Abstract
This study aimed at evaluating the effects of two coating application methods, spraying and dipping, on the quality of fresh-cut melons. An alginate-based coating containing both ascorbic and citric acid was applied at two concentrations (5% and 10%) with both methods on fresh-cut melon. The nutritional quality of the products was investigated during 11 days of storage at 10 °C. The suitability and adaptability of the applied coatings on the fruit were evaluated based on rheological and microstructural properties. Moisture, carotenoids, total polyphenols and ascorbic acid content were analyzed on melon samples during storage. Results showed that the coating solution applied by the dipping method and at the highest concentration (10%), allowed to better maintain some quality characteristics of fresh-cut melon, thanks also to the better coating homogeneity and higher thickness observed through microstructural analysis.
1. Introduction
Melon, particularly Cucumis melo var. reticulatus, is highly appreciated for its aroma and nutritional value, being a source of carotenoids, polyphenols, and ascorbic acid; however, the amount of these compounds is lost quickly during post-harvest storage [1], as well as after minimal processing operations. The cutting inevitably increases respiration and water losses, leading to wilting and softening of fruit tissues, thus limiting the shelf-life. The decrease in carotenoids may be triggered by their degradation since the wounding provokes oxygen exposure and consequently cellular disruption, which promotes the contact between these compounds and lipoxygenase [1,2,3]. Ascorbic acid (AA) and polyphenol losses could be attributed to cellular surface damages, which afterwards promote greater interaction between them and oxidative enzymes (i.e., peroxidase and polyphenol oxidase) [4,5]. Particularly, an increase in the amount of the polyphenols after cutting was observed by different authors in many fruit and vegetables, including lettuce [6], lemon [7], carrot [8], potato [9] and melon [10], as a consequence of a defense mechanism caused by the wounding and other stresses. Moreover, the initial increase in phenolic compounds is normally followed by their decrease because of their interaction with oxidative enzymes promoted by the oxygen presence [11]. Several technologies have been developed and can be applied to maintain the quality of fresh-cut fruit and vegetables during storage, such as modified atmosphere, cold plasma treatments, ultraviolet radiations, pressurized gases and the employment of edible coatings [12,13,14,15]. In particular, edible coatings have a high potential to carry active ingredients such as anti-browning agents, colorants, flavors, nutrients, spices, etc., able to extend product shelf-life, reducing the risk of pathogen growth and improving the general quality of products also from a nutritional point of view [16,17,18]. As known by literature [19], the coating of fresh-cut products can be obtained by using different techniques such as dipping, brushing, fluidized-bed coating, electrostatic coating, vacuum impregnation, spraying, electrospraying, panning, etc. All these techniques exhibit several advantages and disadvantages, (e.g., the vacuum impregnation technique gives rise to a more thick coating, in comparison to dipping, but harms the quality of fruit in terms of texture and color; spraying methods are suitable for multilayer coating applications because they create very thin coatings, but are not recommended for high viscous liquids; fluidized bed coating and panning produce uniform coatings, but are expensive and need a high amount of coating solutions) and the selection of an appropriate method depends on the characteristics of food product to be covered as well as its surface features, and on the managing cost [14,20,21]. However, the most widely and commonly used methods to cover fresh-cut fruit are dipping and spraying due to their low costs and user-friendliness [11,14,20,21,22,23]. Unfortunately, only a few studies have compared the performances of these two different coating methods applied to the same food product. Zhong et al. [22] evaluated the effects of chitosan-, sodium alginate- and soy protein-based coatings, applied by both dipping and spraying on texture, color and mass losses of a mozzarella cheese.
Moreover, despite some works concerned with the use of enriched coatings realized with ascorbic acid and other ingredients on some intact and fresh-cut fruits, there is limited research focused on the application of enriched coatings on fresh-cut melon [18,24,25,26,27,28]. Therefore, to the best of our knowledge, there are no studies concerning the influence of different coating application methods on the microstructural and nutritional properties of fresh-cut melon. As a result, this work aimed at evaluating the influence of two different application methods (spraying and dipping) of enriched coatings on the moisture content and nutritional properties of fresh-cut melon during storage. The coatings have been developed with ascorbic and citric acids at two different concentrations.
2. Materials and Methods
2.1. Raw Material and Coating
Fresh muskmelons (Cucumis melo var. reticolatus “Raptor”) were purchased from a local market. The soluble solid content and pH were respectively 9.7 ± 0.2 Brix and 6.5 ± 0.3, expressed in an average of 10 fruits. The basic coating formulation was composed of distilled water (50 g/100 g), sodium alginate (10 g/100 g), ascorbic acid (20 g/100 g), citric acid (10 g/100 g) and emulsifier Tween 80 (10 g/100 g). Before applying the coating solution on the fruit samples, it was diluted to 5 and 10% w/w with distilled water, to facilitate its application.
2.2. Fruit Processing and Coating Application
Whole melons were washed and scrubbed with a sponge to remove impurities and then were immersed in a 200 mg/L sodium hypochlorite solution for 2 min, to sanitize the peel and decrease the initial microbial load. Afterwards, the fruits were dried with a tissue paper and halved; skin and internal seeds were removed. Slices of about 2 cm were obtained by using a sharp knife and each slice was cored in 4 cylindrical pieces with a diameter of 2 cm (each weighing about 10 g). Specifically half of the samples were dipped and half were sprayed with the coating solution at 5% and 10% (w/w), according to the studies of Atieno et al. [29], with some modification concerning the treatment and the drying times. Both dipping and spraying were performed for 30 s at room temperature (23 ± 1 °C) and then the samples were left in a fridge for 30 min at 4 °C to allow the air drying of the samples. The different coated and uncoated (controls) samples were packed separately into rectangular polyethylene (PET) trays and covered with a transparent polypropylene PP film having a medium barrier to oxygen. The PET tray had a thickness of 3 mm, an oxygen transmission rate (OTR) of 60 cm3 m−2 day−1 and a water transmission rate (WTR) of 27 g3 m−2 day−1, while PP film presented a thickness of 30 μm, an oxygen and water transmission rate respectively of 860 cm3 m−2 day−1 and 19 g3 m−2 day−1 [30].
Samples were stored at 10 °C, to simulate thermal abuse conditions, for up to 11 d. Each tray contained twelve pieces, samples of three trays per treatment and per storage time were analyzed at 0 (T0), 2 (T2), 4 (T4), 7 (T7), 9 (T9) and 11 (T11) days. A total of 216 melon pieces were obtained. Moisture content was analyzed on fresh samples, while the other analytical determinations were performed on the freeze-dried materials (LI0-2000P, Cinquepascal s.r.l., Milan, Italy; 0.25 h Pa, −30 °C) at each sampling time and subsequently stored at −18 °C until analyses.
In Table 1 all the obtained melon samples are reported.

Table 1.
Melon samples and their description.
2.3. Rheological Analysis on Coating Solutions
Rheological measurements were carried out on coating solutions at both concentrations (5% and 10%), to characterize them in terms of viscosity. Measurements were performed at 10 °C by using a controlled stress–strain rheometer (MCR 300, Physica/Anton Paar, Ostfildern, Germany) equipped with a system of coaxial cylinders (CC27). The rheological behavior of the differently concentrated coating solutions was analyzed in steady-state conditions. After a pre-shearing of 500 s at 5 s−1, viscosity was measured increasing shear rate from 2 to 50 s−1 within 180 s, taking 18 point measurements (ICA 2000). Obtained flow curves were fitted according to the Ostwald model commonly referred to as the Power Law model (Equation (1)) [31].
where σ is the shear stress (Pa), K is consistency index (Pa·sn), is the shear rate (s−1) and n is the dimensionless flow behavior index.
2.4. Microscopic Examination of Coated Samples
Microscopic observations were carried out on coated fresh-cut melon samples to evaluate the thickness and homogeneity of the coating during storage. From each piece, a transversal cut using a stainless-steel blade was made to obtain slices of about 1 mm of thickness.
The coating thickness was evaluated by using an inverted light microscope (Eclipse Ti-U, Nikon Co, Tokyo, Japan) equipped with a Nikon digital video camera (digital sight DS-Qi1Mc, Nikon Co, Tokyo, Japan) at a magnification of 4×. Ten melon pieces were analyzed for each different sample (uncoated and differently coated) at 0, 4 and 11 days of storage and for each sample of ten, four different measurements of film thickness were performed [32].
2.5. Moisture Content
Moisture content was determined gravimetrically in triplicate by the difference in mass before (fresh mass) and after drying (dry mass) at 70 °C to constant mass [33].
2.6. Carotenoids Contents
For the determination of carotenoids contents, approx. 100 mg of freeze-dried sample were mixed with 1 mL of distilled water in a centrifuge tube; after 1 min of vortexing, 2.5 mL of 80% (v/v) acetone was added and the mixture was centrifugated for 5 min at 10 °C and 4500 rpm (Beckman Coulter, mod. Allegra 2IR, Indianapolis, IN, USA). The supernatant was measured by spectrophotometer (Shimadzu UV-1601, Kyoto, Japan) [34] at three different wavelengths (470, 633 and 645 nm). The total carotenoids contents were calculated by the following formulas:
where Ca is “Chlorophyll a” concentration (mg L−1); Cb is “Chlorophyll b” concentration (mg L−1); Cx + c is carotenoids concentration (mg L−1); A663 = Absorbance at 663 nm; A645 = Absorbance at 645 nm; A470 = Absorbance at 470 nm. The mass-based concentrations were calculated based on the dry mass of freeze-dried samples as µg/kg. The measurements were performed in three repetitions for each sample.
Ca = 12.75 × A663 − 2.81 × A645
Cb = 20.13 × A645 − 5.03 × A663
Cx + c = ((1000 × A470) − (3.27 × Ca) − (104 × Cb))
2.7. Total Polyphenols Content (TPC)
The freeze-dried melon samples (0.2 g) were dissolved in a methanol/water mixture 80:20 v/v (5 mL) and the total polyphenols content (TPC) was measured according to the Folin-Ciocalteau method described in Tylewicz et al. [35]. Gallic acid (Sigma-Aldrich, Milan, Italy) was used as a standard (0–800 mg/L). The obtained results were expressed as mg of gallic acid equivalents (GAE)/100 g of dry matter. The measurements were performed in three repetitions for each sample.
2.8. Ascorbic Acid Content
Ascorbic acid content was measured according to the method proposed by Laur and Tian [36] with some modifications. The freeze-dried melon samples (0.1 g) were dissolved in 1.9 mL of 6% (w/v) metaphosphoric acid (Sigma Aldrich, Milan, Italy). The mixture was centrifuged for 10 min at 1000 g and 4 °C and the supernatant was filtered under vacuum through a Whatman filter paper n° 4 and then diluted in metaphosphoric acid (1:5 w/v). These solutions were filtered again through a 0.45 µm nylon membrane (Thermo Scientific, Shanghai, China) and transferred in vials for HPLC measurement. Ascorbic acid content was measured by HPLC LC-1500 (Jasco, Carpi, MO, Italy) equipped with thermostatted autosampler and diode array detector (DAD).
The chromatographic conditions were chosen according to the method proposed by Odriozola-Serrano et al. [37]. The measurements were performed in triplicate for each sample.
2.9. Statistical Analysis
Using Statistica for Windows, version 10.0 (Statsoft Inc., Tulsa, UK), one-way analysis of variance (ANOVA) was carried out and Tukey’s test (HSD) was used to evaluate the significance (p < 0.05) between means.
3. Results
3.1. Rheological Analysis on Coatings
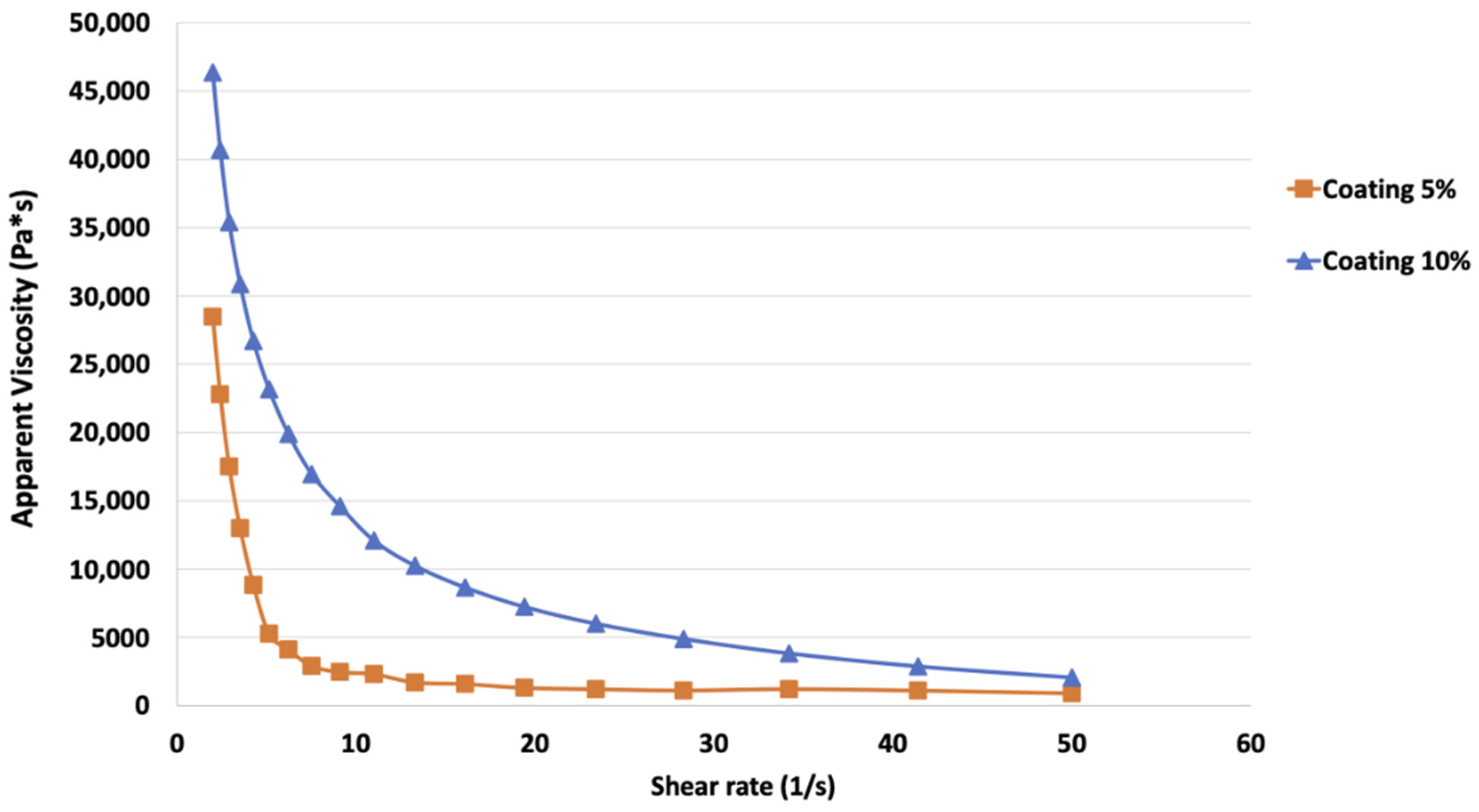
In Figure 1, the flow curves of the coating solutions at two different concentrations (5% and 10%) obtained, reveal increasing shear rates from 2 to 50 s−1, as reported. A higher initial value of apparent viscosity (around 48,000 Pa·sn) was observed in coating solution at 10% if compared to the sample at a lower concentration, which showed viscosity values around 30,000 Pa·sn.
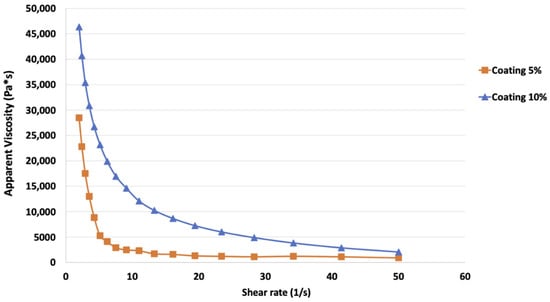
Figure 1.
Flow curves of the alginate coating solution at 5% and 10% concentrations (w/w distilled water), obtained increasing the shear rate from 2 to 50 s−1.
To better explain the rheological behavior of the coating samples, the Power Law model was used and the K and n parameters were calculated (Table 2). Viscosity data of each sample were well fitted by the aforementioned model showing high determination coefficients varying from 0.98 to 0.99. Both samples had a n values < 1, while K parameter was higher for the coating at 10% (48.19 Pa·sn) compared to 5% one (28.12 Pa·sn).

Table 2.
Flow behavior index (n) and consistency index (K) of the coatings at two concentrations, obtained with Power Law model applied on viscosity data.
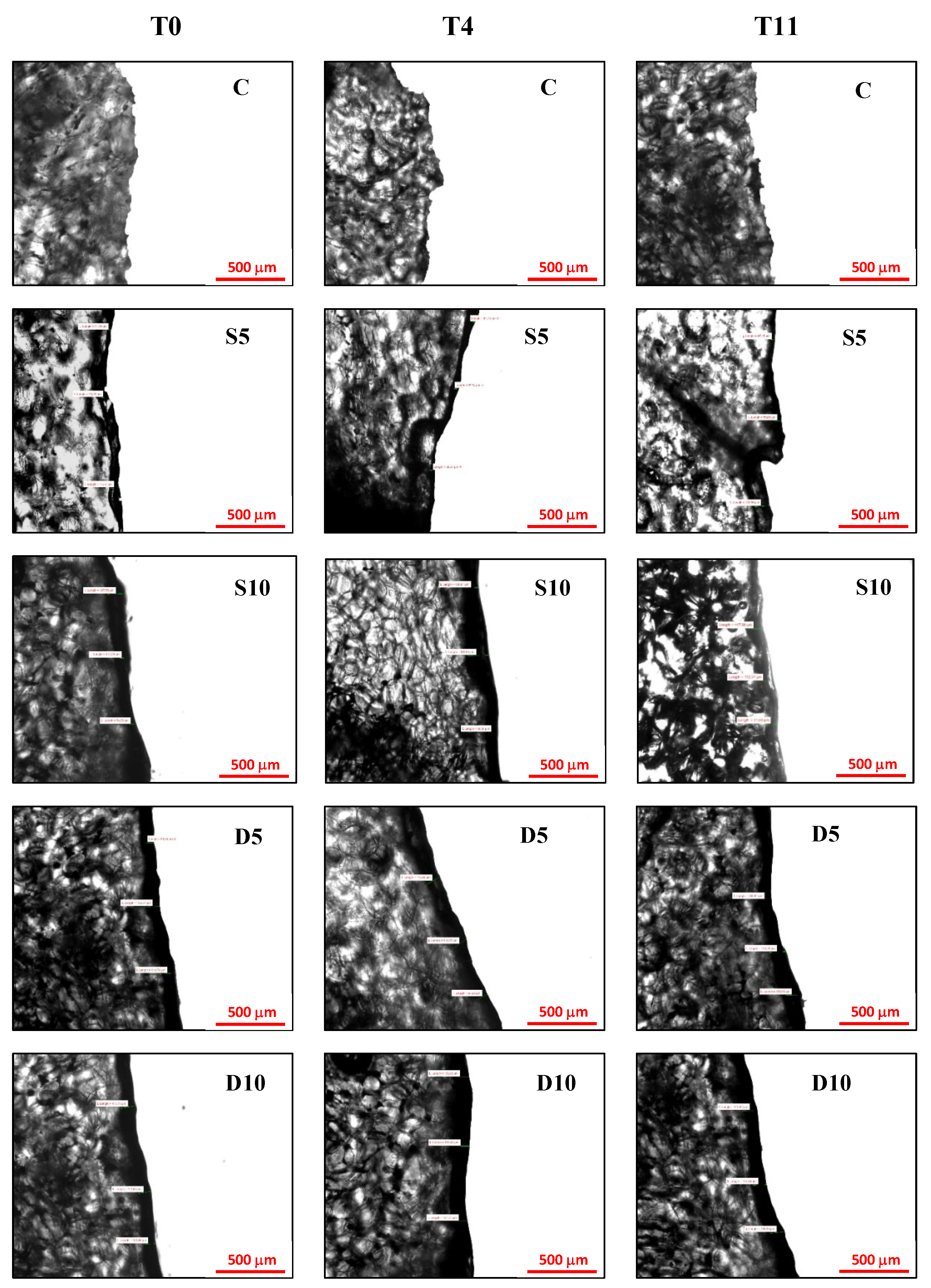
3.2. Microstructural Analysis of Coating
Figure 2 and Table 3 report, respectively, micrographs of cross-linking section and coating thickness of melon samples at 0, 4, 11 d of storage. A higher coating thickness during the entire storage was observed in melon samples with the coating applied by dipping (D10 and D5) as showed by both visual analysis of micrographs (Figure 2) and the measures of thickness presented in Table 3. Moreover, they showed also a more uniform and homogeneous surface, as highlighted by their lower standard deviation compared to sprayed samples (Table 3) [38,39]. Melon samples with a coating at 10% applied by spraying (S10) showed intermediate thickness values (94.89–96.40 µm) even if not statistically different from D10 and D5 samples at each considered storage time. The lowest coating thickness values, in the range of 64.42–65.36 μm, were observed in melon samples with a coating at 5% applied by spraying (S5). During storage, the coating thickness remained almost unchanged for all samples, showing good stability and adhesiveness of the coatings to the melon samples.
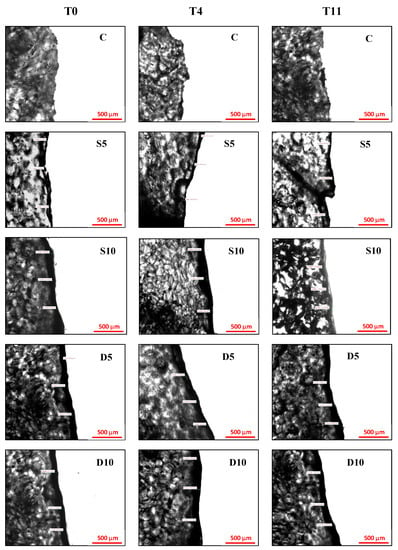
Figure 2.
Micrographs of sections of coated fresh-cut melon samples (C = control, S5 = Sprayed with 5% coating; S10 = Sprayed with 10% coating; D5 = Dipped with 5% coating; D10 = Dipped with 10% coating) at 0, 4 and 11 days of refrigerated storage.

Table 3.
Coating thickness (µm) obtained from the microscopic examination of the cross-linking section of fresh-cut melon samples during 0, 4, 11 days of storage.
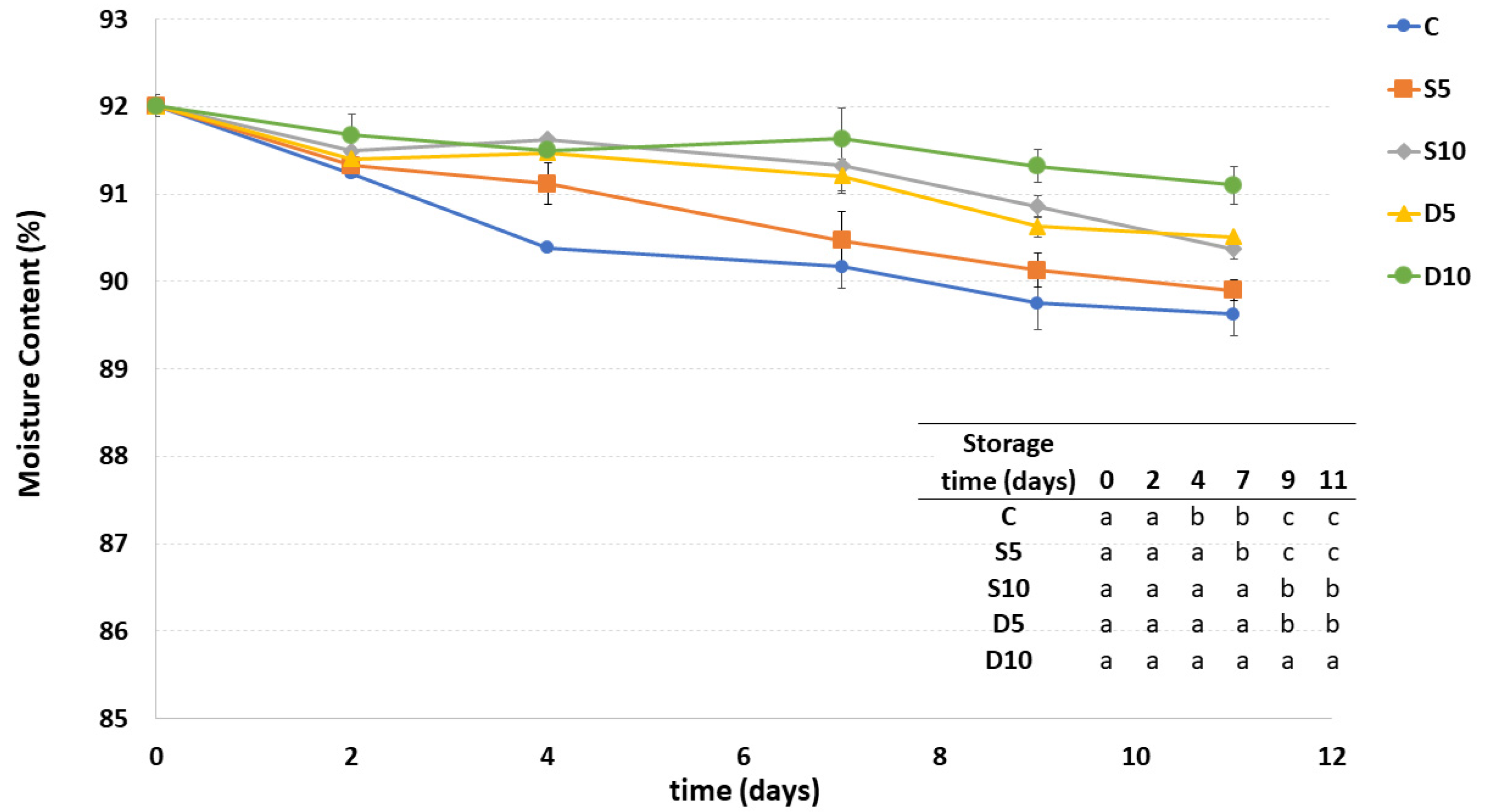
3.3. Moisture Content
Moisture content of all the samples showed a general decrease during storage (Figure 3). The lowest decrease of moisture was observed in the D10 sample. The D5 and S10 samples had a similar trend to D10 samples until the seventh day of storage, decreasing more markedly hereafter. A higher decrease in moisture content during storage was observed in the S5 sample, probably due to both the irregular and inhomogeneous distribution of coating on its surface and its lowest thickness. In general, the uncoated C sample presented the highest moisture decrease during storage, thus promoting the greatest dehydration like the S5 sample starting from the seventh day of storage.
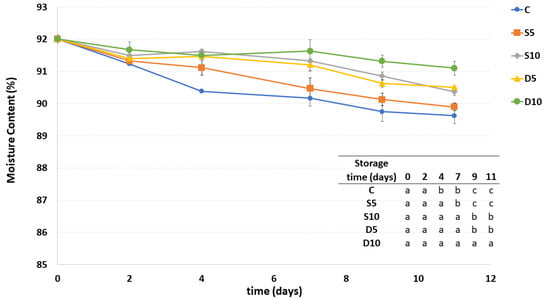
Figure 3.
Moisture content of fresh-cut melon samples (C, D5, D10, S5 and S10) during storage. a–c Different letters in the same column indicate significant differences (p < 0.05) between all considered samples at each storage time.
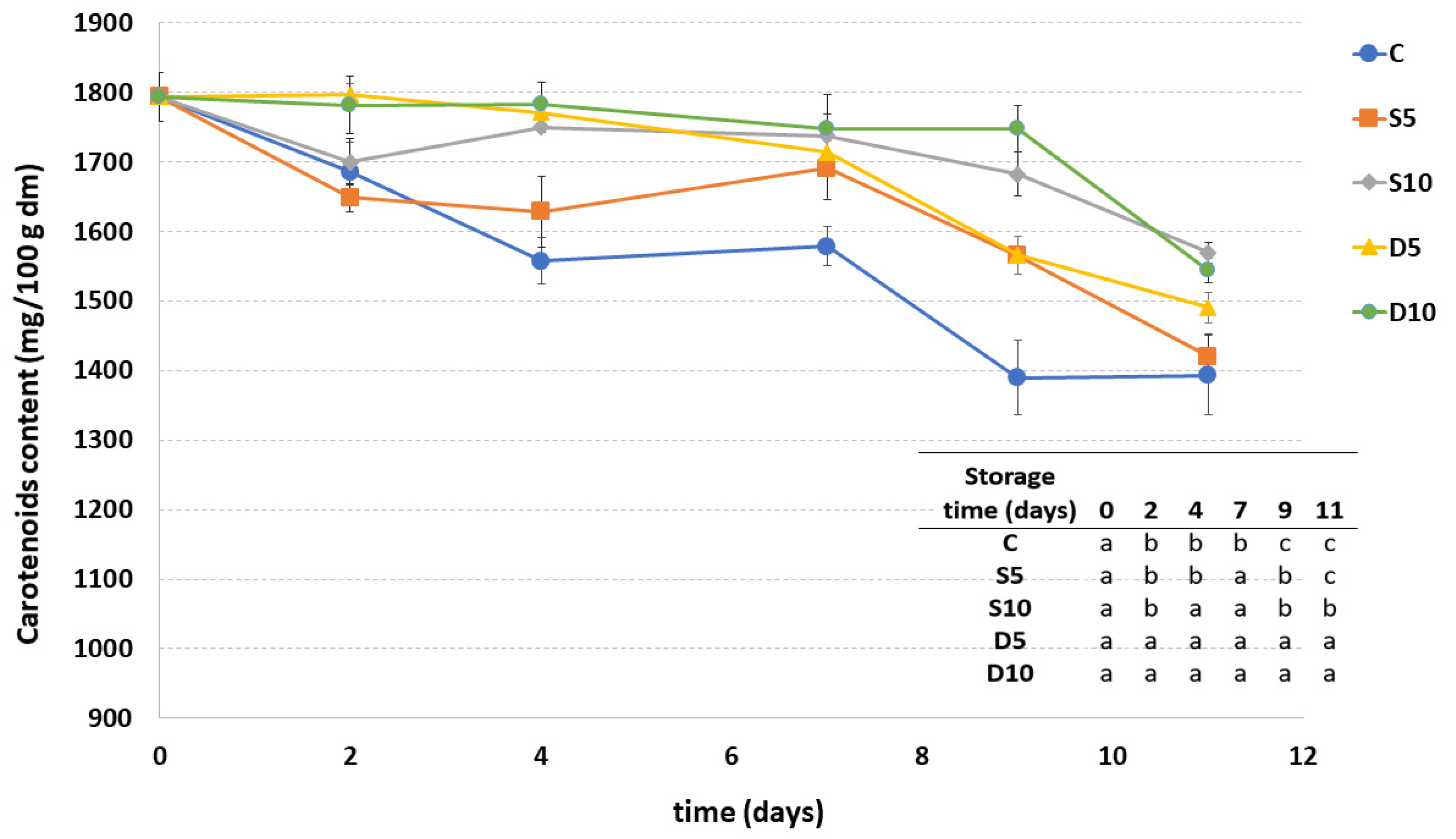
3.4. Carotenoids Content
Carotenoids content of all coated samples showed a decrease during the storage, mainly during the last days (Figure 4). In general, melon samples treated with higher coating concentration (D10 and S10) maintained the highest carotenoid content, regardless of the method of application. The carotenoids content in these samples were also quite stable up to the ninth day of storage. A higher decrease in carotenoids was observed in the samples coated with lower concentration (5%) of coating followed by the control sample.
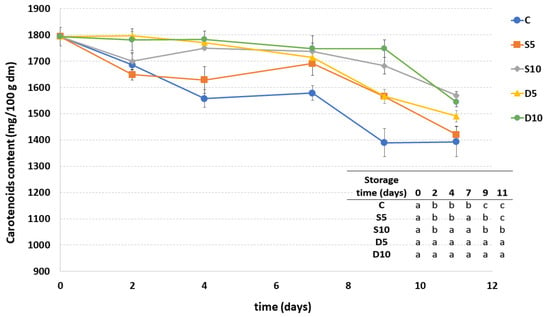
Figure 4.
Carotenoids content of fresh-cut melon samples (C, D5, D10, S5 and S10) during storage. a–c Different letters in the same column indicate significant differences (p < 0.05) between all considered samples at each storage time.
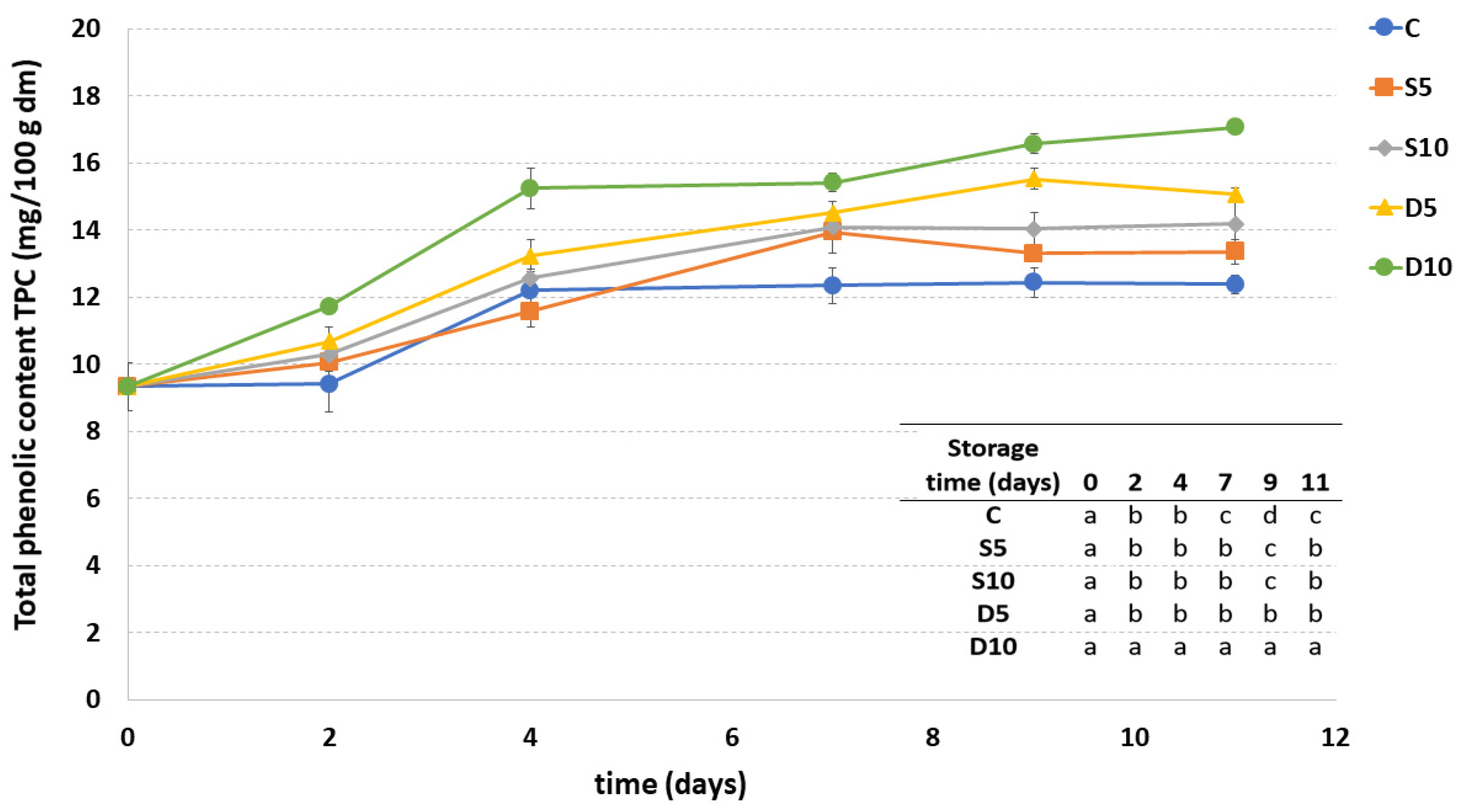
3.5. Total Polyphenol Content (TPC)
Figure 5 reports the amount of TPC in uncoated and differently coated fresh-cut melon samples during the storage. The highest TPC was observed in the D10 sample during the entire shelf-life period, while the lowest content was observed in the uncoated sample (C) from the seventh d of storage. Concerning the application method, in general, a higher TPC was observed in melon samples treated by dipping.
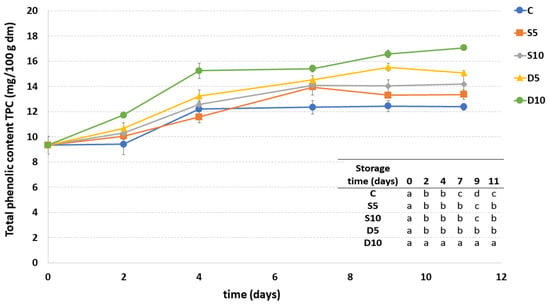
Figure 5.
Total phenolic content (TPC) of fresh-cut melon samples (C, D5, D10, S5 and S10) during storage. a–c Different letters in the same column indicate significant differences (p < 0.05) between all considered samples at each storage time.
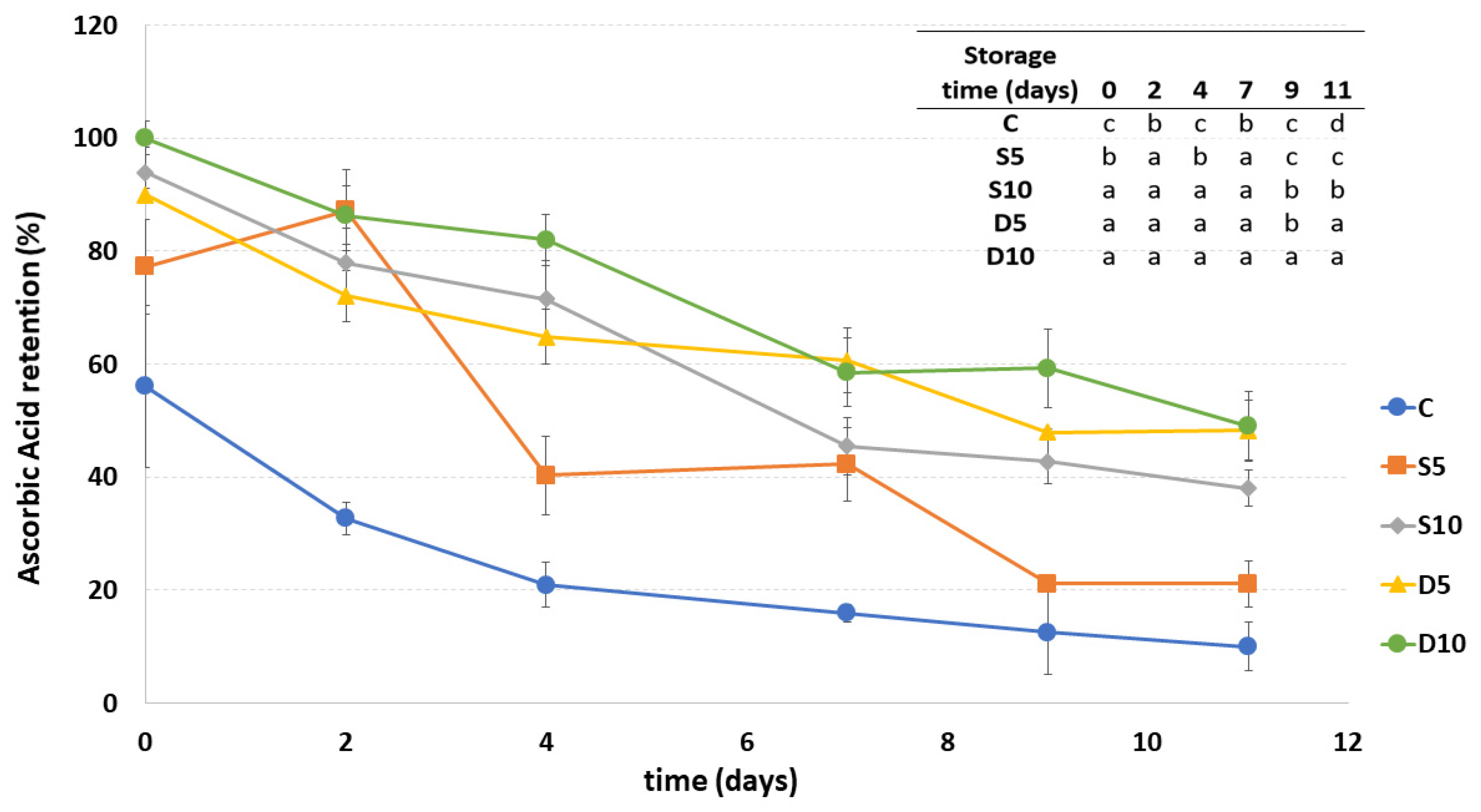
3.6. Ascorbic Acid Content
Figure 6 shows the ascorbic acid content in all the samples during storage. The amount of AA is expressed in % considering the D10 sample (with the highest AA content) as the one with 100% of AA.
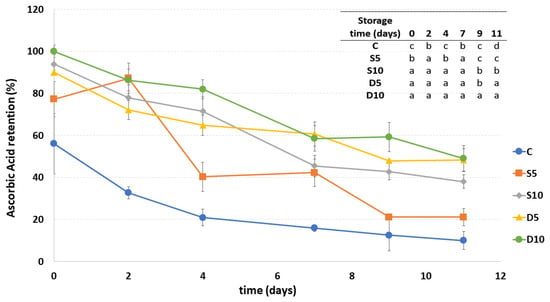
Figure 6.
Ascorbic acid retention (%) of fresh-cut melon samples (C, D5, D10, S5 and S10) during storage. a–d Different letters in the same column indicate significant differences (p < 0.05) between all considered samples at each storage time.
As expected, the uncoated C sample showed significantly lower ascorbic acid content values compared to the others starting from the first day of storage, showing a decrease in AA amount equal to 46% at the end of storage. All coated samples presented a higher AA amount than the control. Moreover, some differences between coated samples were observed already on the first day of storage, in fact, samples D10, D5 and S10, presented higher AA content compared to S5. In particular, dipped fresh-cut melons (both at 5% and 10%) were those with higher AA content at the end of the storage, showing an ascorbic acid degradation compared to the initial value, of respectively 42% and 51%. Both sprayed samples presented, instead, a decrease in the ascorbic acid amount of about 55%. The differences between the coating concentrations were more highlighted in the sprayed samples, showing a significantly lower AA content in S5 if compared with S10 during the almost entire storage duration.
4. Discussion
An appropriate viscosity of the coating solutions is very important to ensure good adhesiveness to the fruit surface. Therefore, in the present study, the rheological behavior of the solutions at two different concentrations (5% and 10%) were analyzed to understand their applicability as an edible coating. In both samples, apparent viscosity decreased as the shear rate increased, underling the presence of a pseudoplastic behavior (shear thinning) [40]. Both samples had n values < 1, that is typical for shear thinning behavior, as previously demonstrated by Glicerina et al. [41]. The K parameter, which is an index of the consistency of the product [42] was higher for the coating at 10% compared to the one at 5%. This result, according to the literature [43,44], could be explained considering the linear relationship existing between viscosity and soluble solid concentration. In fact, in the present work, higher soluble solid content of alginate solution led to higher viscosity of the coating solution. However, as known by the literature, [22] the obtained viscosity values of both solutions can be considered appropriate for their use as edible coating, promoting an appropriate adhesiveness to the fruit surface.
The application of an edible coating on the fruit and vegetable surface can modify the atmosphere surrounding the fruit, by acting as a semipermeable barrier that allows controlling the gas exchange (between fruits and environment) and water loss. As a consequence, it maintains tissue firmness guaranteeing, in addition, the inhibition of microbial spoilage [16,45]. Polysaccharide-based coatings, realized with sodium alginate, modulate the internal tissue atmosphere, once they represent an obstacle to moisture, O2, CO2 and volatiles exchange, slowing down the metabolism and delaying fruit senescence [14]. In the present study, sodium alginate-based coatings applied to fruit, in particular by the dipping method, contributed to give a barrier effect limiting water loss and controlling fruit dehydration, as already observed by Mannozzi et al. [46].
Melon, particularly Cucumis melo var. reticulatus, is highly appreciated due to its nutritional value, and indeed is a source of carotenoids characterized by high antioxidant activity [47]. The decrease in carotenoids during shelf-life may be attributed to their degradation because of the oxygen exposure, and cellular disruption due to wounding that exposes the carotenoids to lipoxygenase action [1]. As also demonstrated by moisture results (Figure 2), up to seventh d of storage, coatings applied on fresh-cut melon by dipping at the highest concentration had a barrier effect reducing the water loss and the oxygen exposure, thus contributing to a reduction of carotenoids degradation. Moreover, the coating applied at a concentration of 10% preserved better carotenoid’s content than the one at 5%, probably because of its higher ascorbic acid content, which exerted antioxidant activity.
The observed increase in TPC in all melon samples during storage could probably be related to the wounding mechanism response of fruits [6,7,8,9,10]. It is known that the coating contributes to reducing the interaction between polyphenols and oxidative enzymes, by oxygen restriction, maintaining high polyphenols levels [11]. Altunkaya, A., & Gökmen [48], in a study conducted on minimally processed lettuce, highlighted the presence of synergism between ascorbic acid (AA) and phenols, causing an increase of the latter in a higher presence of AA. Concerning the application method, in general, a higher TPC was observed in melon samples treated by dipping, in agreement with the study of Tahler et al. [49] that observed an immersion of fresh fruits in the edible coating by dipping was correlated with an increase in the polyphenols content. Moreover, the homogenous distribution of coating solution by dipping contributes to the TPC maintenance, avoiding their oxidative degradation as also reported by Bico et al., Zam et al. and Suhag et al. [21,50,51].
Concerning the AA content, all coated samples presented a higher AA amount than the control one, thanks to the addition of this compound in the coating formulation and to the oxygen barrier of the coating itself, which likely helped to reduce the deteriorative oxidation of ascorbic acid [52]. Yousuf et al. [28] observed even an increase of vitamin C in melon samples following the application of an edible coating based on lemon extract and soy protein isolate during storage. Moreover, the differences observed between coated samples at time zero could probably be related to the presence of soluble solids concentration and the application method. The differences in AA content between the coating concentrations were more highlighted in the sprayed samples in comparison to dipped ones. In fact, the dipping method showed a higher coating thickness and determined a better and homogeneous coverage of fruit pieces than spraying, due to the possible local liquid entrainment during dip-coating procedure [39,53].
5. Conclusions
The obtained results showed that the coating application method has a greater influence on the maintenance of the quality of fresh-cut melon during storage. The dipping method showed a positive effect on maintaining both high moisture levels and higher carotenoids and ascorbic acid contents during the considered storage period. In particular, the application of the enriched coating by dipping treatment at higher concentration (10%) was the best solution to obtain fresh-cut melon with enhanced nutritional quality, that was best maintained during storage, thanks also to the better coating homogeneity and higher thickness observed through microstructural analysis.
However, further studies are necessary to deeply understand the effect of the coating type and application method on other important fresh-cut melon quality characteristics, such as physical, sensorial and microbiological ones.
Author Contributions
Conceptualization, V.G. and S.R.; methodology, G.C.; software, J.M.C.; validation, C.M., V.G., G.C.; formal analysis, C.M., V.G.; investigation, V.G.; resources, S.R., M.D.R.; data curation, V.G.; writing—original draft preparation, C.M., V.G., G.C., U.T.; writing—review and editing, J.M.C., U.T., S.R., M.D.R.; visualization, C.M, V.G., G.C., U.T.; supervision, S.R., M.D.R.; project administration, U.T.; funding acquisition, U.T., S.R. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the financial support for this research provided by transnational funding bodies, partners of the H2020 ERA-NETs SUSFOOD2 and CORE Organic Cofunds, under the Joint SUSFOOD2/CORE Organic Call 2019 (MILDSUSFRUIT).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Amaro, A.L.; Spadafora, N.D.; Pereira, M.J.; Dhorajiwala, R.; Herbert, R.J.; Müller, C.T.; Rogers, H.J.; Pintado, M. Multitrait analysis of fresh-cut cantaloupe melon enables discrimination between storage times and temperatures and identifies potential markers for quality assessments. Food Chem. 2018, 241, 222–231. [Google Scholar] [CrossRef]
- Falah, M.A.F.; Nadine, M.D.; Suryandono, A. Effects of storage conditions on quality and shelf-life of fresh-cut melon (Cucumis melo, L.) and papaya (Carica papaya, L.). Procedia Food Sci. 2015, 3, 313–322. [Google Scholar] [CrossRef] [Green Version]
- Jideani, A.I.; Anyasi, T.; Mchau, G.R.; Udoro, E.O.; Onipe, O.O. Processing and preservation of fresh-cut fruit and vegetable products. In Postharvest Handling; Kahramanoglu, I., Ed.; IntechOpen: London, UK, 2017. [Google Scholar]
- And, O.L.; Watson, M. Effects of ascorbic acid on peroxidase and polyphenoloxidase activities in fresh-cut cantaloupe melon. J. Food Sci. 2001, 66, 1283–1286. [Google Scholar] [CrossRef]
- Plaza, L.; Altisent, R.; Alegre, I.; Viñas, I.; Abadias, M. Changes in the quality and antioxidant properties of fresh-cut melon treated with the biopreservative culture Pseudomonas graminis CPA-7 during refrigerated storage. Postharvest Biol. Technol. 2016, 111, 25–30. [Google Scholar] [CrossRef]
- Campos-Vargas, R.; Saltveit, M.E. Involvement of putative chemical wound signals in the induction of phenolic metabolism in wounded lettuce. Physiol. Plant. 2002, 114, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artés-Hernández, F.; Rivera-Cabrera, F.; Kader, A.A. Quality retention and potential shelf-life of fresh-cut lemons as affected by cut type and temperature. Postharvest Biol. Technol. 2007, 43, 245–254. [Google Scholar] [CrossRef]
- Surjadinata, B.B.; Cisneros-Zevallos, L. Biosynthesis of phenolic antioxidants in carrot tissue increases with wounding intensity. Food Chem. 2012, 134, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Torres-Contreras, A.M.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Plants as biofactories: Stress-induced production of chlorogenic acid isomers in potato tubers as affected by wounding intensity and storage time. Ind. Crop. Prod. 2014, 62, 61–66. [Google Scholar] [CrossRef]
- Oliu, G.O.; Soliva-Fortuny, R.; Martín-Belloso, O. Using polysaccharide-based edible coatings to enhance quality and antioxidant properties of fresh-cut melon. LWT 2008, 41, 1862–1870. [Google Scholar] [CrossRef]
- Robles-Sánchez, R.M.; Rojas-Graü, M.A.; Odriozola-Serrano, I.; González-Aguilar, G.; Martin-Belloso, O. Influence of alginate-based edible coating as carrier of antibrowning agents on bioactive compounds and antioxidant activity in fresh-cut Kent mangoes. LWT 2013, 50, 240–246. [Google Scholar] [CrossRef]
- Perdones, Á.; Sánchez-González, L.; Chiralt, A.; Vargas, M. Effect of chitosan-lemon essential oil coatings on storage-keeping quality of strawberry. Postharvest Biol. Technol. 2012, 70, 32–41. [Google Scholar] [CrossRef]
- Bonilla, J.; Talón, E.; Atarés, L.; Vargas, M.; Chiralt, A. Effect of the incorporation of antioxidants on physicochemical and antioxidant properties of wheat starch-chitosan films. J. Food Eng. 2013, 118, 271–278. [Google Scholar] [CrossRef]
- Yousuf, B.; Qadri, O.S.; Srivastava, A.K. Recent developments in shelf-life extension of fresh-cut fruits and vegetables by application of different edible coatings: A review. LWT 2018, 89, 198–209. [Google Scholar] [CrossRef]
- Giannakourou, M.; Tsironi, T. Application of processing and packaging hurdles for fresh-cut fruits and vegetables preservation. Foods 2021, 10, 830. [Google Scholar] [CrossRef]
- González-Aguilar, G.A.; Valenzuela-Soto, E.; Lizardi-Mendoza, J.; Goycoolea, F.M.; Martínez-Téllez, M.Á.; Villegas-Ochoa, M.A.; Monroy-García, I.N.; Ayala-Zavala, J.F. Effect of chitosan coating in preventing deterioration and preserving the quality of fresh-cut papaya ‘Maradol’. J. Sci. Food Agric. 2008, 89, 15–23. [Google Scholar] [CrossRef]
- Zúñiga, G.; Junqueira-Gonçalves, M.; Pizarro, M.; Contreras, R.; Tapia, A.; Silva, S. Effect of ionizing energy on extracts of Quillaja saponaria to be used as an antimicrobial agent on irradiated edible coating for fresh strawberries. Radiat. Phys. Chem. 2012, 81, 64–69. [Google Scholar] [CrossRef]
- Baraiya, N.S.; Rao, T.V.R.; Thakkar, V. Improvement of postharvest quality and storability of jamun fruit (Syzygium cumini L. Var. Paras) by zein coating enriched with antioxidants. Food Bioprocess Technol. 2015, 8, 2225–2234. [Google Scholar] [CrossRef]
- Andrade, R.D.; Skurtys, O.; Osorio, F. Atomizing spray systems for application of edible coatings. Compr. Rev. Food Sci. Food Saf. 2012, 11, 323–337. [Google Scholar] [CrossRef]
- Parreidt, T.S.; Schmid, M.; Müller, K. Effect of dipping and vacuum impregnation coating techniques with alginate based coating on physical quality parameters of cantaloupe melon. J. Food Sci. 2018, 83, 929–936. [Google Scholar] [CrossRef]
- Suhag, R.; Kumar, N.; Petkoska, A.T.; Upadhyay, A. Film formation and deposition methods of edible coating on food products: A review. Food Res. Int. 2020, 136, 109582. [Google Scholar] [CrossRef]
- Zhong, Y.; Cavender, G.; Zhao, Y. Investigation of different coating application methods on the performance of edible coatings on mozzarella cheese. LWT 2014, 56, 1–8. [Google Scholar] [CrossRef]
- Maringgal, B.; Hashim, N.; Tawakkal, I.S.M.A.; Mohamed, M.T.M. Recent advance in edible coating and its effect on fresh/fresh-cut fruits quality. Trends Food Sci. Technol. 2020, 96, 253–267. [Google Scholar] [CrossRef]
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Martín-Belloso, O. Edible alginate-based coating as carrier of antimicrobials to improve shelf-life and safety of fresh-cut melon. Int. J. Food Microbiol. 2008, 121, 313–327. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Chen, C.-H.; Lai, L.-S. Effect of tapioca starch/decolorized hsian-tsao leaf gum-based active coatings on the qualities of fresh-cut apples. Food Bioprocess Technol. 2012, 6, 2059–2069. [Google Scholar] [CrossRef]
- Poverenov, E.; Danino, S.; Horev, B.; Granit, R.; Vinokur, Y.; Rodov, V. Layer-by-layer electrostatic deposition of edible coating on fresh cut melon model: Anticipated and unexpected effects of alginate–chitosan combination. Food Bioprocess Technol. 2014, 7, 1424–1432. [Google Scholar] [CrossRef]
- Carvalho, R.L.; Cabral, M.F.; Germano, T.A.; de Carvalho, W.M.; Brasil, I.M.; Gallão, M.I.; Moura, C.F.H.; Lopes, M.M.A.; de Miranda, M.R. Chitosan coating with trans-cinnamaldehyde improves structural integrity and antioxidant metabolism of fresh-cut melon. Postharvest Biol. Technol. 2016, 113, 29–39. [Google Scholar] [CrossRef]
- Yousuf, B.; Srivastava, A.K.; Ahmad, S. Application of natural fruit extract and hydrocolloid-based coating to retain quality of fresh-cut melon. J. Food Sci. Technol. 2020, 57, 3647–3658. [Google Scholar] [CrossRef] [PubMed]
- Atieno, L.; Owino, W.; Ateka, E.M.; Ambuko, J. Influence of coating application methods on the postharvest quality of cassava. Int. J. Food Sci. 2019, 2019, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Glicerina, V.; Tylewicz, U.; Canali, G.; Siroli, L.; Rosa, M.D.; Lanciotti, R.; Romani, S. Influence of two different cocoa-based coatings on quality characteristics of fresh-cut fruits during storage. LWT 2019, 101, 152–160. [Google Scholar] [CrossRef]
- Holdsworth, S.D. Rheological models used for the prediction of the flow properties of food products: A literature review. Food Bioprod. Process. Trans. Inst. Chem. Eng. Part C 1993, 71, 139–179. [Google Scholar]
- Mantilla, N.; Castell-Perez, M.; Gomes, C.; Moreira, R.G. Multilayered antimicrobial edible coating and its effect on quality and shelf-life of fresh-cut pineapple (Ananas comosus). LWT 2013, 51, 37–43. [Google Scholar] [CrossRef]
- Szaszák, M.; Gáborik, Z.; Turu, G.; McPherson, P.S.; Clark, A.J.L.; Catt, K.J.; Hunyady, L. Role of the proline-rich domain of dynamin-2 and its interactions with Src homology 3 domains during endocytosis of the AT1 angiotensin receptor. J. Biol. Chem. 2002, 277, 21650–21656. [Google Scholar] [CrossRef] [Green Version]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Tylewicz, U.; Mannozzi, C.; Romani, S.; Castagnini, J.M.; Samborska, K.; Rocculi, P.; Rosa, M.D. Chemical and physicochemical properties of semi-dried organic strawberries enriched with bilberry juice-based solution. LWT 2019, 114, 108377. [Google Scholar] [CrossRef]
- Laur, L.M.; Tian, L. Provitamin A and vitamin C contents in selected California-grown cantaloupe and honeydew melons and imported melons. J. Food Compos. Anal. 2011, 24, 194–201. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Hernández-Jover, T.; Martin-Belloso, O. Comparative evaluation of UV-HPLC methods and reducing agents to determine vitamin C in fruits. Food Chem. 2007, 105, 1151–1158. [Google Scholar] [CrossRef]
- Vizsnyiczai, G.; Kelemen, L.; Ormos, P. Holographic multi-focus 3D two-photon polymerization with real-time calculated holograms. Opt. Express 2014, 22, 24217–24223. [Google Scholar] [CrossRef] [Green Version]
- Balzarotti, R.; Cristiani, C.; Francis, L.F. Combined dip-coating/spin-coating depositions on ceramic honeycomb monoliths for structured catalysts preparation. Catal. Today 2019, 334, 90–95. [Google Scholar] [CrossRef]
- Izidoro, D.R.; Scheer, A.P.; Sierakowski, M.-R.; Haminiuk, C.W. Influence of green banana pulp on the rheological behaviour and chemical characteristics of emulsions (mayonnaises). LWT 2008, 41, 1018–1028. [Google Scholar] [CrossRef]
- Glicerina, V.; Balestra, F.; Rosa, M.D.; Romani, S. Rheological, textural and calorimetric modifications of dark chocolate during process. J. Food Eng. 2013, 119, 173–179. [Google Scholar] [CrossRef]
- Nindo, C.; Tang, J.; Powers, J.; Takhar, P. Rheological properties of blueberry puree for processing applications. LWT 2007, 40, 292–299. [Google Scholar] [CrossRef]
- Walstra, P. Studying Food Colloids: Past, Present and Future; Royal Society of Chemistry (RSC): London, UK, 2007; pp. 391–400. [Google Scholar]
- Comaposada, J.; Gou, P.; Marcos, B.; Arnau, J. Physical properties of sodium alginate solutions and edible wet calcium alginate coatings. LWT 2015, 64, 212–219. [Google Scholar] [CrossRef]
- Parreidt, T.S.; Müller, K.; Schmid, M. Alginate-based edible films and coatings for food packaging applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef] [Green Version]
- Mannozzi, C.; Cecchini, J.; Tylewicz, U.; Siroli, L.; Patrignani, F.; Lanciotti, R.; Rocculi, P.; Dalla Rosa, M.; Romani, S. Study on the efficacy of edible coatings on quality of blueberry fruits during shelf-life. LWT Food Sci. Technol. 2017, 85, 440–444. [Google Scholar] [CrossRef]
- Fundo, J.F.; Miller, F.A.; Tremarin, A.; Garcia, E.; Brandão, T.R.S.; Silva, C.L.M. Quality assessment of Cantaloupe melon juice under ozone processing. Innov. Food Sci. Emerg. Technol. 2018, 47, 461–466. [Google Scholar] [CrossRef]
- Altunkaya, A.; Gokmen, V. Effect of various inhibitors on enzymatic browning, antioxidant activity and total phenol content of fresh lettuce (Lactuca sativa). Food Chem. 2008, 107, 1173–1179. [Google Scholar] [CrossRef]
- Taher, M.A.; MennatAllah, E.A.; Tadros, L.K.; Sanad, M.I. The effects of new formulations based on Gum Arabic on antioxidant capacity of tomato (Solanum lycopersicum L.) fruit during storage. J. Food Meas. Charact. 2020, 14, 2489–2502. [Google Scholar] [CrossRef]
- Bico, S.; Raposo, M.F.J.; Morais, R.; Morais, A. Combined effects of chemical dip and/or carrageenan coating and/or controlled atmosphere on quality of fresh-cut banana. Food Control 2009, 20, 508–514. [Google Scholar] [CrossRef]
- Zam, W. Effect of alginate and chitosan edible coating enriched with olive leaves extract on the shelf life of sweet cherries (Prunus avium L.). J. Food Qual. 2019, 2019, 8192964. [Google Scholar] [CrossRef] [Green Version]
- Ayranci, E.; Tunç, S. A method for the measurement of the oxygen permeability and the development of edible films to reduce the rate of oxidative reactions in fresh foods. Food Chem. 2003, 80, 423–431. [Google Scholar] [CrossRef]
- Prasad, K.; Guarav, A.K.; Preethi, P.; Neha, P. Edible coating technology for extending market life of horticultural produce. Acta Sci. Agric. 2018, 2, 55–64. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).