Bilateral Deficits during Maximal Grip Force Production in Late Postmenopausal Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Particiapants
2.2. Experimental Setup
2.3. Data Analysis
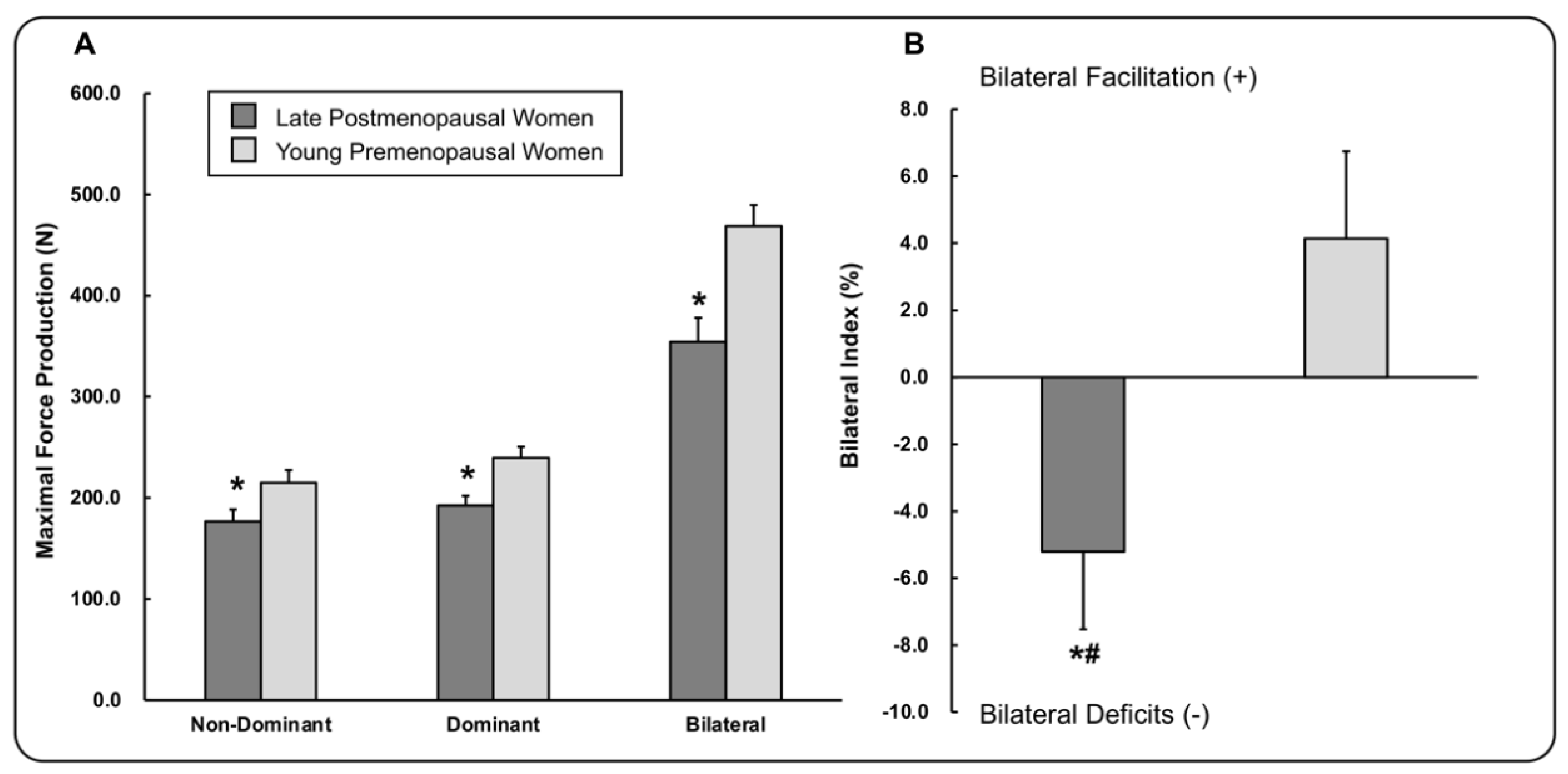
3. Results
3.1. Maximal Hand-Grip Force Production
3.2. Correlation Findings for Late Postmenopausal Women
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, C.Y.; Lim, J.-Y.; Park, H.-Y. Age at natural menopause in Koreans: Secular trends and influences thereon. Menopause 2018, 25, 423–429. [Google Scholar] [CrossRef]
- Global Aging into the 21st Century. 1996. Available online: https://www.census.gov/library/publications/1996/demo/96wchart.html (accessed on 18 August 2021).
- Davy, K.P.; Desouza, C.A.; Jones, P.P.; Seals, D.R. Elevated heart rate variability in physically active young and older adult women. Clin. Sci. 1998, 94, 579–584. [Google Scholar] [CrossRef]
- Huikuri, H.V.; Pikkujämsä, S.M.; Airaksinen, K.E.; Ikäheimo, M.J.; Rantala, A.O.; Kauma, H.; Lilja, M.; Kesäniemi, Y.A. Sex-related differences in autonomic modulation of heart rate in middle-aged subjects. Circulation 1996, 94, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, P.; Mascagni, G.; Giannini, A.; Genazzani, A.R.; Simoncini, T. Symptoms of menopause—Global prevalence, physiology and implications. Nat. Rev. Endocrinol. 2018, 14, 199–215. [Google Scholar] [CrossRef]
- Ribeiro, T.; Azevedo, G.; Crescêncio, J.; Marães, V.; Papa, V.; Catai, A.; Verzola, R.; Oliveira, L.; Silva de Sá, M.; Gallo, L., Jr. Heart rate variability under resting conditions in postmenopausal and young women. Braz. J. Med. Biol. Res. 2001, 34, 871–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ditroilo, M.; Forte, R.; Benelli, P.; Gambarara, D.; de Vito, G. Effects of age and limb dominance on upper and lower limb muscle function in healthy males and females aged 40–80 years. J. Sports Sci. 2010, 28, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, A.; Nimmo, M.A.; Foster, J.E.; Cockburn, M.; McMillan, N.C.; de Vito, G. Contractile muscle volume and agonist-antagonist coactivation account for differences in torque between young and older women. Muscle Nerve 2002, 25, 858–863. [Google Scholar] [CrossRef]
- Vandervoort, A.A. Aging of the human neuromuscular system. Muscle Nerve 2002, 25, 17–25. [Google Scholar] [CrossRef]
- Beurskens, R.; Gollhofer, A.; Muehlbauer, T.; Cardinale, M.; Granacher, U. Effects of Heavy-Resistance Strength and Balance Training on Unilateral and Bilateral Leg Strength Performance in Old Adults. PLoS ONE 2015, 10, e0118535. [Google Scholar] [CrossRef]
- Kilbreath, S.L.; Heard, R.C. Frequency of hand use in healthy older persons. Aust. J. Physiother. 2005, 51, 119–122. [Google Scholar] [CrossRef] [Green Version]
- Calmels, P.; Vico, L.; Alexandre, C.; Minaire, P. Cross-sectional study of muscle strength and bone mineral density in a population of 106 women between the ages of 44 and 87 years: Relationship with age and menopause. Eur. J. Appl. Physiol. Occup. Physiol. 1995, 70, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Lindle, R.S.; Metter, E.J.; Lynch, N.A.; Fleg, J.L.; Fozard, J.L.; Tobin, J.; Roy, T.A.; Hurley, B.F. Age and gender comparisons of muscle strength in 654 women and men aged 20–93 yr. J. Appl. Physiol. 1997, 83, 1581–1587. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.K.; Rook, K.M.; Siddle, N.C.; Bruce, S.A.; Woledge, R.C. Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin. Sci. 1993, 84, 95–98. [Google Scholar] [CrossRef] [Green Version]
- Stanley, S.N.; Taylor, N.A.S. Isokinematic muscle mechanics in four groups of women of increasing age. Eur. J. Appl. Physiol. Occup. Physiol. 1993, 66, 178–184. [Google Scholar] [CrossRef]
- Cipriani, C.; Romagnoli, E.; Carnevale, V.; Raso, I.; Scarpiello, A.; Angelozzi, M.; Tancredi, A.; Russo, S.; de Lucia, F.; Pepe, J.; et al. Muscle strength and bone in healthy women: Effect of age and gonadal status. Hormones 2012, 11, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Henry, F.M.; Smith, L.E. Simultaneous vs. Separate Bilateral Muscular Contractions in Relation to Neural Overflow Theory and Neuromoter Specificity. Res. Q. Am. Assoc. Health Phys. Educ. Recreat. 1961, 32, 42–46. [Google Scholar] [CrossRef]
- Taniguchi, Y. Effect of practice in bilateral and unilateral reaction-time tasks. Percept. Mot. Skills 1999, 88, 99–109. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Burle, B.; Vidal, F.; Bonnet, M. Deficit in motor cortical activity for simultaneous bimanual responses. Exp. Brain Res. 2001, 137, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Škarabot, J.; Cronin, N.; Strojnik, V.; Avela, J. Bilateral deficit in maximal force production. Eur. J. Appl. Physiol. Occup. Physiol. 2016, 116, 2057–2084. [Google Scholar] [CrossRef] [PubMed]
- Bobbert, M.F.; de Graaf, W.W.; Jonk, J.N.; Casius, L.J.R. Explanation of the bilateral deficit in human vertical squat jumping. J. Appl. Physiol. 2006, 100, 493–499. [Google Scholar] [CrossRef]
- Samozino, P.; Rejc, E.; di Prampero, P.E.; Belli, A.; Morin, J.-B. Force–Velocity Properties’ Contribution to Bilateral Deficit during Ballistic Push-off. Med. Sci. Sports Exerc. 2014, 46, 107–114. [Google Scholar] [CrossRef]
- Van Dieen, J.H.; Ogita, F.; de Haan, A. Reduced Neural Drive in Bilateral Exertions: A Performance-Limiting Factor? Med. Sci. Sports Exerc. 2003, 35, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Pääsuke, M.; Ereline, J.; Gapeyeva, H.; Joost, K.; Mõttus, K.; Taba, P. Leg-Extension Strength and Chair-Rise Performance in Elderly Women with Parkinson’s Disease. J. Aging Phys. Act. 2004, 12, 511–524. [Google Scholar] [CrossRef]
- Ruiz-Cárdenas, J.; Rodríguez-Juan, J.; Jakobi, J.; Ríos-Díaz, J.; Marín-Cascales, E.; Rubio-Arias, J. Bilateral deficit in explosive force related to sit-to-stand performance in older postmenopausal women. Arch. Gerontol. Geriatr. 2018, 74, 145–149. [Google Scholar] [CrossRef]
- Janzen, C.L.; Chilibeck, P.D.; Davison, K.S. The effect of unilateral and bilateral strength training on the bilateral deficit and lean tissue mass in post-menopausal women. Eur. J. Appl. Physiol. Occup. Physiol. 2006, 97, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Kuruganti, U.; Seaman, K. The bilateral leg strength deficit is present in old, young and adolescent females during isokinetic knee extension and flexion. Eur. J. Appl. Physiol. Occup. Physiol. 2006, 97, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, J.; Mishima, C.; Nakayama, S.; Ishii, N. Force–velocity, force–power relationships of bilateral and unilateral leg multi-joint movements in young and elderly women. J. Biomech. 2009, 42, 2151–2157. [Google Scholar] [CrossRef] [PubMed]
- Bayer, U.; Hausmann, M. Estrogen treatment affects brain functioning after menopause. Menopause Int. 2011, 17, 148–152. [Google Scholar] [CrossRef]
- Sherman, S. Defining the menopausal transition. Am. J. Med. 2005, 118, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Carbone, S.; Kirkman, D.L.; Garten, R.S.; Rodriguez-Miguelez, P.; Artero, E.G.; Lee, D.-C.; Lavie, C.J. Muscular strength and cardiovascular disease: An updated state-of-the-art narrative review. J. Cardiopulm. Rehabil. Prev. 2020, 40, 302–309. [Google Scholar] [CrossRef]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Lopez-Jaramillo, P.; Avezum, A., Jr.; Orlandini, A.; Seron, P.; Ahmed, S.H.; Rosengren, A.; Kelishadi, R. Prognostic value of grip strength: Findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015, 386, 266–273. [Google Scholar] [CrossRef]
- García-Hermoso, A.; Cavero-Redondo, I.; Ramírez-Vélez, R.; Ruiz, J.R.; Ortega, F.B.; Lee, D.-C.; Martínez-Vizcaíno, V. Mus-cular strength as a predictor of all-cause mortality in an apparently healthy population: A systematic review and meta-analysis of data from approximately 2 million men and women. Arch. Phys. Med. Rehabil. 2018, 99, 2100–2113. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.D.; Enoka, R.M. Maximum bilateral contractions are modified by neurally mediated interlimb effects. J. Appl. Physiol. 1991, 70, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Butlin, M.; Qasem, A. Large artery stiffness assessment using SphygmoCor technology. Pulse 2016, 4, 180–192. [Google Scholar] [CrossRef] [Green Version]
- Butlin, M.; Qasem, A.; Avolio, A.P. Estimation of central aortic pressure waveform features derived from the brachial cuff volume displacement waveform. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 2591–2594. [Google Scholar]
- Karamanoglu, M.; O’Rourke, M.F.; Avolio, A.P.; Kelly, R.P. An analysis of the relationship between central aortic and peripheral upper limb pressure waves in man. Eur. Heart J. 1993, 14, 160–167. [Google Scholar] [CrossRef]
- Roman, M.J.; Devereux, R.B.; Kizer, J.R.; Okin, P.M.; Lee, E.T.; Wang, W.; Umans, J.G.; Calhoun, D.; Howard, B.V. High Central Pulse Pressure Is Independently Associated with Adverse Cardiovascular Outcome: The Strong Heart Study. J. Am. Coll. Cardiol. 2009, 54, 1730–1734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlachopoulos, C.; Aznaouridis, K.; O’Rourke, M.F.; Safar, M.E.; Baou, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: A systematic review and meta-analysis. Eur. Heart J. 2010, 31, 1865–1871. [Google Scholar] [CrossRef] [Green Version]
- Weber, T.; Auer, J.; O’Rourke, M.F.; Kvas, E.; Laßnig, E.; Berent, R.; Eber, B. Arterial Stiffness, Wave Reflections, and the Risk of Coronary Artery Disease. Circulation 2004, 109, 184–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, J.P.; Nelson-Whalen, N.L.; Franke, W.D.; McLean, S.P. Bilateral index expressions and iEMG activity in older versus young adults. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2003, 58, M536–M541. [Google Scholar] [CrossRef] [Green Version]
- Perez, M.A.; Butler, J.E.; Taylor, J.L. Modulation of transcallosal inhibition by bilateral activation of agonist and antagonist proximal arm muscles. J. Neurophysiol. 2014, 111, 405–414. [Google Scholar] [CrossRef] [Green Version]
- Soteropoulos, D.S.; Perez, M.A. Physiological changes underlying bilateral isometric arm voluntary contractions in healthy humans. J. Neurophysiol. 2011, 105, 1594–1602. [Google Scholar] [CrossRef] [Green Version]
- Cincotta, M.; Ziemann, U. Neurophysiology of unimanual motor control and mirror movements. Clin. Neurophysiol. 2008, 119, 744–762. [Google Scholar] [CrossRef]
- Vercauteren, K.; Pleysier, T.; van Belle, L.; Swinnen, S.P.; Wenderoth, N. Unimanual muscle activation increases interhe-mispheric inhibition from the active to the resting hemisphere. Neurosci. Lett. 2008, 445, 209–213. [Google Scholar] [CrossRef]
- DeJong, S.L.; Lang, C.E. The bilateral movement condition facilitates maximal but not submaximal paretic-limb grip force in people with post-stroke hemiparesis. Clin. Neurophysiol. 2012, 123, 1616–1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oda, S.; Moritani, T. Movement-related cortical potentials during handgrip contractions with special reference to force and electromyogram bilateral deficit. Eur. J. Appl. Physiol. Occup. Physiol. 1995, 72, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Oda, S.; Moritani, T. Cross-correlation studies of movement-related cortical potentials during unilateral and bilateral muscle contractions in humans. Eur. J. Appl. Physiol. Occup. Physiol. 1996, 74, 29–35. [Google Scholar] [CrossRef]
- Weis, S.; Hausmann, M. Sex Hormones: Modulators of Interhemispheric Inhibition in the Human Brain. Neuroscience 2009, 16, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Weis, S.; Hausmann, M.; Stoffers, B.; Vohn, R.; Kellermann, T.; Sturm, W. Estradiol Modulates Functional Brain Organization during the Menstrual Cycle: An Analysis of Interhemispheric Inhibition. J. Neurosci. 2008, 28, 13401–13410. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.E.; Kim, K.W.; Paik, N.-J.; Jang, H.C.; Chang, C.B.; Baek, G.H.; Lee, Y.H.; Gong, H.S. Evaluation of Factors Influencing Grip Strength in Elderly Koreans. J. Bone Metab. 2012, 19, 103–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Aloia, J.F.; McGowan, D.M.; Vaswani, A.N.; Ross, P.; Cohn, S.H. Relationship of menopause to skeletal and muscle mass. Am. J. Clin. Nutr. 1991, 53, 1378–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with func-tional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sitnick, M.; Foley, A.M.; Brown, M.; Spangenburg, E.E. Ovariectomy prevents the recovery of atrophied gastrocnemius skeletal muscle mass. J. Appl. Physiol. 2006, 100, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.J. Interactions Between Reactive Oxygen Species Generated by Contractile Activity and Aging in Skeletal Muscle? Antioxid. Redox Signal. 2013, 19, 804–812. [Google Scholar] [CrossRef] [Green Version]
- Maltais, M.L.; Desroches, J.; Dionne, I.J. Changes in muscle mass and strength after menopause. J. Musculoskelet. Neuronal Interact. 2009, 9, 186–197. [Google Scholar]
- Powers, S.K.; Jackson, M.J. Exercise-Induced Oxidative Stress: Cellular Mechanisms and Impact on Muscle Force Production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [Green Version]
- Brown, M. Skeletal muscle and bone: Effect of sex steroids and aging. Adv. Physiol. Educ. 2008, 32, 120–126. [Google Scholar] [CrossRef]
- Wiik, A.; Ekman, M.; Johansson, O.; Jansson, E.; Esbjörnsson, M. Expression of both oestrogen receptor alpha and beta in human skeletal muscle tissue. Histochem. Cell Biol. 2008, 131, 181–189. [Google Scholar] [CrossRef]
- Pfeilschifter, J.; Scheidt-Nave, C.; Leidig-Bruckner, G.; Woitge, H.W.; Blum, W.F.; Wüster, C.; Haack, D.; Ziegler, R. Relationship between circulating insulin-like growth factor components and sex hormones in a population-based sample of 50-to 80-year-old men and women. J. Clin. Endocrinol. Metab. 1996, 81, 2534–2540. [Google Scholar]
- Kallman, D.A.; Plato, C.C.; Tobin, J.D. The Role of Muscle Loss in the Age-Related Decline of Grip Strength: Cross-Sectional and Longitudinal Perspectives. J. Gerontol. 1990, 45, M82–M88. [Google Scholar] [CrossRef]
- Coelho, H.J., Jr.; Aguiar, S.; Gonçalves, I.D.O.; Sampaio, R.A.C.; Uchida, M.C.; Moraes, M.R.; Asano, R.Y. Sarcopenia Is Associated with High Pulse Pressure in Older Women. J. Aging Res. 2015, 2015, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuttle, C.S.; Thang, L.A.; Maier, A.B. Markers of inflammation and their association with muscle strength and mass: A sys-tematic review and meta-analysis. Ageing Res. Rev. 2020, 64, 101185. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Khera, R.; Corrales-Medina, V.F.; Townsend, R.R.; Chirinos, J.A. “Inflammation and arterial stiffness in humans”. Atherosclerosis 2014, 237, 381–390. [Google Scholar] [CrossRef]
- Zhang, C. The role of inflammatory cytokines in endothelial dysfunction. Basic Res. Cardiol. 2008, 103, 398–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Said, M.A.; Eppinga, R.N.; Lipsic, E.; Verweij, N.; van der Harst, P. Relationship of Arterial Stiffness Index and Pulse Pressure with Cardiovascular Disease and Mortality. J. Am. Heart Assoc. 2018, 7, e007621. [Google Scholar] [CrossRef] [Green Version]
- Lewis, S.J. Cardiovascular disease in postmenopausal women: Myths and reality. Am. J. Cardiol. 2002, 89, 5–10. [Google Scholar] [CrossRef]
- Martin, H.J.; Syddall, H.E.; Dennison, E.M.; Cooper, C.; Sayer, A.A. Relationship between customary physical activity, muscle strength and physical performance in older men and women: Findings from the Hertfordshire Cohort Study. Age Ageing 2008, 37, 589–593. [Google Scholar] [CrossRef] [Green Version]

| Group | Late Postmenopausal Women | Young Premenopausal Women |
|---|---|---|
| Sample Size (n) | 20 | 20 |
| Age (years) | 66 (63–73) | 23 (22–25) |
| Handedness | 20 right | 20 right |
| Skeletal Muscle Mass (kg) | 19.9 ± 1.6 | 23.6 ± 2.1 |
| Body Fat Mass (kg) | 20.6 ± 5.1 | 15.9 ± 4.0 |
| Body Mass Index (kg/m2) | 23.9 ± 2.7 | 22.0 ± 2.3 |
| Time Since Menopause (years) | 14.4 ± 6.2 | - |
| Central Pulse Pressure (mmHg) | 35.6 ± 5.9 | - |
| Augmentation index (%) | 32.0 ± 7.5 | - |
| Bilateral Index | Central Pulse Pressure | |
|---|---|---|
| Nondominant Hand MF | r = 0.388; p = 0.091 | r = −0.483; p = 0.031 * |
| Dominant Hand MF | r = 0.524; p = 0.018 * | r = −0.500; p = 0.025 * |
| Bilateral Hand MF | r = 0.705; p = 0.001 * | r = −0.510; p = 0.022 * |
| Bilateral Index | - | r = −0.280; p = 0.232 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-S.; Hwang, M.-H.; Kang, N. Bilateral Deficits during Maximal Grip Force Production in Late Postmenopausal Women. Appl. Sci. 2021, 11, 8426. https://doi.org/10.3390/app11188426
Kim J-S, Hwang M-H, Kang N. Bilateral Deficits during Maximal Grip Force Production in Late Postmenopausal Women. Applied Sciences. 2021; 11(18):8426. https://doi.org/10.3390/app11188426
Chicago/Turabian StyleKim, Jin-Su, Moon-Hyon Hwang, and Nyeonju Kang. 2021. "Bilateral Deficits during Maximal Grip Force Production in Late Postmenopausal Women" Applied Sciences 11, no. 18: 8426. https://doi.org/10.3390/app11188426






