Positive Effects of Highly Graphitized Porous Carbon Loaded with PbO on Cycle Performance of Negative Plates of Lead-Acid Batteries
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Materials
2.3. Electrochemical Test
2.4. Physical Characterization
2.5. Method of Content Analysis
3. Results and Discussion
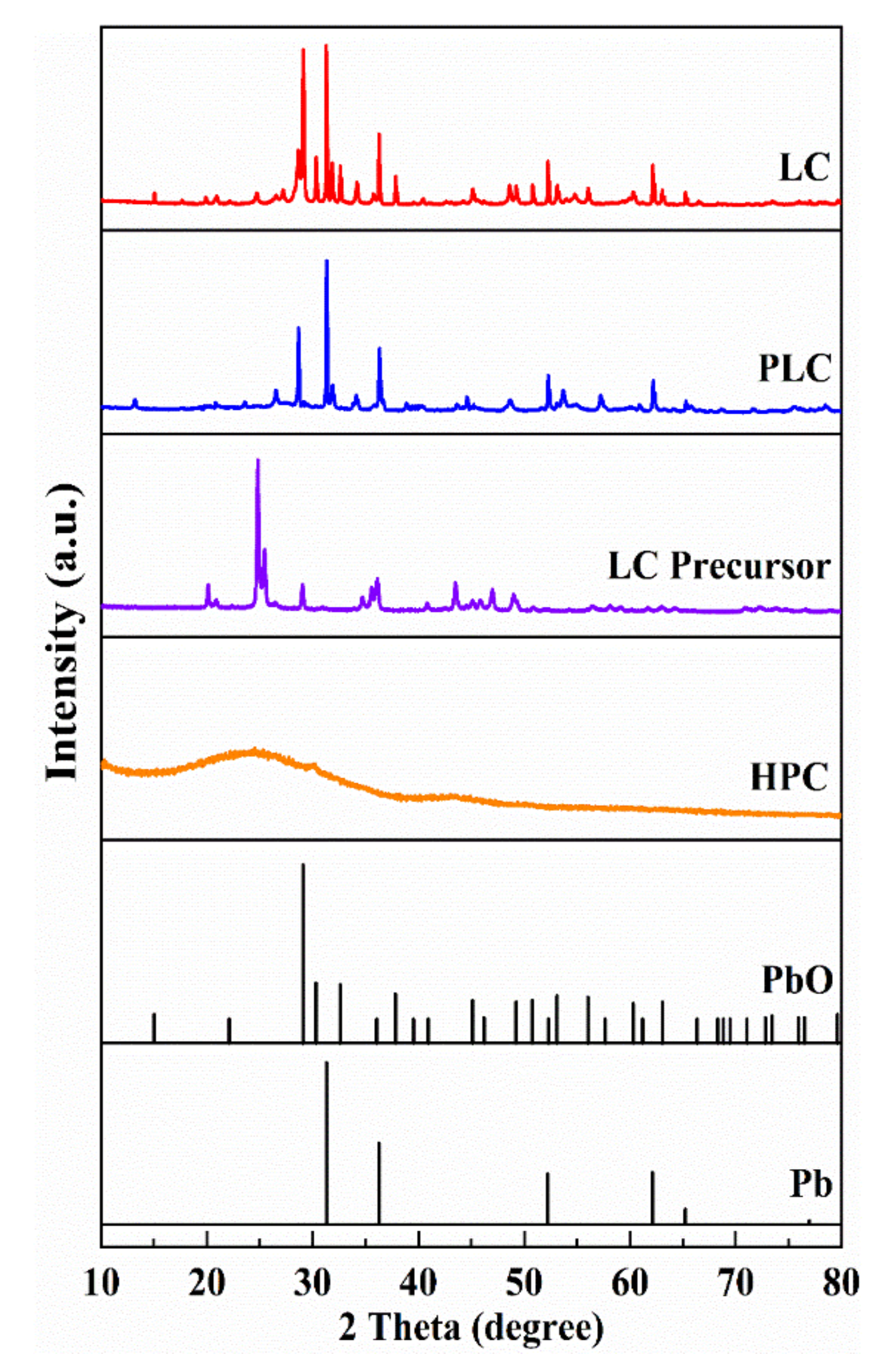
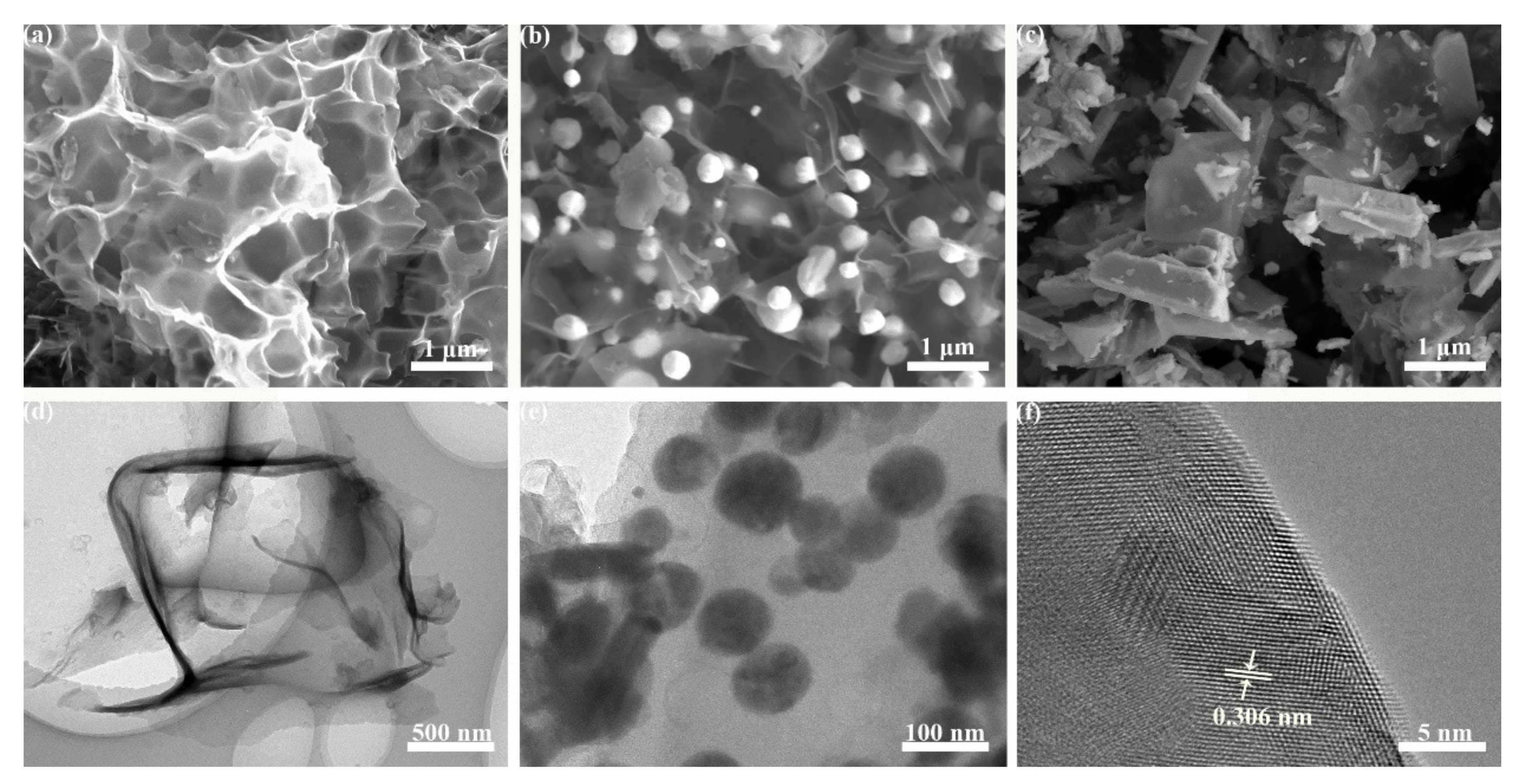
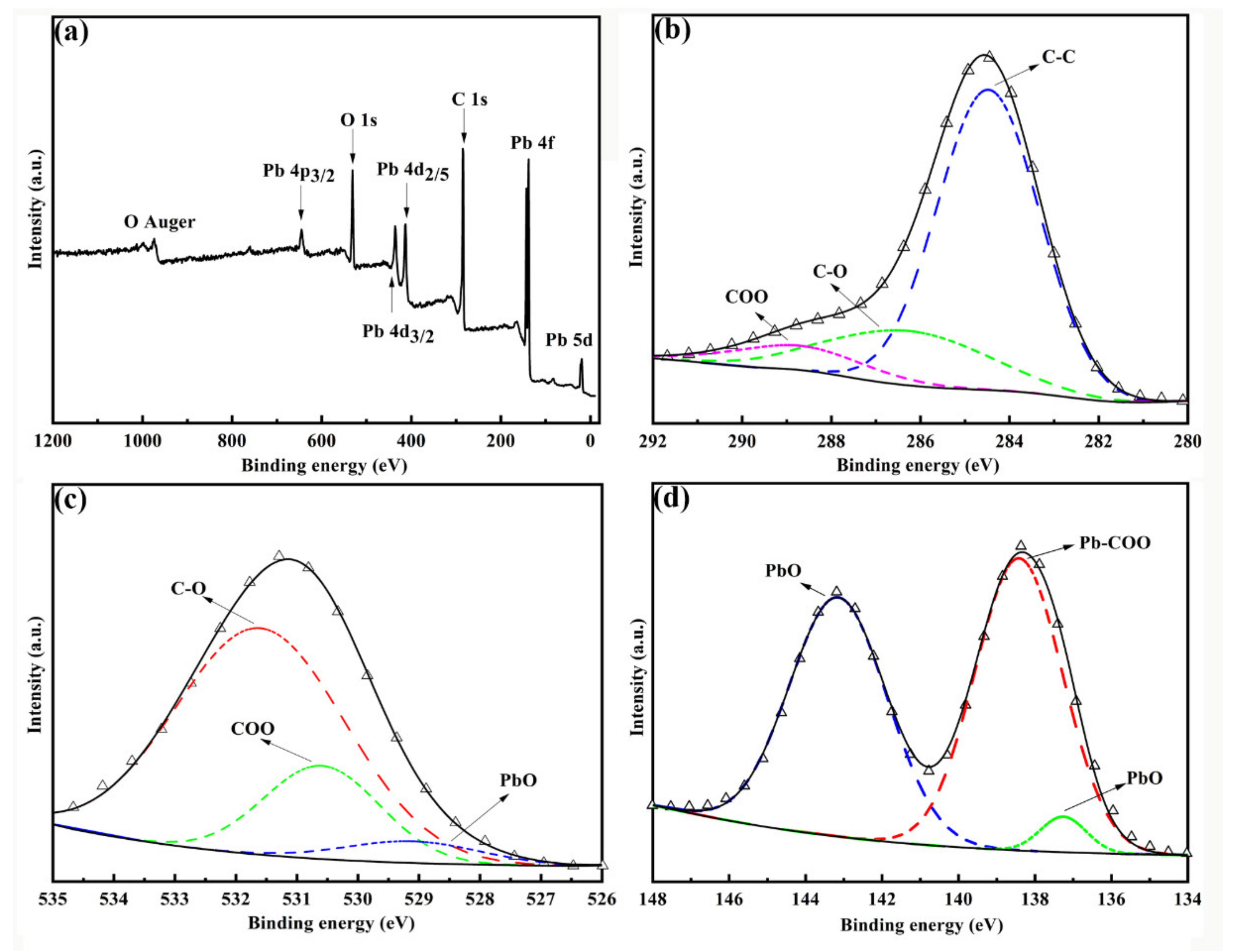
3.1. Characterization of Materials
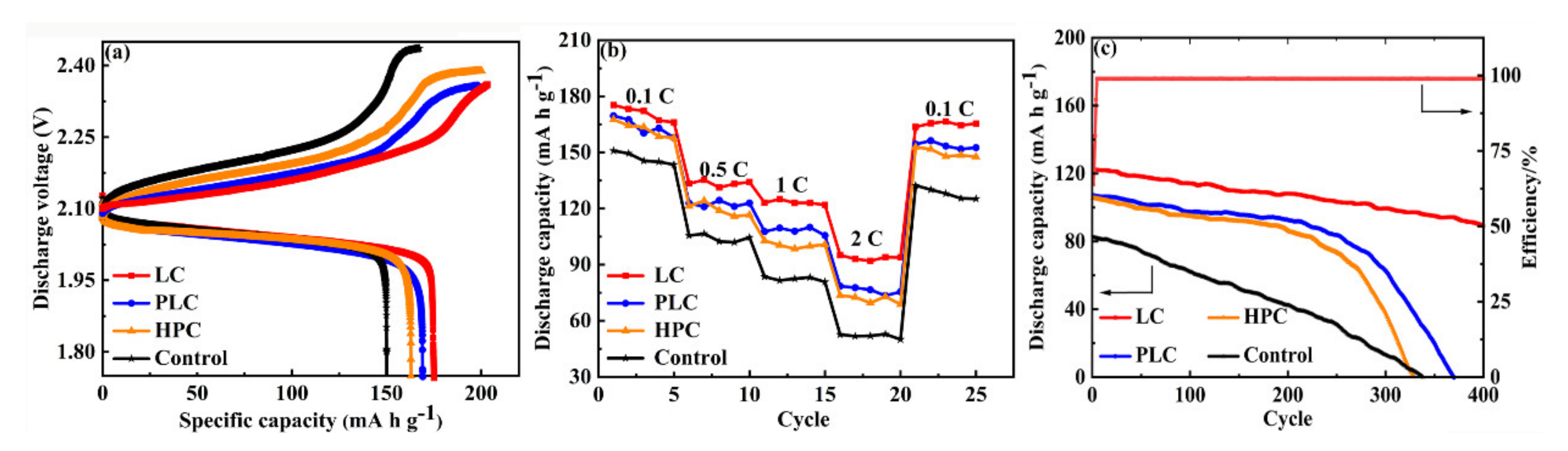
3.2. Electrochemical Performance of Negative Plates
3.3. Analysis of NAM
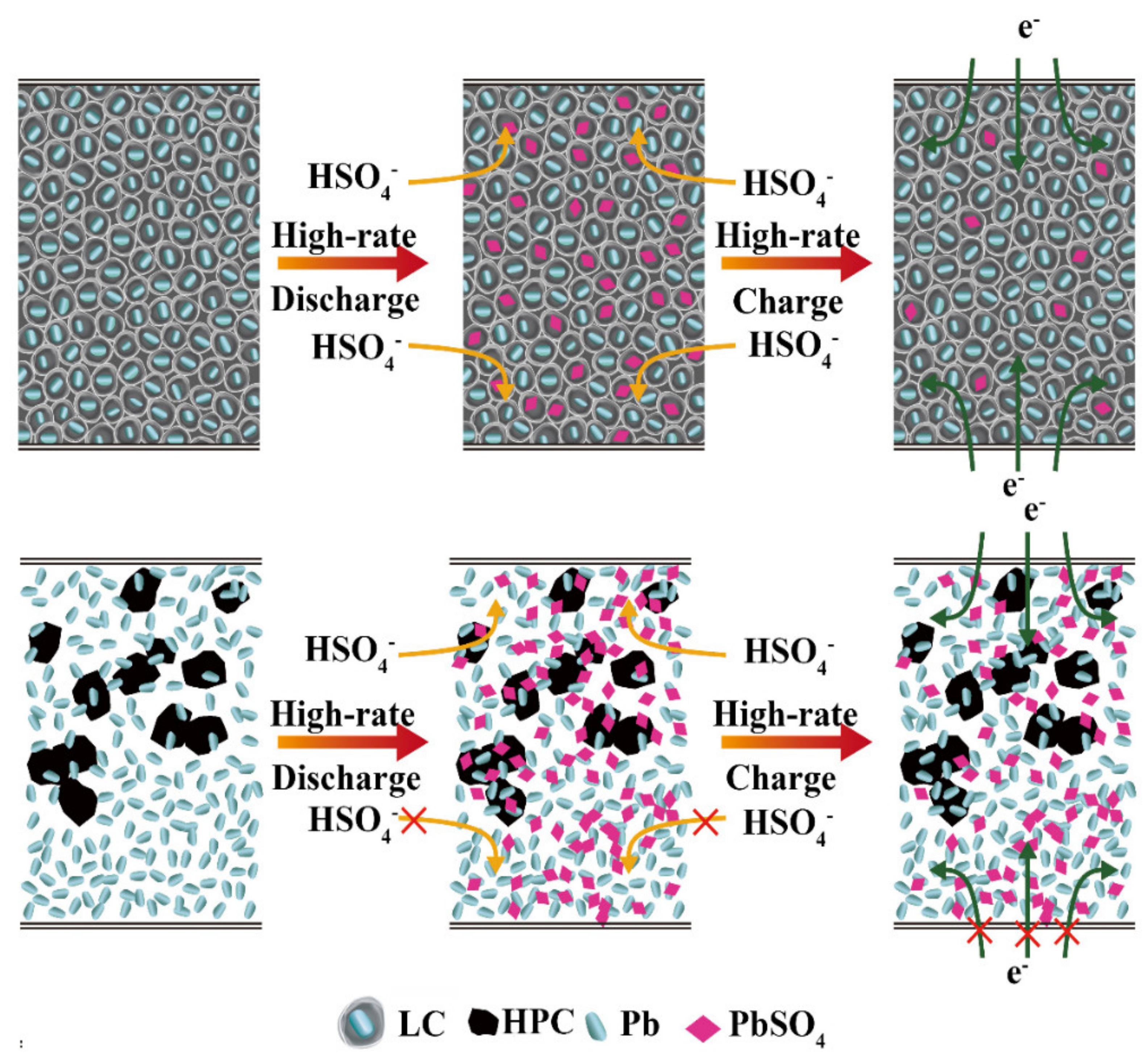
3.4. Possible Action Principle of the LC Material
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blanco, H.; Faaij, A. A review at the role of storage in energy systems with a focus on Power to Gas and long-term storage. Renew. Sustain. Energy Rev. 2018, 81, 1049–1086. [Google Scholar] [CrossRef]
- Hamelink, M.; Opdenakker, R. How business model innovation affects firm performance in the energy storage market. Renew. Energy 2019, 131, 120–127. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, C. Cost-effective iron-based aqueous redox flow batteries for large-scale energy storage application: A review. J. Power Sources 2021, 493, 229445. [Google Scholar] [CrossRef]
- Garche, J. Advanced battery systems—The end of the lead–acid battery? Phys. Chem. Chem. Phys. 2001, 3, 356–367. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, J.; Hu, J.; Wang, J.; Liang, S.; Hou, H.; Wu, X.; Liu, B.; Yu, W.; He, X.; et al. Synthesis of Nanostructured PbO@C Composite Derived from Spent Lead-Acid Battery for Next-Generation Lead-Carbon Battery. Adv. Funct. Mater. 2018, 28, 1705294. [Google Scholar] [CrossRef]
- Yu, J.; Yang, J.; Jiang, Z.; Zhang, H.; Wang, Y. Emergy based sustainability evaluation of spent lead acid batteries recycling. J. Clean. Prod. 2020, 250, 119467. [Google Scholar] [CrossRef]
- Xing, P.; Wang, C.; Wang, L.; Ma, B.; Chen, Y. Hydrometallurgical recovery of lead from spent lead-acid battery paste via leaching and electrowinning in chloride solution. Hydrometallurgy 2019, 189, 105134. [Google Scholar] [CrossRef]
- Carroquino, J.; Escriche-Martinez, C.; Valino, L.; Dufo-Lopez, R. Comparison of Economic Performance of Lead-Acid and Li-Ion Batteries in Standalone Photovoltaic Energy Systems. Appl. Sci. 2021, 11, 3587. [Google Scholar] [CrossRef]
- Dinesh, A.; Olivera, S.; Venkatesh, K.; Santosh, M.S.; Priya, M.G.; Inamuddin; Asiri, A.M.; Muralidhara, H.B. Iron-based flow batteries to store renewable energies. Environ. Chem. Lett. 2018, 16, 683–694. [Google Scholar] [CrossRef]
- Kebede, A.A.; Coosemans, T.; Messagie, M.; Jemal, T.; Behabtu, H.A.; Van Mierlo, J.; Berecibar, M. Techno-economic analysis of lithium-ion and lead-acid batteries in stationary energy storage application. J. Energy Storage 2021, 40, 102748. [Google Scholar] [CrossRef]
- Lam, L.T.; Haigh, N.P.; Phyland, C.G.; Urban, A.J. Failure mode of valve-regulated lead-acid batteries under high-rate partial-state-of-charge operation. J. Power Sources 2004, 133, 126–134. [Google Scholar] [CrossRef]
- Xiang, J.; Ding, P.; Zhang, H.; Wu, X.; Chen, J.; Yang, Y. Beneficial effects of activated carbon additives on the performance of negative lead-acid battery electrode for high-rate partial-state-of-charge operation. J. Power Sources 2013, 241, 150–158. [Google Scholar] [CrossRef]
- Pavlov, D.; Nikolov, P.; Rogachev, T. Influence of carbons on the structure of the negative active material of lead-acid batteries and on battery performance. J. Power Sources 2011, 196, 5155–5167. [Google Scholar] [CrossRef]
- Zou, X.; Kang, Z.; Shu, D.; Liao, Y.; Gong, Y.; He, C.; Hao, J.; Zhong, Y. Effects of carbon additives on the performance of negative electrode of lead-carbon battery. Electrochim. Acta 2015, 151, 89–98. [Google Scholar] [CrossRef]
- Hu, H.-Y.; Xie, N.; Wang, C.; Wu, F.; Pan, M.; Li, H.-F.; Wu, P.; Wang, X.-D.; Zeng, Z.; Deng, S.; et al. Enhancing the Performance of Motive Power Lead-Acid Batteries by High Surface Area Carbon Black Additives. Appl. Sci. 2019, 9, 186. [Google Scholar] [CrossRef] [Green Version]
- Fernández, M.; Valenciano, J.; Trinidad, F.; Muñoz, N. The use of activated carbon and graphite for the development of lead-acid batteries for hybrid vehicle applications. J. Power Sources 2010, 195, 4458–4469. [Google Scholar] [CrossRef]
- Kumar, S.M.; Ambalavanan, S.; Mayavan, S. Effect of graphene and carbon nanotubes on the negative active materials of lead acid batteries operating under high-rate partial-state-of-charge operation. RSC Adv. 2014, 4, 36517–36521. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, W.; Lin, N.; Lin, H.; Shi, J. Long-Life Lead-Acid Battery for High-Rate Partial-State-of-Charge Operation Enabled by a Rice-Husk-Based Activated Carbon Negative Electrode Additive. ChemistrySelect 2020, 5, 2551–2558. [Google Scholar] [CrossRef]
- Sadhasivam, T.; Park, M.-J.; Shim, J.-Y.; Jin, J.-E.; Kim, S.-C.; Kurkuri, M.D.; Roh, S.-H.; Jung, H.-Y. High charge acceptance through interface reaction on carbon coated negative electrode for advanced lead-carbon battery system. Electrochim. Acta 2019, 295, 367–375. [Google Scholar] [CrossRef]
- Dhanabalan, K.; Sadhasivam, T.; Kim, S.C.; Eun, J.J.; Shim, J.; Jeon, D.; Roh, S.-H.; Jung, H.-Y. Novel core–shell structure of a lead-activated carbon (Pb@AC) for advanced lead–acid battery systems. J. Mater. Sci. Mater. Electron. 2017, 28, 10349–10356. [Google Scholar] [CrossRef]
- Swogger, S.W.; Everill, P.; Dubey, D.P.; Sugumaran, N. Discrete carbon nanotubes increase lead acid battery charge acceptance and performance. J. Power Sources 2014, 261, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Sugumaran, N.; Everill, P.; Swogger, S.W.; Dubey, D.P. Lead acid battery performance and cycle life increased through addition of discrete carbon nanotubes to both electrodes. J. Power Sources 2015, 279, 281–293. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Hu, G.; Yue, X.; Wang, D. Study of Graphene as a Negative Additive for Valve-Regulated Lead-Acid Batteries Working under High-Rate Partial-StateOf-Charge Conditions. Int. J. Electrochem. Sci. 2016, 11, 700–709. [Google Scholar]
- Yeung, K.K.; Zhang, X.; Kwok, S.C.T.; Ciucci, F.; Yuen, M.M.F. Enhanced cycle life of lead-acid battery using graphene as a sulfation suppression additive in negative active material. RSC Adv. 2015, 5, 71314–71321. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Su, Z.; Zhao, Y.; Zhao, X.; Wang, R. Graphene nanosheets as backbones to build a 3D conductive network for negative active materials of lead–acid batteries. J. Appl. Electrochem. 2017, 47, 619–630. [Google Scholar] [CrossRef]
- Pavlov, D.; Nikolov, P.; Rogachev, T. Influence of expander components on the processes at the negative plates of lead-acid cells on high-rate partial-state-of-charge cycling. Part II. Effect of carbon additives on the processes of charge and discharge of negative plates. J. Power Sources 2010, 195, 4444–4457. [Google Scholar] [CrossRef]
- Long, Q.; Ma, G.; Xu, Q.; Ma, C.; Nan, J.; Li, A.; Chen, H. Improving the cycle life of lead-acid batteries using three-dimensional reduced graphene oxide under the high-rate partial-state-of-charge condition. J. Power Sources 2017, 343, 188–196. [Google Scholar] [CrossRef]
- Saravanan, M.; Ganesan, M.; Ambalavanan, S. A Modified Lead-Acid Negative Electrode for High-Rate Partial-State-of-Charge Applications. J. Electrochem. Soc. 2012, 159, A452–A458. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.J.; Zhu, Y.W.; An, J.H.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Tong, P.; Zhao, R.; Zhang, R.; Yi, F.; Shi, G.; Li, A.; Chen, H. Characterization of lead (II)-containing activated carbon and its excellent performance of extending lead-acid battery cycle life for high-rate partial-state-of-charge operation. J. Power Sources 2015, 286, 91–102. [Google Scholar] [CrossRef]
- Yang, J.; Hu, C.; Wang, H.; Yang, K.; Liu, J.B.; Yan, H. Review on the research of failure modes and mechanism for lead-acid batteries. Int. J. Energy Res. 2017, 41, 336–352. [Google Scholar] [CrossRef]
- Li, J.; Hu, Y.; Zhang, Y.; Xie, J.; Shen, P.K. Construction of a novel three-dimensional porous lead-carbon network for improving the reversibility of deep discharge lead-carbon batteries. J. Electroanal. Chem. 2021, 883, 115065. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Shen, P.K. Simultaneous Formation of Ultrahigh Surface Area and Three-Dimensional Hierarchical Porous Graphene-Like Networks for Fast and Highly Stable Supercapacitors. Adv. Mater. 2013, 25, 2474–2480. [Google Scholar] [CrossRef]
- Pavlov, D.; Nikolov, P. Capacitive carbon and electrochemical lead electrode systems at the negative plates of lead-acid batteries and elementary processes on cycling. J. Power Sources 2013, 242, 380–399. [Google Scholar] [CrossRef]
- Moseley, P.T.; Rand, D.A.J.; Peters, K. Enhancing the performance of lead-acid batteries with carbon—In pursuit of an understanding. J. Power Sources 2015, 295, 268–274. [Google Scholar] [CrossRef]
- Spanos, C.; Turney, D.E.; Fthenakis, V. Life-cycle analysis of flow-assisted nickel zinc-, manganese dioxide-, and valve-regulated lead-acid batteries designed for demand-charge reduction. Renew. Sustain. Energy Rev. 2015, 43, 478–494. [Google Scholar] [CrossRef]
- Probstle, H.; Schmitt, C.; Fricke, J. Button cell supercapacitors with monolithic carbon aerogels. J. Power Sources 2002, 105, 189–194. [Google Scholar] [CrossRef]
- Vangapally, N.; Jindal, S.; Gaffoor, S.A.; Martha, S.K. Titanium dioxide-reduced graphene oxide hybrid as negative electrode additive for high performance lead-acid batteries. J. Energy Storage 2018, 20, 204–212. [Google Scholar] [CrossRef]
- Zhang, W.-L.; Yin, J.; Lin, Z.-Q.; Shi, J.; Wang, C.; Liu, D.-B.; Wang, Y.; Bao, J.-P.; Lin, H.-B. Lead-carbon electrode designed for renewable energy storage with superior performance in partial state of charge operation. J. Power Sources 2017, 342, 183–191. [Google Scholar] [CrossRef]
- Pavlov, D.; Rogachev, T.; Nikolov, P.; Petkova, G. Mechanism of action of electrochemically active carbons on the processes that take place at the negative plates of lead-acid batteries. J. Power Sources 2009, 191, 58–75. [Google Scholar] [CrossRef]
- Naresh, V.; Elias, L.; Martha, S.K. Poly(3,4-ethylenedioxythiophene) coated lead negative plates for hybrid energy storage systems. Electrochim. Acta 2019, 301, 183–191. [Google Scholar] [CrossRef]

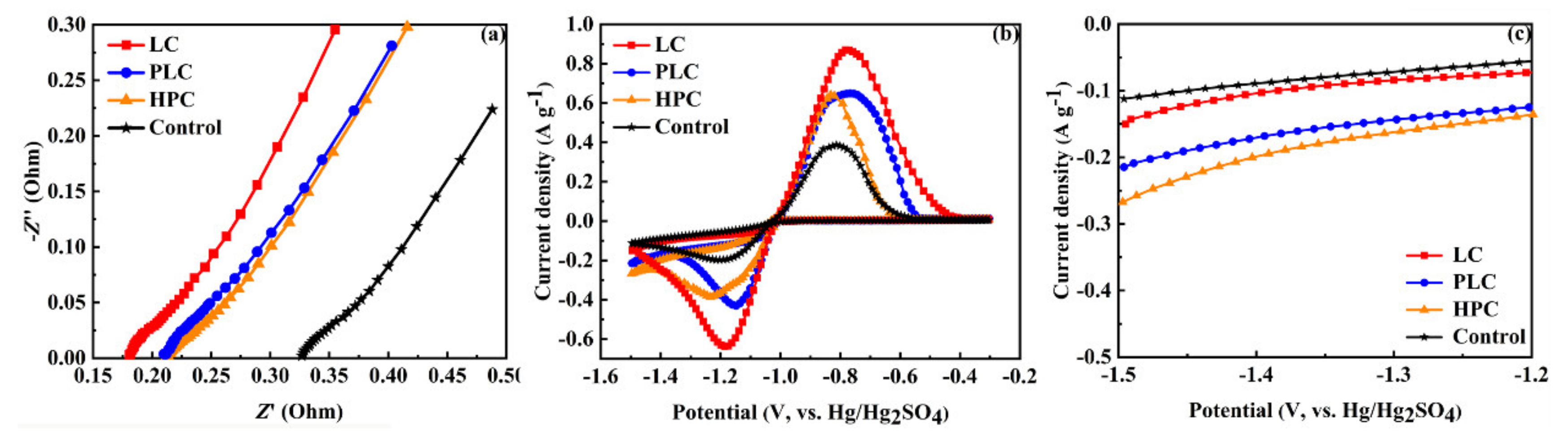
| Sample | Ipa (A g−1) | Ipc (A g−1) | Ipc/Ipa |
|---|---|---|---|
| LC | 0.87 | 0.64 | 0.73 |
| PLC | 0.65 | 0.43 | 0.66 |
| HPC | 0.64 | 0.39 | 0.60 |
| Control | 0.39 | 0.20 | 0.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, J.; Hu, Y.; Wu, X.; Ali, A.; Shen, P. Positive Effects of Highly Graphitized Porous Carbon Loaded with PbO on Cycle Performance of Negative Plates of Lead-Acid Batteries. Appl. Sci. 2021, 11, 8469. https://doi.org/10.3390/app11188469
Xie J, Hu Y, Wu X, Ali A, Shen P. Positive Effects of Highly Graphitized Porous Carbon Loaded with PbO on Cycle Performance of Negative Plates of Lead-Acid Batteries. Applied Sciences. 2021; 11(18):8469. https://doi.org/10.3390/app11188469
Chicago/Turabian StyleXie, Jiaming, Yitao Hu, Xiaoli Wu, Asad Ali, and Peikang Shen. 2021. "Positive Effects of Highly Graphitized Porous Carbon Loaded with PbO on Cycle Performance of Negative Plates of Lead-Acid Batteries" Applied Sciences 11, no. 18: 8469. https://doi.org/10.3390/app11188469
APA StyleXie, J., Hu, Y., Wu, X., Ali, A., & Shen, P. (2021). Positive Effects of Highly Graphitized Porous Carbon Loaded with PbO on Cycle Performance of Negative Plates of Lead-Acid Batteries. Applied Sciences, 11(18), 8469. https://doi.org/10.3390/app11188469





