Influence of Sulphate Attack on Properties of Modified Cement Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Solution Preparation
2.4. Testing Methods
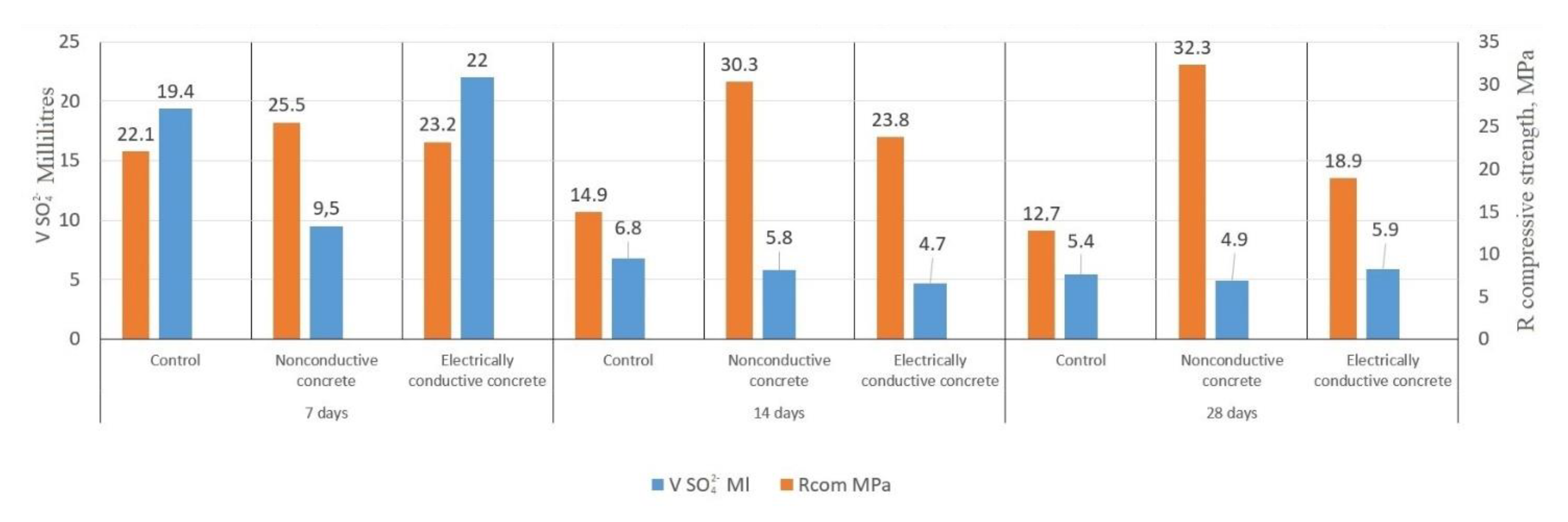
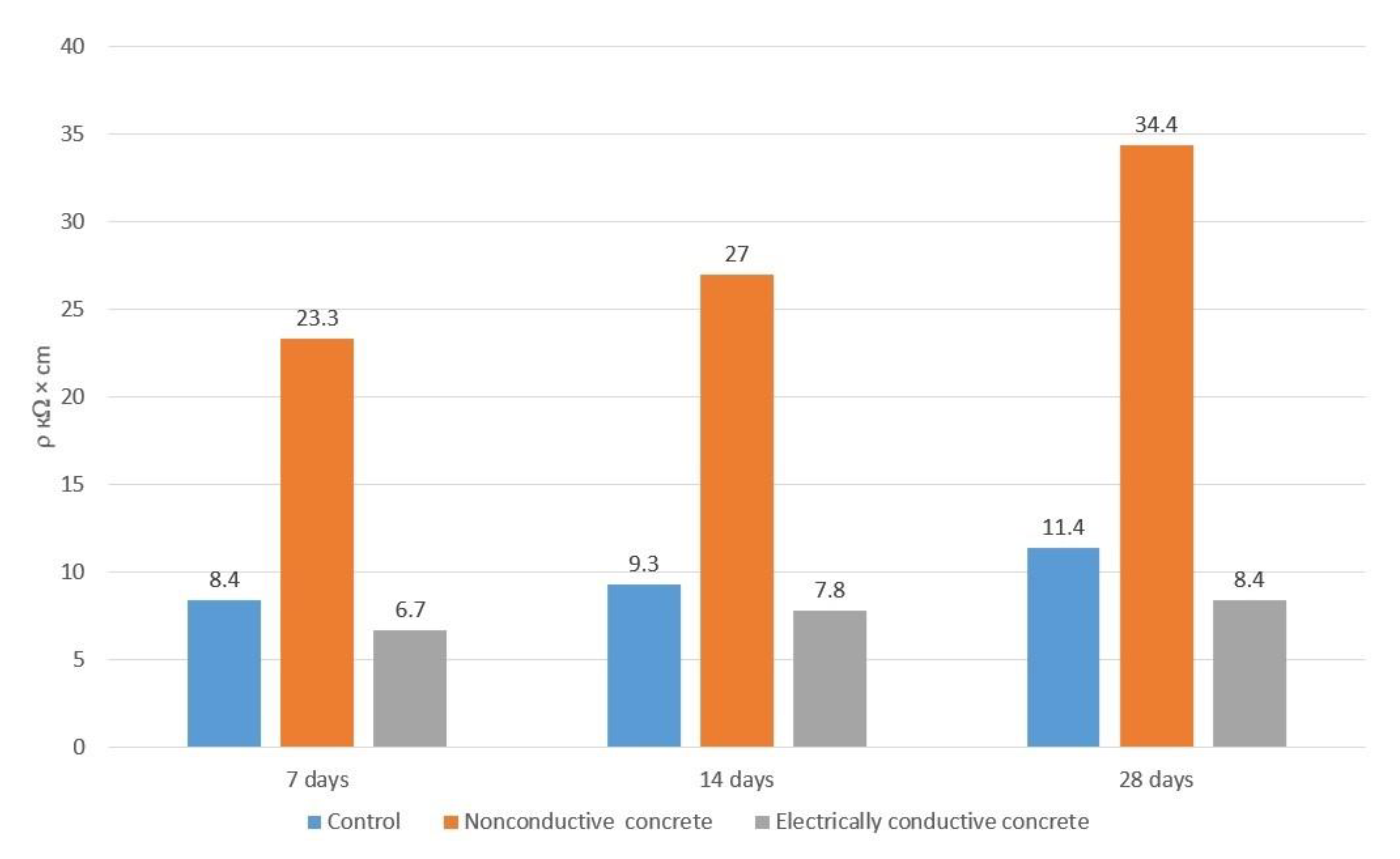
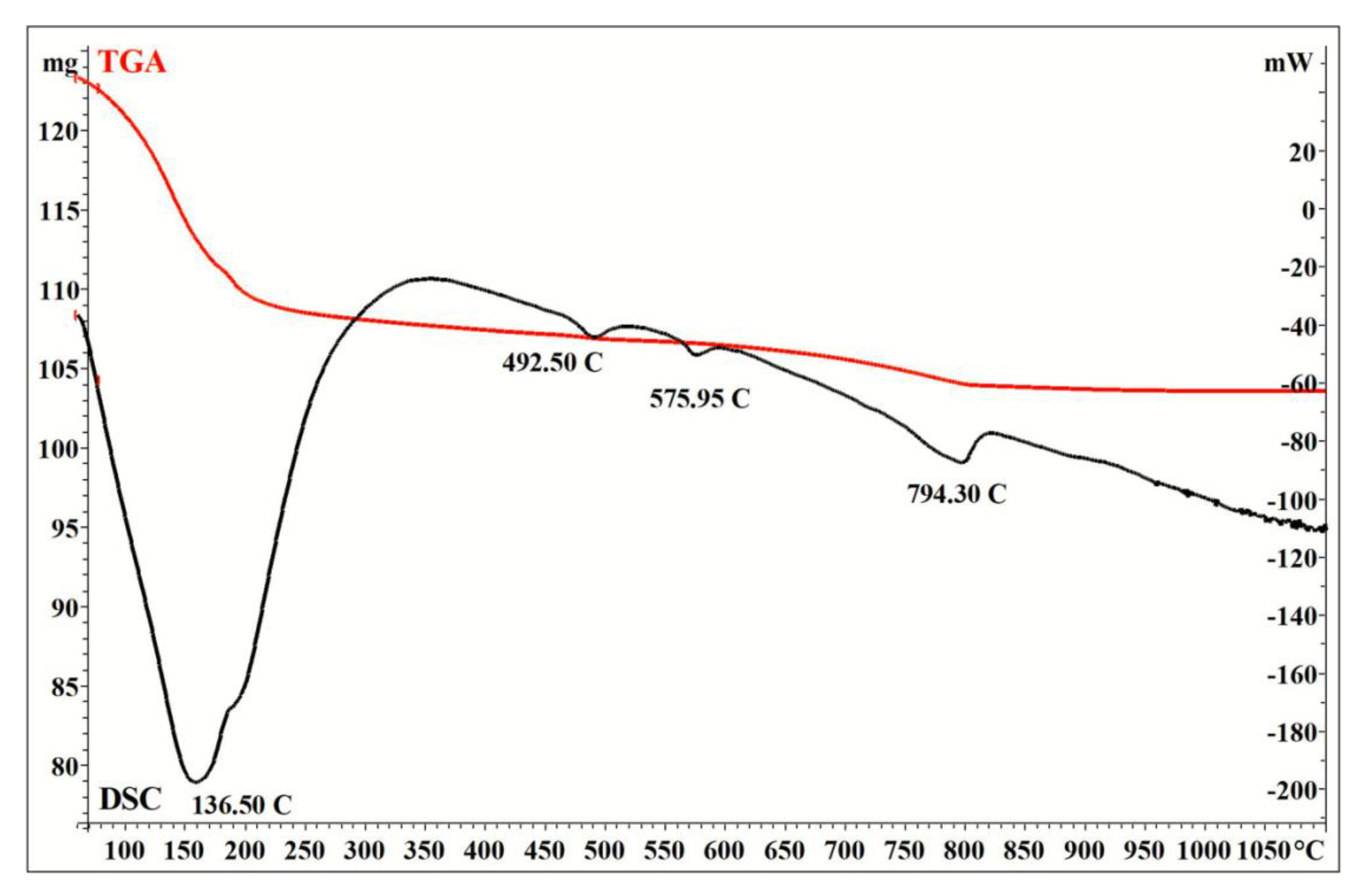
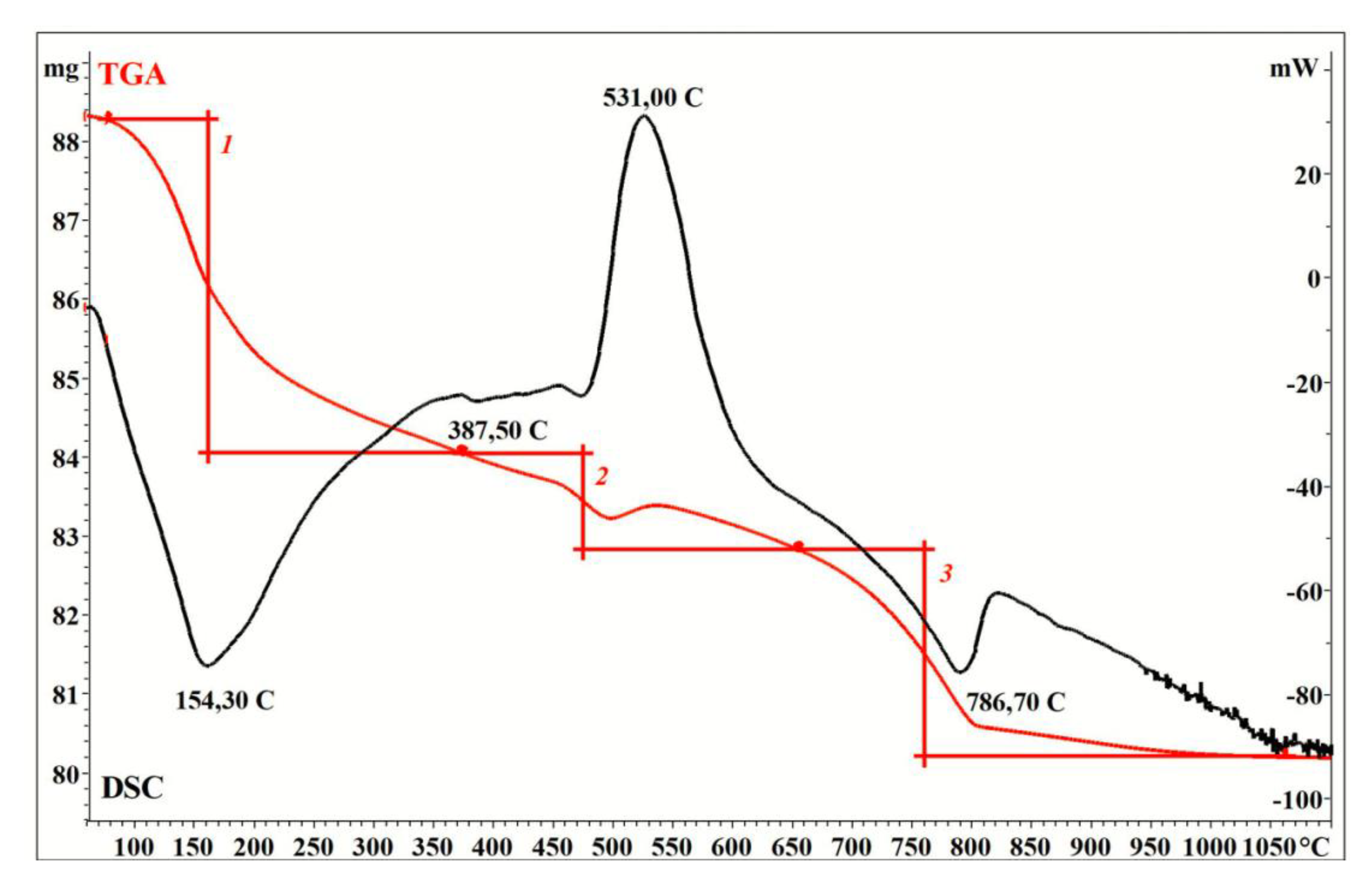
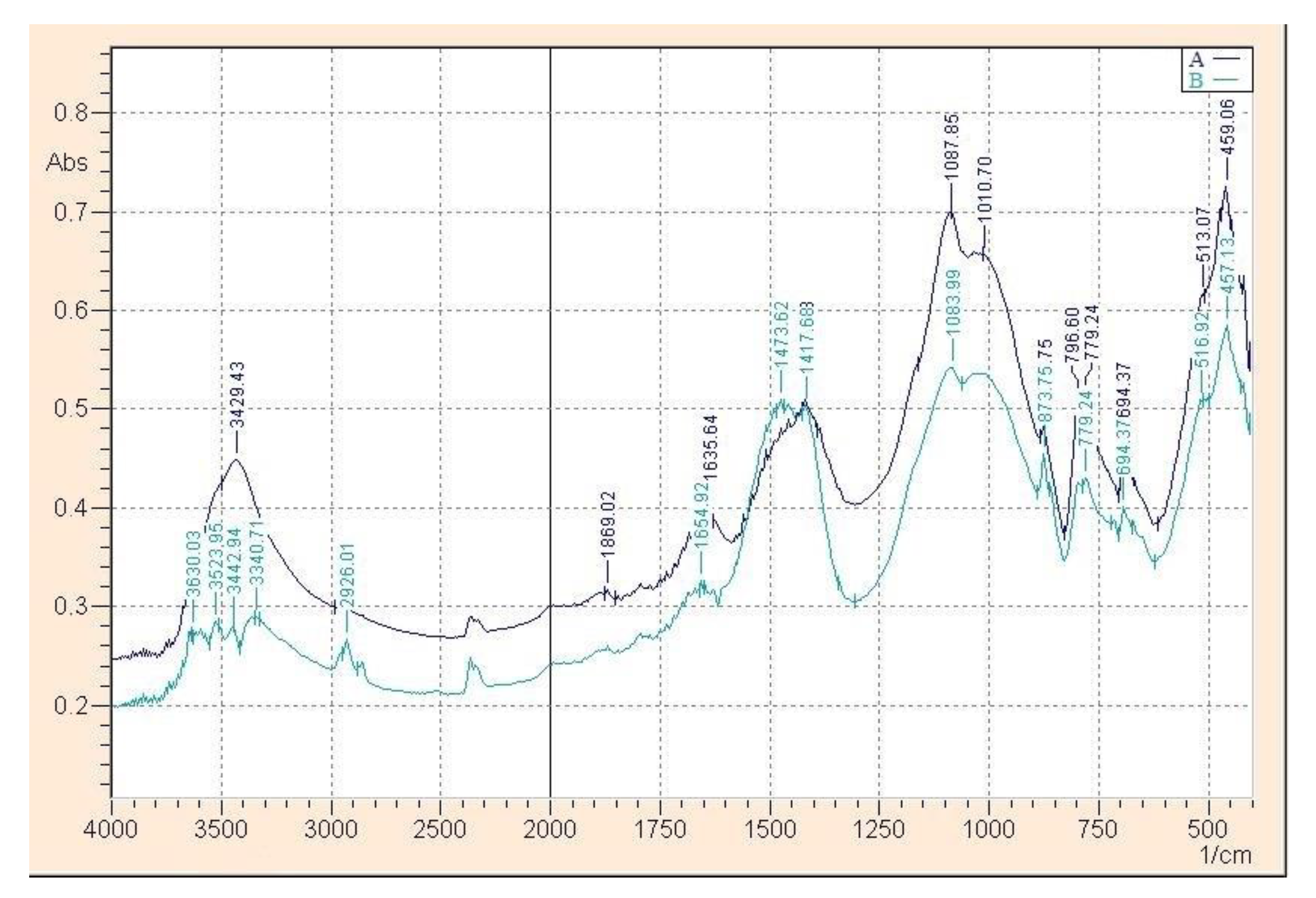
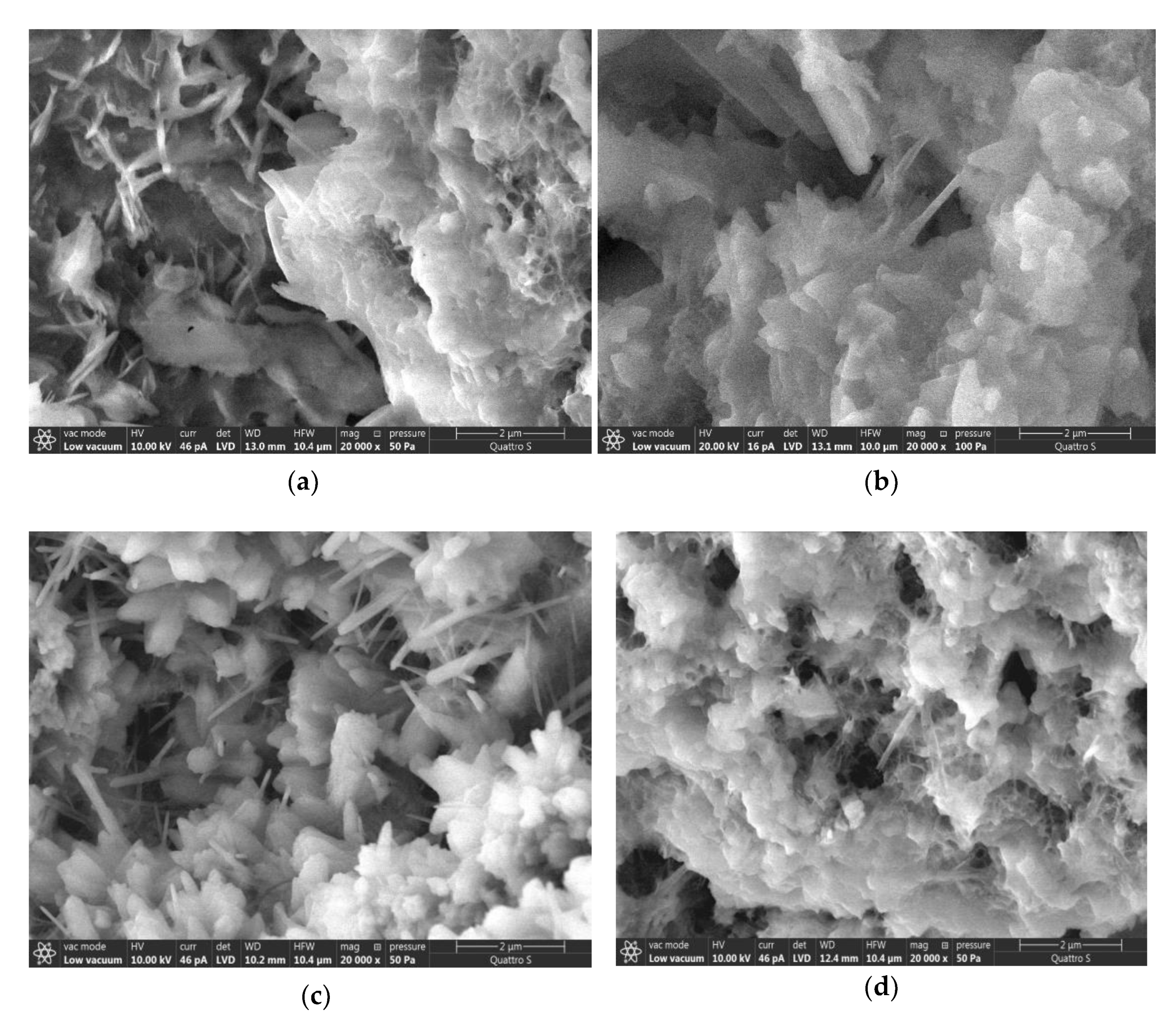
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Cleven, S.; Raupach, M.; Matschei, T. Electrical Resistivity of Steel Fibre-Reinforced Concrete—Influencing Parameters. Materials 2021, 14, 3408. [Google Scholar] [CrossRef]
- Zhou, R.; Li, Q.; Wang, J.; Zhou, K.; He, R.; Fu, C. Assessment of Electrical Resistivity and Oxygen Diffusion Coefficient of Cementitious Materials from Microstructure Features. Materials 2021, 14, 3141. [Google Scholar] [CrossRef]
- Dong, W.; Huang, Y.; Lehane, B.; Aslani, F.; Ma, G. Mechanical and electrical properties of concrete incorporating an iron-particle contained nano-graphite by-product. Constr. Build. Mater. 2021, 270, 121377. [Google Scholar] [CrossRef]
- Trushko, O.V.; Trushko, V.L.; Demenkov, P.A. Arrangement of multistory underground parking garages in complex engineering and geological environment. Int. J. Math. Eng. Manag. Sci. 2020, 5, 897–912. [Google Scholar]
- Goldobina, L.A.; Demenkov, P.A.; Trushko, O.V. Ensuring the safety of construction works during the erection of buildings and structures. J. Min. Inst. 2019, 239, 583–595. [Google Scholar] [CrossRef] [Green Version]
- Shagiakhmetov, A.M.; Raupov, I.R.; Terleev, A.V. Investigation of selective properties of the gel-forming composition for the limitation of water inflow to carbonate reservoirs conditions. Int. J. Civ. Eng. Technol. 2019, 10, 485–492. [Google Scholar]
- Liu, S.; Zhu, M.; Ding, X.; Ren, Z.; Zhao, S.; Zhao, M.; Dang, J. High-Durability Concrete with Supplementary Cementitious Admixtures Used in Corrosive Environments. Crystals 2021, 11, 196. [Google Scholar] [CrossRef]
- Zhao, G.; Shi, M.; Guo, M.; Fan, H. Degradation Mechanism of Concrete Subjected to External Sulfate Attack: Comparison of Different Curing Conditions. Materials 2020, 13, 3179. [Google Scholar] [CrossRef]
- Du, J.; Li, G.; Wu, J. Concrete sulfate corrosion coupled with hydraulic pressure. Mar. Georesour. Geotechnol. 2020, 38, 40–47. [Google Scholar] [CrossRef]
- Zhao, G.; Li, J.; Shi, M.; Fan, H.; Cui, J.; Xie, F. Degradation mechanisms of cast-in-situ concrete subjected to internal-external combined sulfate attack. Constr. Build. Mater. 2020, 248, 118683. [Google Scholar] [CrossRef]
- Li, T.; Zhang, B. Stochastic Dynamic Model of Sulfate Corrosion Reactions in Concrete Materials considering the Effects of Colored Gaussian Noises. Complexity 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Ikumi, T.; Cavalaro, S.H.P.; Segura, I.; Aguado, A. Alternative methodology to consider damage and expansions in external sulfate attack modeling. Cem. Concr. Res. 2014, 63, 105–116. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Martin, R.P.; Metalssi, O.O.; Fen-Chong, T.; Dangla, P. Pore size analyses of cement paste exposed to external sulfate attack and delayed ettringite formation. Cem. Concr. Res. 2019, 123, 105766. [Google Scholar] [CrossRef]
- Ochkurov, V.I.; Vilenskii, M.Y. Comparative evaluation of the saving of binder with fine ground slag. IOP Conf. Ser. Mater. Sci. Eng. 2019, 666, 012026. [Google Scholar] [CrossRef]
- Kazanskaya, L.F.; Isakovsky, V.I.; Fadeeva, S.A. Technological properties of self-compacting concrete mixtures with ground quartz sand. Int. J. Innov. Technol. Explor. Eng. 2019, 8, 799–803. [Google Scholar]
- Barnett, S.J.; Adam, C.D.; Jackson, A.R.W. Solid solutions between ettringite, Ca6Al2(SO4)3(OH)12·26H2O, and thaumasite, Ca3SiSO4CO3(OH)6·12H2O. J. Mater. Sci. 2000, 35, 4109–4114. [Google Scholar] [CrossRef]
- Barnett, S.J.; Macphee, D.E.; Lachowski, E.E.; Crammond, N.J. XRD, EDX and IR analysis of solid solutions between thaumasite and ettringite. Cem. Concr. Res. 2002, 32, 719–730. [Google Scholar] [CrossRef]
- Urkhanova, L.A.; Buyantuev, S.L.; Urkhanova, A.A.; Lkhasaranov, S.A.; Ardashova, G.R.; Fediuk, R.S.; Svintsov, A.P.; Ivanov, I.A. Mechanical and electrical properties of concrete modified by carbon nanoparticles. Mag. Civ. Eng. 2019, 92, 163–172. [Google Scholar] [CrossRef]
- Sassani, A.; Ceylan, H.; Kim, S.; Arabzadeh, A.; Taylor, P.C.; Gopalakrishnan, K. Development of carbon fiber-modified electrically conductive concrete for implementation in Des Moines International Airport. Case Stud. Constr. Mater. 2018, 8, 277–291. [Google Scholar] [CrossRef]
- Baeza, F.J.; Galao, O.; Vegas, I.J.; Cano, M.; Garcés, P. Influence of recycled slag aggregates on the conductivity and strain sensing capacity of carbon fiber reinforced cement mortars. Constr. Build. Mater. 2018, 184, 311–319. [Google Scholar] [CrossRef]
- El-Dieb, A.S.; El-Ghareeb, M.A.; Abdel-Rahman, M.A.H.; El Sayed, A.S. Multifunctional electrically conductive concrete using different fillers. J. Build. Eng. 2018, 15, 61–69. [Google Scholar] [CrossRef]
- Dehghanpour, H.; Yilmaz, K.; Ipek, M. Evaluation of recycled nano carbon black and waste erosion wires in electrically conductive concretes. Constr. Build. Mater. 2019, 221, 109–121. [Google Scholar] [CrossRef]
- Cordon, H.C.F.; Tadini, F.B.; Akiyama, G.A.; de Andrade, V.O.; da Silva, R.C. Development of electrically conductive concrete. Cerâmica 2019, 66, 88–92. [Google Scholar] [CrossRef]
- Bernatskii, A.F.; Tselebrovskii, I.U.N.; Chunchin, V.A. Electrical Properties of Concrete; Energiya: Moscow, Russia, 1980. [Google Scholar]
- Wang, H.; Yang, J.; Liao, H.; Chen, X. Electrical and mechanical properties of asphalt concrete containing conductive fibers and fillers. Constr. Build. Mater. 2016, 12, 2184–2190. [Google Scholar] [CrossRef] [Green Version]
- Santhanam, M.; Cohen, M.D.; Olek, J. Modeling the effects of solution temperature and concentration during sulfate attack on cement mortars. Cem. Concr. Res. 2002, 32, 585–592. [Google Scholar] [CrossRef]
- Diab, A.M.; Abd Elmoaty, A.E.M.; Aly, A.A. Long term study of mechanical properties, durability and environmental impact of limestone cement concrete. Alex. Eng. J. 2016, 55, 1465–1482. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Xie, F.; Zhao, G.; Li, L. Experimental and numerical investigation of cast-in-situ concrete under external sulfate attack and drying-wetting cycles. Constr. Build. Mater. 2020, 249, 118789. [Google Scholar] [CrossRef]
- Gopalakrishnan, R.; Chinnaraju, K. Durability of ambient cured alumina silicate concrete based on slag/fly ash blends against sulfate environment. Constr. Build. Mater. 2019, 204, 70–83. [Google Scholar] [CrossRef]
- Xu, P.; Jiang, L.; Guo, M.Z.; Zha, J.; Chen, L.; Chen, C.; Xu, N. Influence of sulfate salt type on passive film of steel in simulated concrete pore solution. Constr. Build. Mater. 2019, 223, 352–359. [Google Scholar] [CrossRef]
- Averbakh, E.E.; Luginina, I.G.; Smogorzhevsky, V.I. Study of electrical conductivity of clinker minerals and cements. Cement 1963, 6, 6–7. [Google Scholar]
- Ghazizadeh, S.; Hanein, T.; Provis, J.L.; Matschei, T. Estimation of standard molar entropy of cement hydrates and clinker minerals. Cem. Concr. Res. 2020, 136, 106188. [Google Scholar] [CrossRef]
- Dehghanpour, H.; Yilmaz, K. Heat behavior of electrically conductive concretes with and without rebar reinforcement. Mater. Sci. 2020, 26, 4. [Google Scholar] [CrossRef]
- Mobili, A.; Giosuè, C.; Bellezze, T.; Revel, G.M.; Tittarelli, F. Gasification char and used foundry sand as alternative fillers to graphene nanoplatelets for electrically conductive mortars with and without virgin. Recycl. Carbon Fibres Appl. Sci. 2021, 11, 50. [Google Scholar] [CrossRef]
- Hong, S.; Yuan, T.; Choi, J.; Yoon, Y. Effects of steelmaking slag and moisture on electrical properties of concrete. Materials 2020, 13, 2675. [Google Scholar] [CrossRef] [PubMed]
- Justnes, H. Properties of gypsum-free Portland cement. Cem. Based Mater. 2014, 3, 128–139. [Google Scholar] [CrossRef]
- Justnes, H. Calcium nitrate as a multi-functional concrete admixture. Concrete 2010, 44, 34–36. [Google Scholar]
- Karagöl, F.; Demirboǧa, R.; Kaygusuz, M.A.; Yadollahi, M.M.; Polat, R. The influence of calcium nitrate as antifreeze admixture on the compressive strength of concrete exposed to low temperatures. Cold Reg. Sci. Technol. 2013, 89, 30–35. [Google Scholar] [CrossRef]
- Milla, J.; Hassan, M.M.; Rupnow, T.; Al-Ansari, M.; Arce, G. Effect of self-healing calcium nitrate microcapsules on concrete properties. Transp. Res. Record. 2016, 2577, 69–77. [Google Scholar] [CrossRef]
- Kovalchuk, V.S.; Nikolaev, N.I. Carbon additives for high-quality well cementing. IOP Conf. Ser. Earth Environ. Sci. 2021, 677, 052035. [Google Scholar] [CrossRef]
- Isildak, Ö.; Özbek, O. Application of potentiometric sensors in real samples. Crit. Rev. Anal. Chem. 2020, 1–14. [Google Scholar] [CrossRef]
- Gumeniuk, A.N.; Polyanskikh, I.S.; Pervushin, G.N.; Gordina, A.F.; Yakovlev, G.I.; Khazeev, D.R. Structuring additive based on production waste for mineral binders. Constr. Mater. 2019, 7, 41–46. [Google Scholar] [CrossRef]
- Yakovlev, G.I.; Černý, V.; Polyanskikh, I.; Gordina, A.F.; Pudov, I.A.; Gumenyuk, A.; Smirnova, O. The effect of complex modification on the impedance of cement matrices. Materials 2021, 14, 557. [Google Scholar] [CrossRef]
- Qasim, O.A. A Review Paper on Specimens Size and Shape Effects on the Concrete Properties. Int. J. Recent Adv. Sci. Technol. 2018, 5, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; Ye, J.; Fang, Z.; Zhao, M. Size effect on cubic and prismatic compressive strength of cement paste. J. Cent. South Univ. 2015, 22, 4090–4096. [Google Scholar] [CrossRef]
- Tian, Y.; Yan, X.; Zhang, M.; Lu, D.; Yang, T.; Wang, Z.; Li, W. Internal transport and corrosion behaviors of sulfate corrosion media carried by recycled aggregate in concrete. Constr. Build. Mater. 2020, 260, 120480. [Google Scholar] [CrossRef]
- Al-Dulaijan, S.U.; Maslehuddin, M.; Al-Zahrani, M.M.; Sharif, A.M.; Shameem, M.; Ibrahim, M. Sulfate resistance of plain and blended cements exposed to varying concentrations of sodium sulfate. Cem. Concr. Compos. 2003, 25, 429–437. [Google Scholar] [CrossRef]
- Hulanicki, A.; Głąb, S. TITRIMETRY|Potentiometric. Encycl. Anal. Sci. 2005, 114–121. [Google Scholar] [CrossRef]
- Pipilikaki, P.; Papageorgiou, D.; Dimitroula, M.; Chaniotakis, E.; Katsioti, M. Microstructure changes in mortars attacked by sulphates at 5 °C. Constr. Build. Mater. 2009, 23, 2259–2264. [Google Scholar] [CrossRef]
- Plugin, A.A.; Pluhin, O.A.; Kasyanov, V.V.; Dyomina, O.I.; Bondarenko, D.O. Portland cement-based penetrating electrically conductive composition for protection against electrocorrosion. Funct. Mater. 2021, 28, 121–130. [Google Scholar]
- Jo, B.W.; Sikandar, M.A.; Chakraborty, S.; Baloch, Z. Investigation of the acid and sulfate resistance performances of hydrogen-rich water based mortars. Constr. Build. Mater. 2017, 137, 1–11. [Google Scholar] [CrossRef]
| Sample | CEM I 42.5 g | Quartz Sand | Industrial Soot, % | Industrial Sulfur, % | Calcium Nitrate % | Water-to- Cement Ratio | Polymer- Cement Ratio |
|---|---|---|---|---|---|---|---|
| Control | 800 | 1600 | - | - | - | 0.5 | - |
| Non-conductive concrete | - | 7 | - | 0.05 | |||
| Electrically conductive concrete | 7 | - | 3 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakovlev, G.; Polyanskikh, I.; Gordina, A.; Pudov, I.; Černý, V.; Gumenyuk, A.; Smirnova, O. Influence of Sulphate Attack on Properties of Modified Cement Composites. Appl. Sci. 2021, 11, 8509. https://doi.org/10.3390/app11188509
Yakovlev G, Polyanskikh I, Gordina A, Pudov I, Černý V, Gumenyuk A, Smirnova O. Influence of Sulphate Attack on Properties of Modified Cement Composites. Applied Sciences. 2021; 11(18):8509. https://doi.org/10.3390/app11188509
Chicago/Turabian StyleYakovlev, Grigoriy, Irina Polyanskikh, Anastasiya Gordina, Igor Pudov, Vít Černý, Alexander Gumenyuk, and Olga Smirnova. 2021. "Influence of Sulphate Attack on Properties of Modified Cement Composites" Applied Sciences 11, no. 18: 8509. https://doi.org/10.3390/app11188509
APA StyleYakovlev, G., Polyanskikh, I., Gordina, A., Pudov, I., Černý, V., Gumenyuk, A., & Smirnova, O. (2021). Influence of Sulphate Attack on Properties of Modified Cement Composites. Applied Sciences, 11(18), 8509. https://doi.org/10.3390/app11188509







