr. Res. 1999, 29, 479–485. [Google Scholar] [CrossRef]
- Aldridge, L.; Day, R.; Leung, S.; Ray, A.; Stevens, M.; Knight, R. 42 C’S retention in zeolites immobilised by portland cement. In Mechanisms of Chemical Degradation of Cement-Based Systems; CRC Press: London, UK, 1997; pp. 358–365. [Google Scholar]
- Bernal, S.A.; San Nicolas, R.; Myers, R.J.; de Gutiérrez, R.M.; Puertas, F.; van Deventer, J.S.; Provis, J.L. MgO content of slag controls phase evolution and structural changes induced by accelerated carbonation in alkali-activated binders. Cem. Concr. Res. 2014, 57, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Václava, T.; Mária, F.; Martin, D.; Ján, M. Immobilisation of cesium in matrices based on the blast furnace slag incorporating zeolitic additives. Ceramics-Silikáty 1995, 39, 155–159. [Google Scholar]
- Hong, S.-Y.; Glasser, F. Alkali binding in cement pastes: Part. I. The CSH phase. Cem. Concr. Res. 1999, 29, 1893–1903. [Google Scholar] [CrossRef]
- Glasser, F.P. Characterisation of the barrier performance of cements. MRS Online Proc. Libr. Arch. 2002, 713. [Google Scholar] [CrossRef]
- Komarneni, S.; Breval, E.; Roy, D.M.; Roy, R. Reactions of some calcium silicates with metal cations. Cem. Concr. Res. 1988, 18, 204–220. [Google Scholar] [CrossRef]
- Pointeau, I.; Piriou, B.; Fedoroff, M.; Barthes, M.G.; Marmier, N.; Fromage, F. Sorption mechanisms of Eu3+ on CSH phases of hydrated cements. J. Colloid Interface Sci. 2001, 236, 252–259. [Google Scholar] [CrossRef]
- Bintintan, A.; Gligor, M.; Radulescu, C.; Dulama, I.D.; Lucian Olteanu, R.; Teodorescu, S.; Stirbescu, R.M.; Bucurica, I.A. Multielemental and Chemical Characterization of Eneolithic Petresti Painted Pottery from the Alba Iulia-Lumea Noua Archaeological Site, Romania. Anal. Lett. 2019, 52, 2348–2364. [Google Scholar] [CrossRef]
- Puertas, F.; Palacios, M.; Manzano, H.; Dolado, J.; Rico, A.; Rodríguez, J. A model for the CASH gel formed in alkali-activated slag cements. J. Eur. Ceram. Soc. 2011, 31, 2043–2056. [Google Scholar] [CrossRef]
- He, D.; Dong, F.; Luo, Y.; Huang, L.; Zhang, B. Preparation of spherical calcium carbonate from phosphorus slag. J. Chin. Ceram. Soc 2010, 38, 1268–1273. [Google Scholar]
- Reig, F.B.; Adelantado, J.G.; Moreno, M.M. FTIR quantitative analysis of calcium carbonate (calcite) and silica (quartz) mixtures using the constant ratio method. Application to geological samples. Talanta 2002, 58, 811–821. [Google Scholar] [PubMed]
- Bintintan, A.; Gligor, M.; Dulama, I.D.; Teodorescu, S.; Stirbescu, R.M.; Radulescu, C. ATR-FTIR and SEM-EDS analyses of lumea noua painted pottery from Alba Iulia-Lumea noua neolithic site. Rev. Chim. 2017, 68, 847–852. [Google Scholar] [CrossRef]
- Nedeljković, M.; Zuo, Y.; Arbi, K.; Ye, G. Carbonation resistance of alkali-activated slag under natural and accelerated conditions. J. Sustain. Metall. 2018, 4, 33–49. [Google Scholar] [CrossRef] [Green Version]
- Bernal, S.A.; de Gutierrez, R.M.; Provis, J.L.; Rose, V. Effect Effect of silicate modulus and metakaolin incorporation on the carbonation of alkali silicate-activated slags. Cem. Concr. Res. 2010, 40, 898–907. [Google Scholar] [CrossRef]
- Nedeljković, M.; Šavija, B.; Zuo, Y.; Luković, M.; Ye, G. Effect of natural carbonation on the pore structure and elastic modulus of the alkali-activated fly ash and slag pastes. Constr. Build. Mater. 2018, 161, 687–704. [Google Scholar] [CrossRef] [Green Version]
- Pecher, C. Biological Investigations with Radioactive Calcium and Strontium. Proc. Soc. Exp. Biol. Med. 1941, 46, 86–91. [Google Scholar] [CrossRef]
- Blaschko, S.D.; Chi, T.; Miller, J.; Flechner, L.; Fakra, S.; Kapahi, P.; Kahn, A.; Stoller, M.L. Strontium substitution for calcium in lithogenesis. J. Urol. 2013, 189, 735–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, N.D. Binding mechanisms of radionuclides to cement. Cem. Concr. Res. 2008, 38, 543–553. [Google Scholar] [CrossRef] [Green Version]

| CaO | SiO2 | Al2O3 | Fe2O3 | SO3 | MgO | K2O | Na2O | P2O5 | MnO | LOI 1 |
|---|---|---|---|---|---|---|---|---|---|---|
| 63.8 | 22.1 | 5.4 | 3.2 | 2.1 | 1.6 | 0.5 | 0.4 | 0.1 | 0.1 | 0.7 |
| Blank | Cs | Sr | Co | Eu | |
|---|---|---|---|---|---|
| Content (mmol) | - | 10 | 10 | 10 | 10 |
| Geometric surface area (cm2) | 194.4 | 194.6 | 195.7 | 196.5 | 197.6 |
| Volume (cm3) | 194.4 | 194.2 | 194.9 | 196.9 | 198.1 |
| Mass (g) | 392.0 | 377.6 | 383.5 | 371.2 | 373.0 |
| Time | Cs | Sr | Co | Eu |
|---|---|---|---|---|
| * | ||||
| * | ||||
| * | ||||
| Cs | Sr | Co | Eu | |
|---|---|---|---|---|
| LI | 7.6 | 10.5 | 17.3 | 21.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goo, J.-Y.; Kim, B.-J.; Kang, M.; Jeong, J.; Jo, H.Y.; Kwon, J.-S. Leaching Behavior of Cesium, Strontium, Cobalt, and Europium from Immobilized Cement Matrix. Appl. Sci. 2021, 11, 8418. https://doi.org/10.3390/app11188418
Goo J-Y, Kim B-J, Kang M, Jeong J, Jo HY, Kwon J-S. Leaching Behavior of Cesium, Strontium, Cobalt, and Europium from Immobilized Cement Matrix. Applied Sciences. 2021; 11(18):8418. https://doi.org/10.3390/app11188418
Chicago/Turabian StyleGoo, Ja-Young, Bong-Ju Kim, Myunggoo Kang, Jongtae Jeong, Ho Young Jo, and Jang-Soon Kwon. 2021. "Leaching Behavior of Cesium, Strontium, Cobalt, and Europium from Immobilized Cement Matrix" Applied Sciences 11, no. 18: 8418. https://doi.org/10.3390/app11188418








