Featured Application
This work presents a helpful tool when dealing with the biodiversity of monumental fountains and other wet lithotype surfaces in the sector of stone conservation.
Abstract
All fountains are inhabited by phototrophic microorganisms, especially if they are functional and located outdoors. This fact, along with the regular presence of water and the intrinsic bioreceptivity of stone material, easily favors the biological development. Many of these organisms are responsible for the biodeterioration phenomena and recognizing them could help to define the best strategies for the conservation and maintenance of monumental fountains. The presence of biological growth involves different activities for the conservation of artistic fountains. This paper is a review of the phototrophic biodiversity reported in 46 fountains and gives a whole vision on coping with biodeteriogens of fountains, being an elementary guide for professionals in the field of stone conservation. It is focused on recognizing the main phototrophs by using simplified dichotomous keys for cyanobacteria, green algae and diatoms. Some basic issues related to the handling of the samples and with the control of these types of microalgae are also briefly described, in order to assist interested professionals when dealing with the biodiversity of monumental fountains.
1. Introduction
Fountains are structures with a functional, decorative or recreation purpose that have been built since ancient times [1]. Monumental and artistic fountains are often impressive constructions built all over the world, belonging to the public and garden art, such as ‘La Joute’ Fountain from Montreal (Canada), Fountain of Giant Wild Goose Pagoda from Xi’an (China), Flora Fountain from Mumbai (India), Keller Fountain from Portland, Oregon (USA), etc. They are mainly made of stone (structure, basin and decorations) and metallic alloys (hydraulic system and decorations). Stones with a local origin are preferred, but valuable stones are also used for the significant decorative elements (e.g., marble). However, other types of materials have been employed in the recent century, such as concrete material (e.g., Villancourt Fountain, San Francisco, CA, USA) or other modern materials such resins (Stravinsky Fountain, Paris, France, or Charybdis’ Vortex Fountain, Sunderland, UK).
The roughness of the stone substrate is one of the main factors that influences the phototrophic colonization by epilithic, chasmolithic or endolithic microorganisms [2]. The last ones include the criptoendolithic microorganisms (which develop in layers, parallel with respect to the surface) and euendolithic microorganisms (which are the most aggressive, as they actively penetrate inside the stone). The epilithic communities are the most frequent. They mainly induce esthetical damage, but often corrosion phenomena can be observed (like little holes in the stones, corresponding to the phototrophic settlement). The chasmolithic development induces damage such as detachments or stone flakes formation and lifting. The biodeterioration phenomena were investigated in different studies [3,4,5,6,7].
The main types of phototrophic communities form pellicles, pustules, mats or layered sedimentary formations [6]. Often, the biological presence of phototrophic microorganisms can be considered a patina [8] or a phototrophic biofilm (due to its mucilaginous aspect).
Artistic fountains are not only heritage items but sociocultural artefacts as well, with a significant importance for the identity of certain places or periods [9,10]. They are part of the community and human treasures and raise preservation demands for transmitting their values to the further generations. Applying monitoring activity with a professional maintenance plan is essential for good and effective preservation of these structures. The preservation strategies should include different activities such as cleaning, repairs and consolidation, application or replacement of protective coatings, controlling of the water quality; all these interventions are related with the degradation problems induced by physical, chemical or biological agents. Coping with the phototrophic colonizers of artistic fountains and other wet surfaces means mainly cleaning and monitoring activity. The morphological identification of the phototrophic presence is usually enough for the documentation before and after the restoration. A detailed mapping of all types of deterioration phenomena present on a certain case study helps to decide the best strategies for both treatment and monitoring plans. Although the control treatments are often common for all the phototrophic groups, it worth mentioning that the principle of minimal intervention should always be kept in mind, being therefore ready to undertake, when necessary, selective treatments on the same stone artefact. This is due especially to two main reasons: (i) the phototrophic growth on the fountains is rarely formed by a singular group of phototrophs, and (ii) the cleaning treatments (chemical, physical or mechanical) are not so selective. However, in particular cases (e.g., the presence of phototrophic endolithic growth) it is possible to improve the cleaning procedures by making changes to the applications time, the concentration used, or to combine different types of cleaning treatments (e.g., mechanical and chemical; mechanical and physical). Moreover, the molecular identification could be necessary despite increasing procedure costs. These types of analysis need a small amount of sample and are definitely more precise than the morphological analysis. They are very useful to control the regrowth of dangerous species after a certain treatment.
An easy to use guide containing identification keys for the main phototrophs growing on monumental fountains and other wet stone surfaces is presented in this work. These new keys resulted after revising and simplifying other different identification keys [11,12,13,14,15,16,17,18,19,20,21] and they were tailored as a new tool for professionals in the field of stone conservation, that are interested in making their first steps in phototrophs identification. It is based on both macroscopic and microscopic characteristics for the main genera belonging to cyanobacteria, green algae and diatoms, the main three phototrophic groups found on the ornamental fountains. Moreover, some basic issues related to the handling of the samples and the control methodologies were also briefly described.
2. Materials and Methods
2.1. Morphological Identification
Phototrophic samples (about 265) were collected from various ornamental fountains and identified by the authors of this paper in precedent works [21,22,23,24,25,26] as indicated in Table 1, Table 2, Table 3 and Table 4. The most frequent genera found by other authors [3,5,7,24,25,27,28,29,30,31,32] are also inserted in these identification keys.

Table 1.
Artistic fountains investigated for the main phototrophic microorganisms.

Table 2.
Cyanobacteria observed in different artistic fountains (see Table 1 for details of the location), where CO = Columbia, IT = Italy, ES = Spain and USA = United States.

Table 3.
Green algae observed in different artistic fountains (see Table 1 for details of the location), where CO = Columbia, IT = Italy, IN = India, SK = Slovakia, ES = Spain and USA = United States.

Table 4.
Diatoms observed in different artistic fountains (see Table 1 for details of the location), where CO = Columbia, IT = Italy, IN = India, ES = Spain and USA = United States.
The collection of the phototrophic biomass is usually made by using sterile scalpels and tubes of different sizes. When possible, in the case of chasmolithic or endolithic developments, small pieces of stone substrata containing microorganisms can be sampled as well. A fixation with formalin (2%, final concentration) is recommended in order to preserve the morphology of the phototrophs [23]. The Lugol’s iodine solution (1% v/v) is also used for preventing contamination [5], being also a useful test under the microscope, as it gives a dark purple/brown/blue black stain to the cells containing starch (green algae and diatoms), favoring the sedimentation of the cells and their easier observation as well [33].
The microscopic observations of the samples can be made in transmission light, and good quality images can be obtained by using DIC (differential interference contrast) microscopy. The fresh samples will often contain more than one genus. The sample preparation for the microscopic analysis can be summarized as following: (i) first of all, place a drop of water on the center of a clean slide; (ii) take a very small part of the sample (e.g., with the help of a needle or tweezers) and place it inside the drop of water; if the sample is solid or too thick (e.g., a dense mat or a crust) it must be crushed and scattered in the water drop in order to be able distinguish individual cells by the light passing through the sample; it is important to not mechanically break the clusters before the slide preparation, as confusion may occur with the non-colonial genera; (iii) once the sample is uniformly distributed, place slowly the cover on top by firstly putting in contact its outer edge with the water drop. If the water overflows, gently remove the excess with a paper towel. When diatoms are present, a cleaning procedure of the frustules can be used before preparing the slides [34,35]. This procedure involves a careful use of some acids (nitric acid, hydrogen peroxide), able to remove the organic matter and carbonates from the sample, and therefore to highlight the morphological characteristics of the diatoms. Once prepared, the slides can be observed under different magnification levels (10×, 20×, 40×, 50× or 100×) in order to distinguish many of the morphological features of the cells. Some of the main features that would be observed are related to the shape of the cells, the presence or absence of the sheaths, the division type, the end cells morphology in the case of the filamentous cyanobacteria, the presence of motility phenomena, the plastids position and their shape in the case of green algae. It is possible to make deeper investigations by evaluating the ratio within the main phototrophic groups, as Popović et al. [36] describe a detailed procedure for the mixed phototrophic biofilms.
2.2. Identification Keys
The keys derive from the specific literature survey on the taxonomy of cyanobacteria [11,12,13,14,15,16,17,18,19], green algae [11,13] and diatoms [11,13,21] and from the authors’ collected material. The keys are dichotomous. Each number describes a couple of contrasting and distinctive characteristics. The reader makes a choice between the two options and follows the indications for the next couplet and so on. The dichotomous chain finishes with finding the genus. It should be specified that many species of cyanobacteria exhibit variable morphology, and their identification till the species level may need specific ultrastructural and molecular features, a fact that it was not considered necessary for the aim of this work and therefore they were not used in this key.
Five plates containing the illustrations of the main common genera were realized in watercolor technique (Arches cotton paper 300 g/m3, Sennelier and Mijello water colors) by F. Bolívar-Galiano, according mainly to our microscopic observations.
3. Results
3.1. First Screening
The first screening can be undertaken by naked eye or by using a portable microscope with about 20× magnification which can help to choose the sampling area and to make a rough morphological discrimination, through the inspection of the color and morphological aspects of the biological colonization (mat aspect with visible filaments, patina aspect with uniform color, spots like growth, etc.). A good camera with a macro objective can also be very useful.
In this step, it is difficult to assign the sample to a single phototrophic group, as on monumental fountains and other wet stone surfaces there are developing communities of microorganisms, which are a mix of different cyanobacteria and/or green algae and/or diatoms, as can be seen in some fresh samples observed under the microscope (Supplementary Materials S1–S5). Diatoms prefer high levels of humidity and are more often associated with green algae, and less only with cyanobacteria, but they can be found beside algae and cyanobacteria as well. Often, the color gives an idea about the possible phototrophic presence, but it is never conclusive [37].
- 1a
- visible vivid green, light green, green or brownish phototrophic growth.....................................2
- 1b
- visible dark green, deep brown, deep grey or black phototrophic growth.....................................3
- 2a
- with a mucilaginous, wet or powdering aspect, it seems not well adhered to the stone surface ...................................................4
- 2b
- with an aspect of patina, pellicle or mat, that seems adhered to the stone surface... ......5
- 3a
- dark green, dark grey or dark brown wet formations, with filamentous or spherical aspect ...................................... see 3.2. Cyanobacteria (blue-green algae) and 3.3. Green Algae
- 3b
- dark patina with intergranular growth on dry surfaces ................... see 3.2. Cyanobacteria
- 4a
- vivid and light green color........................................................................see 3.3. Green Algae
- 4b
- green, yellow-green or light brown color, sometimes with aspect of pustule...................................................................................see 3.3. Green Algae and 3.4. Diatoms
- 5a
- pellicles more or less gelatinous....................... see 3.2. Cyanobacteria and 3.3. Green Algae
- 5b
- aspect of mats and patina, with different thicknesses, dark green or dark blue-green color with many filaments........................................................see mainly 3.2. Cyanobacteria
3.2. Cyanobacteria (Blue-Green Algae)
They are prokaryotic phototrophic microorganisms, without chloroplasts (plastids), with an immense morphological variability, from free and isolated cells to very hard and consistent assemblages. Moreover, the Lugol solution used for sample preservation, does not stain the cells blue/black. Cyanobacteria have a characteristic blue-green color, hence their common name of blue-green algae. They can be divided in two main groups: I. simple cyanobacteria and II. filamentous cyanobacteria.
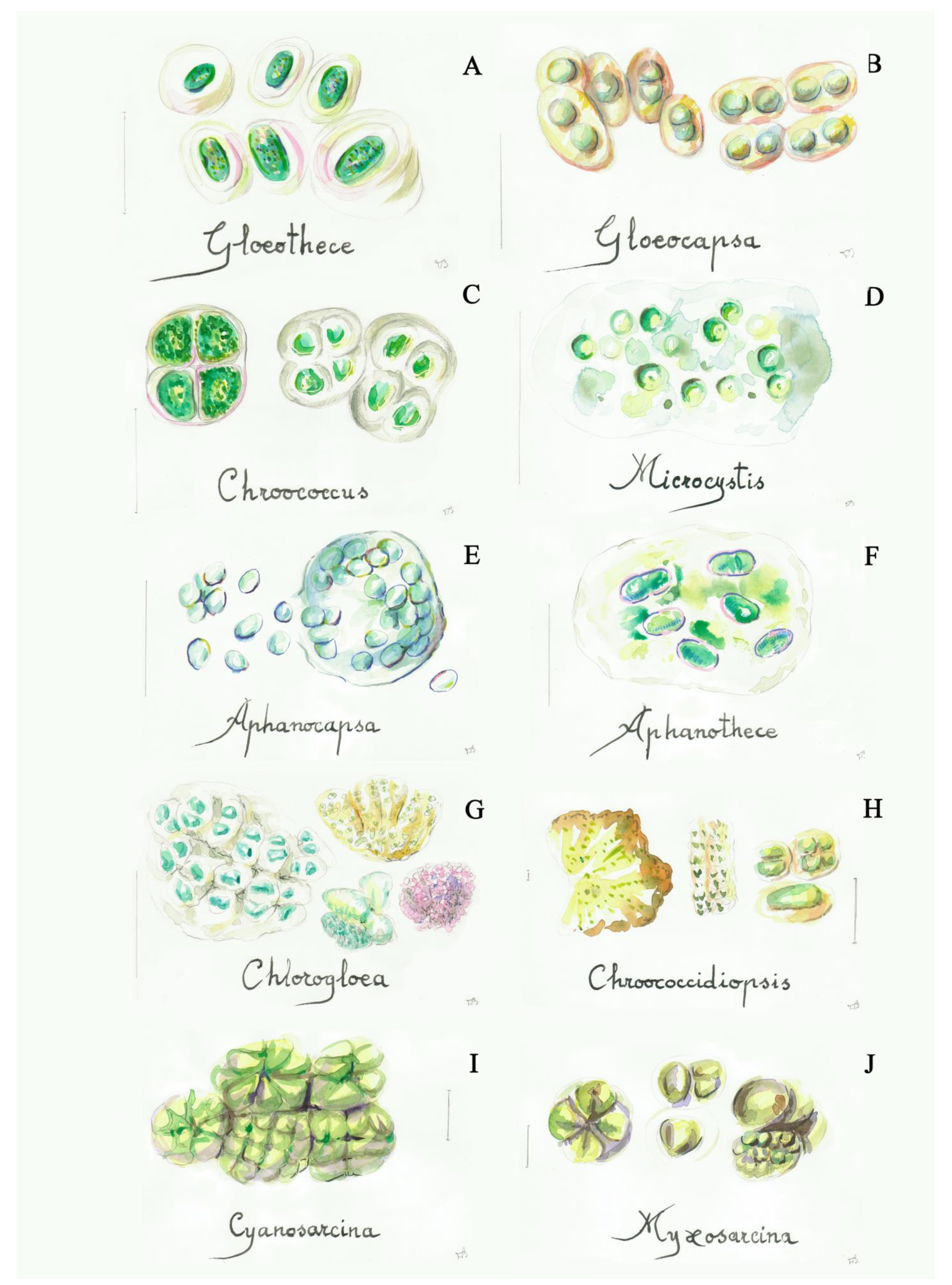
I. simple cyanobacteria (unicellular, mucilaginous groups, strong adherent group of cells) (Figure 1, Figure S6 and [23,38])
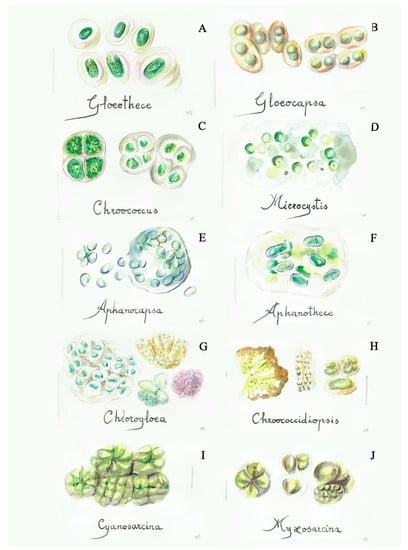
Figure 1.
Some simple cyanobacteria (blue-green algae): (A) Gloeothece (4a), (B) Gloeocapsa (5a), (C) Chroococcus (5b), (D) Microcystis (7a), (E) Aphanocapsa (7b), (F) Aphanothece (8a), (G) Chlorogloea (9a), (H) Chroococcidiopsis (9b), (I) Cyanosarcina (12a), (J) Myxosarcina (12b). Scale bar 10 μm. The number in the brackets corresponds to the one indicated in the identification key.
- 1a
- mucilaginous or compact groups of cells ......………………………....................................2
- 1b
- cells lacking mucilage..........................................................................................................10
- 2a
- irregular agglomerations of cells embedded in mucilage....................................................3
- 2b
- agglomerations of cells with a regular packet-like arrangement .....................................13
- 3a
- concentrically layered gelatinous sheaths around the cells (with distinct or indistinct lamellation).................................................................................................................................4
- 3b
- sheaths without clearly concentric lamellation.....................................................................6
- 4a
- ovoid to rod shaped cells, well delimitated and lamelled sheaths......................Gloeothece
- 4b
- spherical to hemispherical cell shape, with colorless or light yellow-brown sheaths.........................................................................................................................................5
- 5a
- sheaths wide and vesiculous………………………………….........………...……Gloeocapsa
- 5b
- sheaths thin and usually colorless, usually small colonies (2–16 cells, with the shape of a half or a quarter of sphere after division)......................................................Chroococcus
- 6a
- irregular colonies forming amorphous masses ....................................................................7
- 6b
- irregular colonies with their cells distributed in the mucilage............................................8
- 7a
- spherical cells............................................................................................................Microcystis
- 7b
- spherical or slightly elongated cells (oval, ovoid).............................................Aphanocapsa
- 8a
- ellipsoidal cells with homogeneous sheaths.......................................................Aphanothece
- 8b
- spherical cells.............................................................................................................................9
- 9a
- cells with an irregular-rounded to polygonal-rounded outline, and with the margin of the colony in a more or less radial row..................................................................Chlorogloea
- 9b
- cells more or less uniform in size in the colony...........................................Chroococcidiopsis
- 10a
- cells ovate to oblong, single or in small colonies.............................................................11
- 10b
- spherical and solitary cells...............................................................................Synechocystis
- 11a
- cells with a dimension of (1.5)3–15(40) × 0.4–3(6) μm ..............................Synechococcus
- 11b
- very small cells, up to 1.5 × 3 μm..................................................................Gloeobacter
- 12a
- agglomerations of cells with a more regular arrangement (cubical)..........Cyanosarcina
- 12b
- agglomerations of cells with a less regular arrangement ............................Myxosarcina
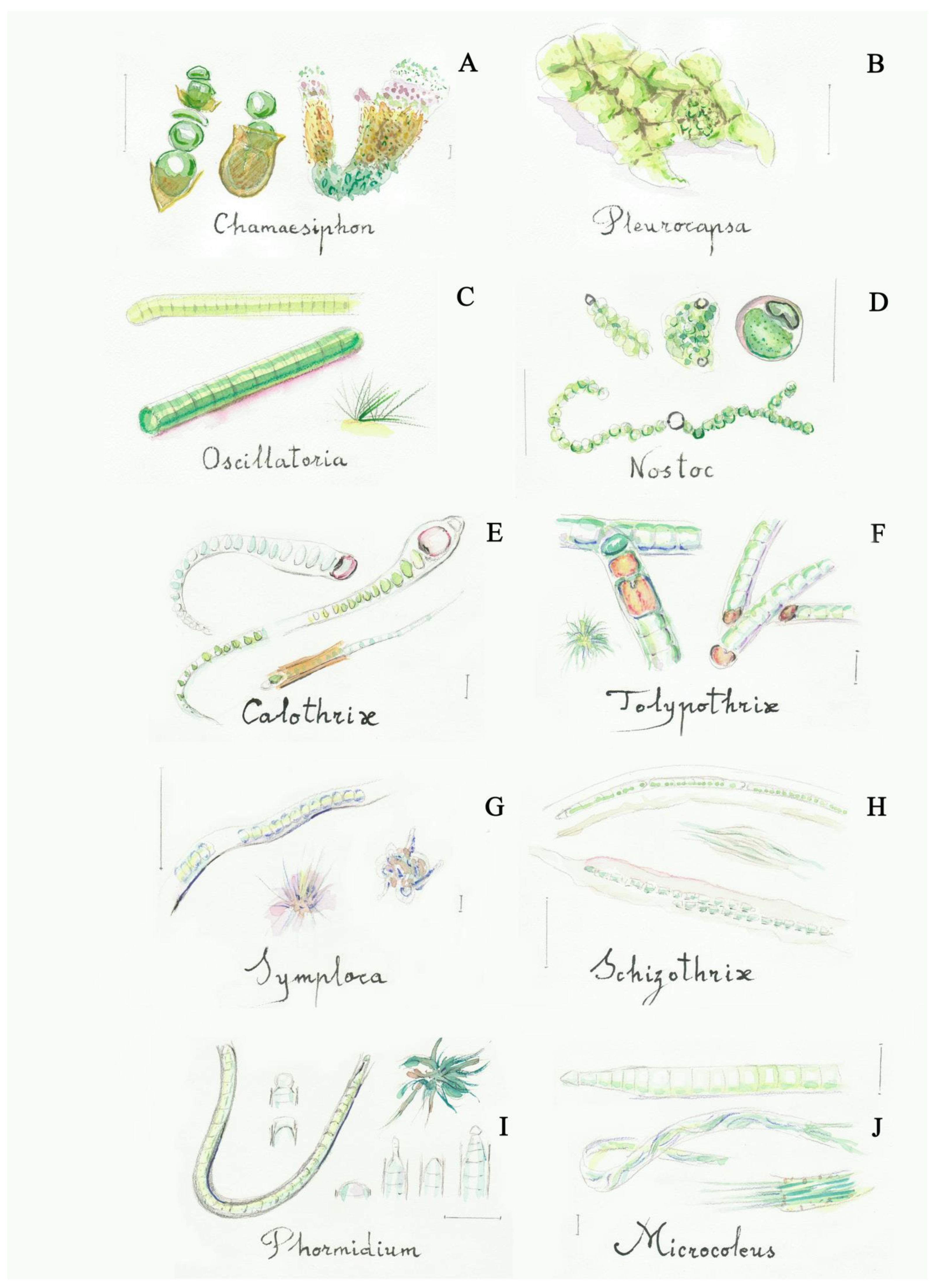
II. Filamentous cyanobacteria (linear groups of cells called trichomes, with one or more rows, surrounded or not by sheaths) (Figure 2, Figures S7 and S8 and [23,38])
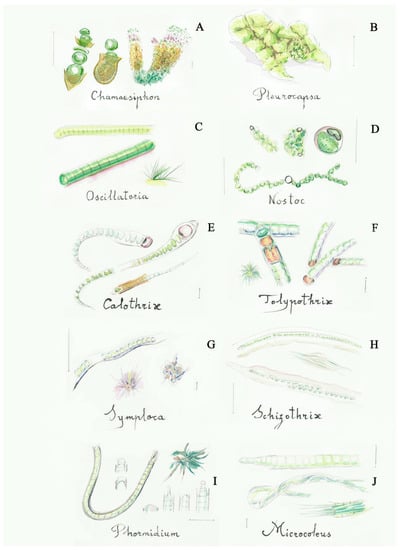
Figure 2.
Some filamentous cyanobacteria (blue-green algae): (A) Chamaesiphon (2a), (B) Pleurocapsa (2b), (C) Oscillatoria (5a), (D) Nostoc (6a), (E) Calothrix (9a), (F) Tolypothrix (10b), (G) Symploca (13b), (H) Schizothrix (14a), (I) Phormidium (16a), (J) Microcoleus (17a). Scale bar 10 μm. The number in the brackets corresponds to the one indicated in the identification key.
- 1a
- trichomes not clearly developed, compact groups of cells.....………….…....………..…..2
- 1b
- trichomes clearly developed, isolated or forming mats or pellicles.......……………....…3
- 2a
- short apical row of cells easily damaged, basal cells with colored sheath, forming macroscopic brown spots……….......................………........................……….Chamaesiphon
- 2b
- irregular groups of rows—pseudofilaments—built by polygonal cells...........Pleurocapsa
- 3a
- trichomes without sheath or with a very diffluent sheath..........................……………….4
- 3b
- trichomes with a visible sheath……………………….………..………….........…………....6
- 4a
- trichomes very short, up to 8 cells (rarely 16)................................................................Borzia
- 4b
- trichomes with a higher number of cells ................................................................................5
- 5a
- trichomes with very short cells, coin shaped and close together, visible moving filaments under the microscope …...............................................……..............….…Oscillatoria
- 5b
- trichomes with long cells more distant from one to another...……….....…Pseudanabaena
- 6a
- trichomes forming firm colonies with mucilage .........................................................Nostoc
- 6b
- trichomes forming compact clusters with a covering character .........................................7
- 7a
- presence of differentiated cells (heterocytes) in addition to the rest of the cells .............8
- 7b
- all cells with the same morphology, sometimes apical cells are differentiated...............11
- 8a
- heterocytes present at the base of polar filaments, filaments narrow into the shape of a hair...............................................................................................................................................9
- 8b
- heterocytes intercalated along the filaments, filaments not narrow.................................10
- 9a
- filaments with thin sheaths, no branching…............................................................Calothrix
- 9b
- filaments with wide sheaths, false-branching .......................................................Dichothrix
- 10a
- filament usually with rounded cells, no branching…………....……..….………..Nostoc
- 10b
- filaments with rectangular or squared cells and false branching, usually prostrated ……………………………………………………………................….............……Tolypothrix
- 11a
- one trichome within each sheath ......................................................................................12
- 11b
- several trichomes within each sheath ..............................................................................17
- 12a
- consistent sheaths with apical cells not differentiated, filaments can form mats.......13
- 12b
- mucilaginous sheaths with apical cells usually differentiated.....................................15
- 13a
- mats formed exclusively by prostrated filaments...........................................................14
- 13b
- mats formed by a base of prostrated filaments that form peripheral erect bundles with a conical shape, cells lack the calypra...............................................................Symploca
- 14a
- strongly intertwined filaments with false branching and wide sheaths, and sometimes more than one trichoma.................................................................................Schizothrix
- 14b
- very intertwined filaments forming less consistent mats, usually between mineral concretions......................................................................................................................Lyngbya
- 15a
- squared or slightly rectangular cells................................................................................16
- 15b
- cylindrical cells longer than wide, less than 3 µm in width, unbranched or with false branching............................................................................................................Leptolyngbya
- 16a
- filaments with no branching, sheath sometimes difficult to see..................Phormidium
- 16b
- filaments with false branching...............................................................Pseudophormidium
- 17a
- numerous trichomes within each sheath..........................................................Microcoleus
- 17b
- one or few trichomes within each sheath.........................................................................18
- 18a
- few-celled and short trichomes, constricted at cross walls, with a more or less cylindrical shape.....................................................................................................Pseudanabaena
- 18b
- trichomes in vegetative state always within distinct sheaths, filaments with typical “scytonematoid” (double and single) false branching, forming slimy mats.....Plectonema
3.3. Green Algae (Chlorophyta)
This division contains very different organisms regarding the morphological, dimensional and reproductive aspects. They have a typical vivid light green color, but the cells with starch will stain dark purple/brown/blue black in Lugol’s iodine solution (if used for preserving the sample). They contain chloroplasts (plastids) and can be divided into two main groups: I. simple green algae (and green flagellate) and II. filamentous green algae.
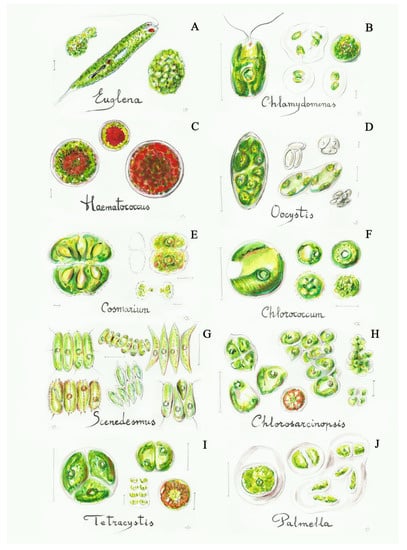
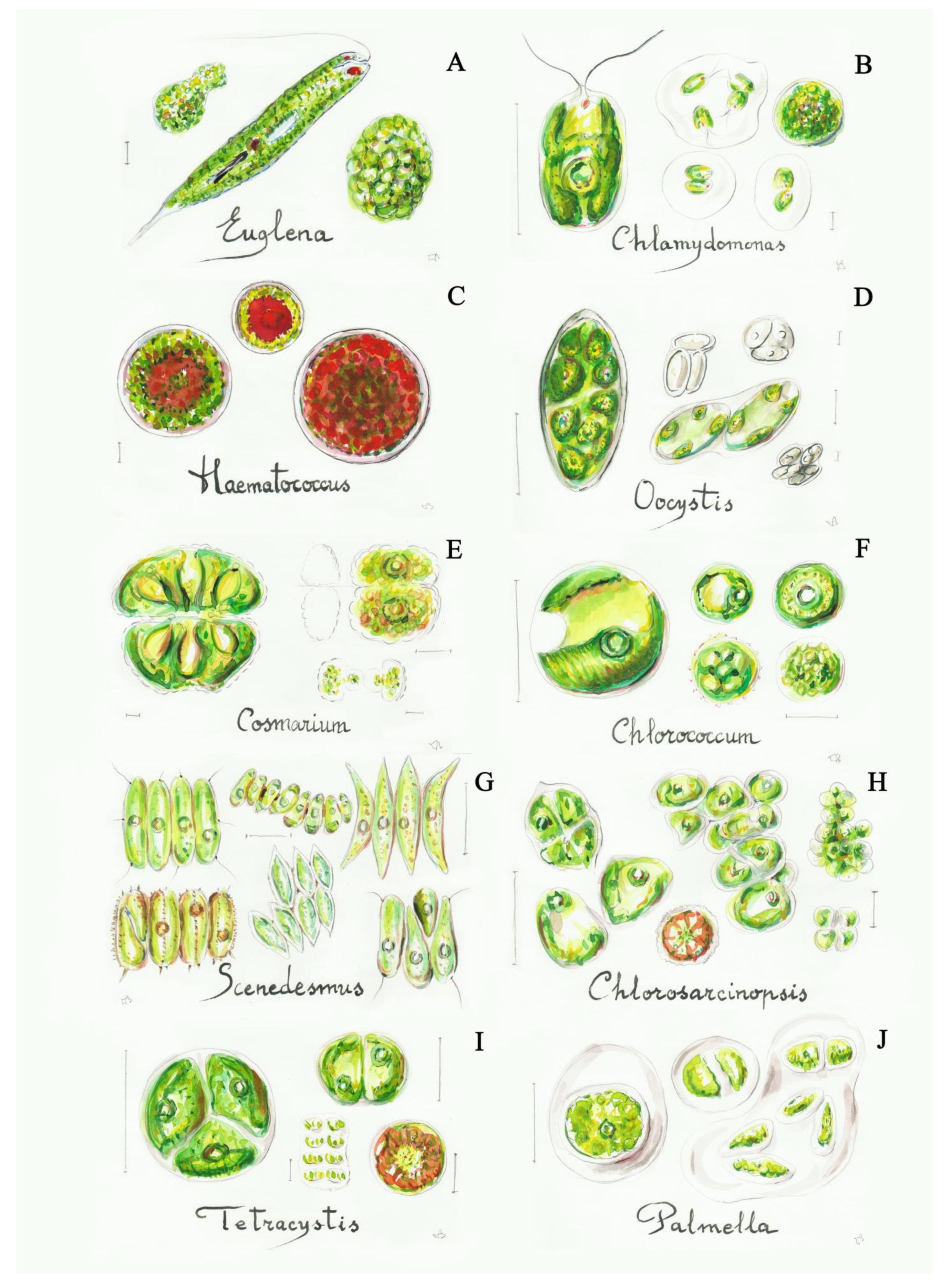
Figure 3.
Some simple green algae: (A) Euglena (3b), (B) Chlamydomonas (4a), (C) Haematococcus (4b), (D) Oocystis (8a), (E) Cosmarium (12a), (F) Chlorococcum (13b), (G) Scenedesmus (17b), (H) Chlorosarcinopsis (19a), (I) Tetracystis (19b), (J) Palmella (20a). Scale bar 10 μm. The number in the brackets corresponds to the one indicated in the identification key.
- 1a
- solitary cells................................................................................................................................2
- 1b
- cells forming colonies..............................................................................................................14
- 2a
- motile cells .................................................................................................................................3
- 2b
- non-motile cells..........................................................................................................................5
- 3a
- cells with two flagella and with a rigid cellular surface.......................................................4
- 3b
- cells with flagella but with elastic (deformable) cellular surface.............................Euglena
- 4a
- solitary cells with an oval or globular shape.................................................Chlamydomonas
- 4b
- cells with a more or less globular shape, with a red spot or totally red-colored in adverse conditions. ………………........................................................................Haematococcus
- 5a
- cells not closely grouped, more or less spherical or globular.............................................6
- 5b
- cells closely and continuously grouped...............................................................................14
- 6a
- elliptical, egg-shaped cells........................................................................................................7
- 6b
- spherical cells, isolated or arranged in unstructured groups ….........................................9
- 7a
- cells clearly elongated, fusiform…………………………………........…......Monoraphidium
- 7b
- cells are ellipsoidal or globular................................................................................................8
- 8a
- cells clearly elliptic, sometimes lemon-shaped, colonies delimitated by a parental wall..................................................................................................................................Oocystis
- 8b
- ovoid cells, almost globular, solitary or in irregular mucilaginous colonies...Coccomyxa
- 9a
- many parietal plastids inside the cell, without pyrenoids................................................10
- 9b
- few plastids inside the cell, usually with pyrenoids………..............................................11
- 10a
- solitary multinucleated large cells, maximum 85 μm in diameter............Bracteococcus
- 10b
- spherical cells with a diameter of 5–18 μm............................................................Muriella
- 11a
- spherical cells with a dimension of 2–25 μm, with a huge star-shaped plastid, usually as a lichen symbiont.............................................................................................Trebouxia
- 11b
- ellipsoidal, globular or sub-spherical cells......................................................................12
- 12a
- cells with a median constriction dividing them into two semi-cells.............Cosmarium
- 12b
- cells without constriction…………………………………………………….....………..13
- 13a
- cells with a cup- or plated-like plastid, maximum diameter of 20 μm..............Chlorella
- 13b
- spherical, sometimes ellipsoidal cells with a variable diameter inside the same population (from 10–15 to 100 μm), with the plastid filling the cell………………………………………...............................................................Chlorococcum
- 14a
- groups of cells that are close together……………...........................................................15
- 14b
- groups of usually separated cells forming mucilaginous structures...........................20
- 15a
- small and bidimensional groups of cells..........................................................................16
- 15b
- large groups of cells, not bidimensional ..........................................................................18
- 16a
- cells are arranged on a single layer, with a two-dimensional structure .....................17
- 16b
- cells are arranged in groups of four (sometimes in a clear gelatinous matrix) or isolated, with strip- or plated-like plastids …………….........…………...…….Pseudochlorella
- 17a
- cells are globular ...................................................................................................Planophila
- 17b
- cells are elliptical, spindle-shaped or pear-shaped, forming groups of 4–8 cells (or multiples of four), arranged linearly or in zig-zag ............................................Scenedesmus
- 18a
- cubical shape of the colony..............................................................................Chlorosarcina
- 18b
- non-cubic colonies……………………………………………...….......………………….19
- 19a
- cells are very compacted, forming angular-shaped colonies.............Chlorosarcinopsis
- 19b
- globular shape of the colony, with its cells isolated or in small groups (two or four cells) usually tetrahedron-like shaped....................................................................Tetracystis
- 20a
- globular or amorphous colonies, cells with a diameter of 10–15 μm.................Palmella
- 20b
- globular, tetrahedron-like or irregular shape of the colony, with a consistent gelatinous matrix and with every cell enclosed by a visible membrane, the diameter varies between 3 and 23 μm ...........................................................................................Gloeocystis
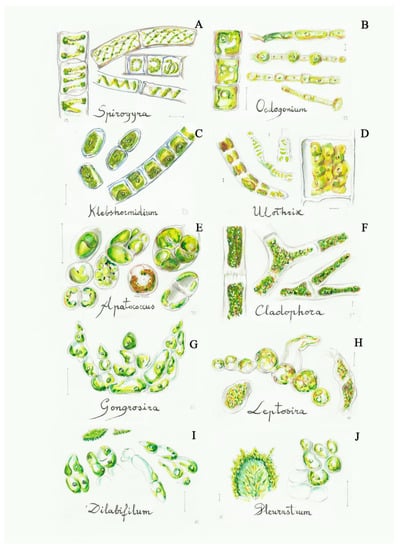
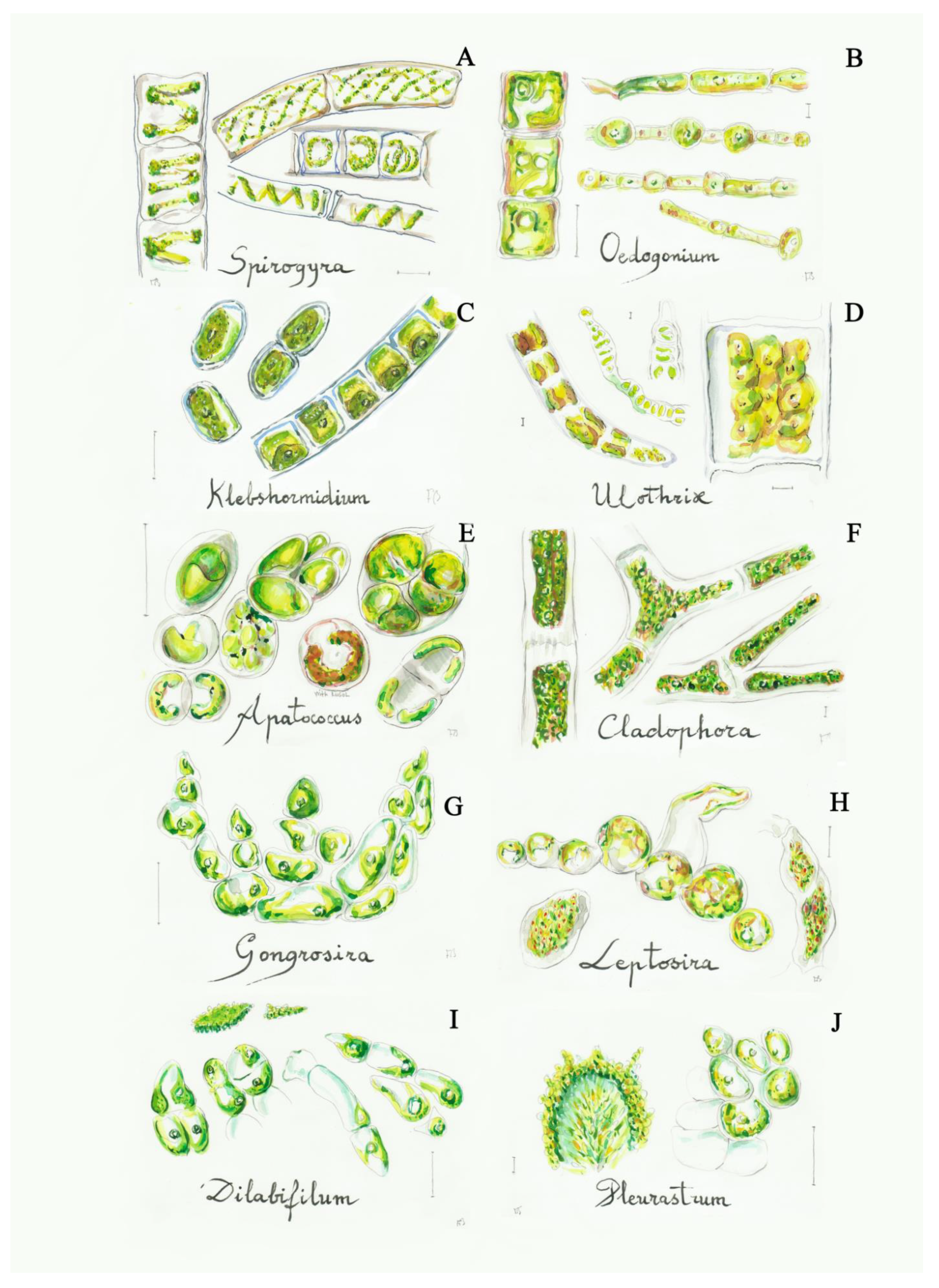
Figure 4.
Some filamentous green algae from the C2 group: (A) Spirogyra (2a), (B) Oedogonium (3a), (C) Klebsormidium (4b), (D) Ulothrix (4a), (E) Apatococcus (5a), (F) Cladophora (6a), (G) Gongrosira (7a), (H) Leptosira (8a), (I) Dilabifilum (9b), (J) Pleurastrum (9b). Scale bar 10 μm. The number in the brackets corresponds to the one indicated in the identification key.
- 1a
- filaments not branched..............................................................................................................2
- 1b
- filaments branched, sometimes forming very compact structures ....................................5
- 2a
- plastids with the shape of a spiral.............................................................................Spirogyra
- 2b
- plastids with another shape .....................................................................................................3
- 3a
- presence of polar rings and intercalary widened cells (oogonia) ….....…...…Oedogonium
- 3b
- all cells with similar morphology and without polar rings…………....………....……….4
- 4a
- long filaments fixed at their base, with a stable structure, annular plastids….....Ulothrix
- 4b
- filaments that disarticulate easily, usually short, annular or elliptic plastids, usually aerophytic..........................................................................................................Klebshormidium
- 5a
- short filaments forming packet-like colonies, sometimes cubical in the first stages, cells globular or elongated..............................................................................................Apatococcus
- 5b
- filaments clearly uniseriate, linear or branched....................................................................6
- 6a
- long filaments made of long cylindrical cells with laminated wall and reticulate plastids with numerous pyrenoids ……….…………….…….……....…..............…..Cladophora
- 6b
- short filaments or small groups of cells usually with a prostrate base and a small erect part, parietal plastids with few or no pyrenoids………………….…....……......................7
- 7a
- cylindrical or club-shaped cells with a blunt apex, usually tangled filaments, built crustose lime impregnated ......................................................................................Gongrosira
- 7b
- globular or cylindrical cells forming compact groups, sometimes without a clear filament structure............................................................................................................................8
- 8a
- globular cells, parietal plastids without pyrenoids, short filaments without branches or with short branches of 1–2 cells……...........................................................................Leptosira
- 8b
- cylindrical or globular cells, with pyrenoids, from irregular groups to small filaments.9
- 9a
- cylindrical-elongated cells with short branches ...................................................Dilabifilum
- 9b
- cylindrical or subglobular cells, forming both irregular and dense groups and branched filaments .................................................................................................Pleurastrum
3.4. Diatoms (Bacillariophyta)
They are unicellular reddish brown or golden algae (Figure 5, Figure S10 and [23,38]). The specific characteristic of this group is the presence of an external layer (the cell wall) made of silica that is called frustule. They are common on permanent wetted surfaces, and on monumental fountains the most encountered diatoms are those with bisymmetrical or monosymmetrical valves, one overlapping the other. The diatoms with radial symmetry are less common.
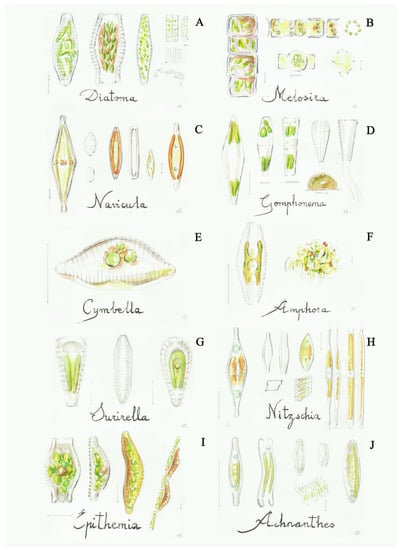
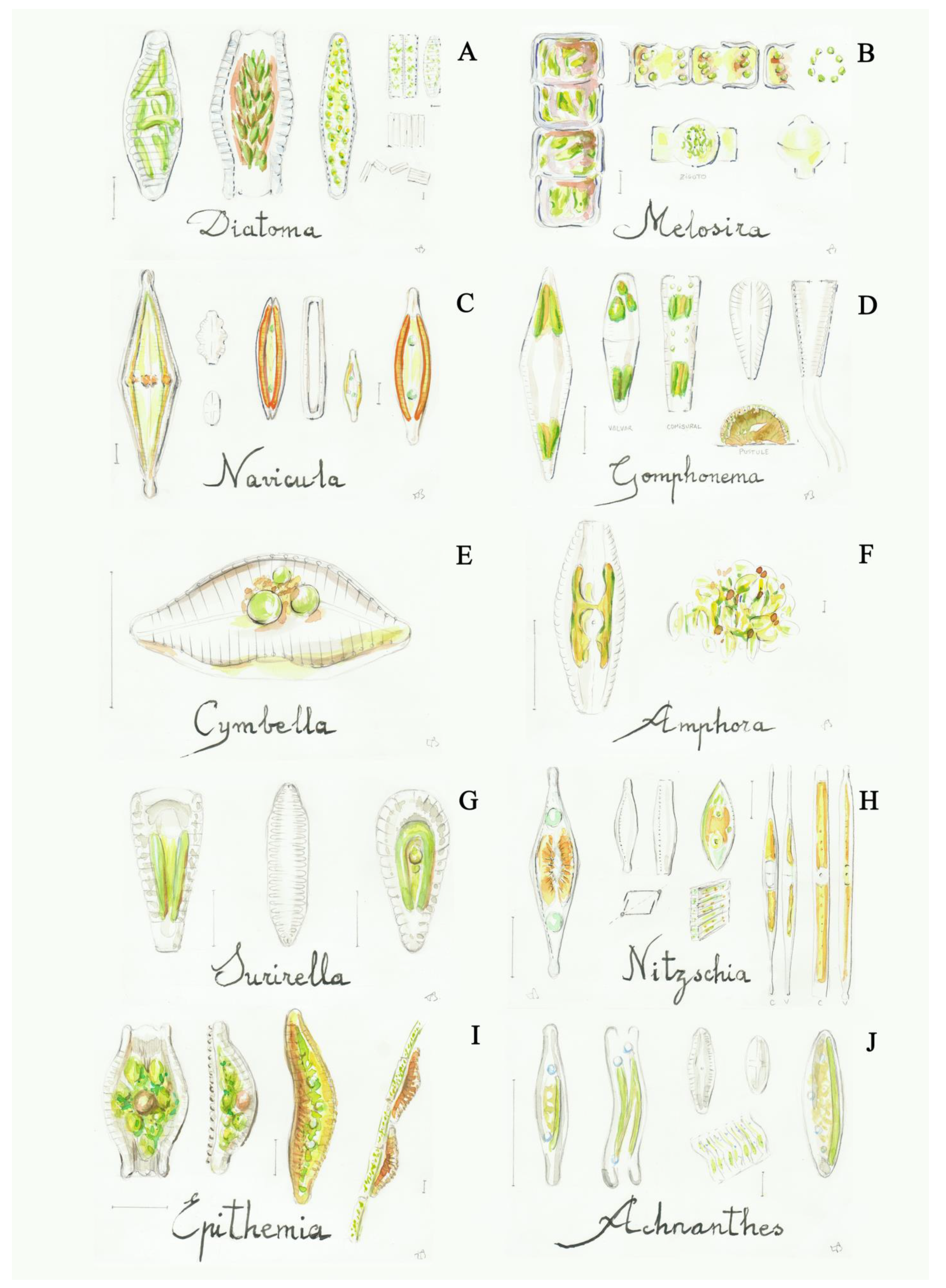
Figure 5.
Some diatoms: (A) Diatoma (2a), (B) Melosira (2b), (C) Navicula (8a), (D) Gomphonema (9b), (E) Cymbella (10a), (F) Amphora (10b), (G) Surirella (11b), (H) Nitzschia (12a), (I) Epithemia (12b), (J) Achnanthes (13a). Scale bar 10 μm. The number in the brackets corresponds to the one indicated in the identification key.
- 1a
- cells with a colonial organization, united in filament-like structures, numerous discoidal plastids.......................................................................................................................2
- 1b
- solitary cells, sometimes forming colonies, with valves of different shapes (elliptic, lanceolate, oblong, etc.), but never circular and with no radial symmetry, two lateral plastids........................................................................................................................................3
- 2a
- short cells, the filaments are easily separated in small groups of cells, valves symmetric both on the frontal and lateral faces, septa are lacking in the frustules .................Diatoma
- 2b
- long cells, valves with a circular shape (only visible in valvar view of isolated cells), the cells form filaments often found inside the shady fountains..................................Melosira
- 3a
- both valves with a raphe (structure composed by two slits or fissures), usually with a striation from the raphe to marginal part..............................................................................4
- 3b
- both valves without a raphe..................................................................................................14
- 4a
- the raphe is located in the middle of both valves.................................................................5
- 4b
- the raphe is located in a different part……..........................................................................12
- 5a
- the raphe is located on both valves.........................................................................................6
- 5b
- the raphe is located along the margin of the valves...........................................................11
- 6a
- bi-symmetric valves, central raphe.........................................................................................7
- 6b
- mono-symmetric valve, central or lateral raphe…………………………...........................9
- 7a
- striation narrow or not very wide that is not visible............................................................8
- 7b
- very wide striations, the raphe bent at both ends..................................................Pinnularia
- 8a
- thin striation, visible in dead cells, valve elliptical to boat-shaped.......................Navicula
- 8b
- very thin striations (horizontal and vertical), difficult to see even in dead cells.................................................................................................................................Frustulia
- 9a
- the valves are longitudinally asymmetric, raphe marginal...............................................10
- 9b
- the valves are transversely asymmetric, with the upper part broader than the lower one, with a trapezoidal girdle view…….............................................................Gomphonema
- 10a
- the valve margins are different, one convex and the other straight or slightly curved, raphe parallel to the straight margin and striations do not strongly radiate in the center .............................................................................................................................Cymbella
- 10b
- elliptic or oblong valves vision, intercalary bands and striations are imperceptible....................................................................................................................................Amphora
- 11b
- the raphe is present on two sides of each valve, with thick striation and a big central channel............................................................................................................................Surirella
- 11a
- the raphe is present on one side of each valve …………………..………………...…..13
- 12a
- hyaline valves, usually linear with lateral refractory dots (fibulae).........Nitzschia
- 12b
- the raphe forms a peak in the ventral part of the valve.....................................Epithemia
- 13a
- linear-elliptic valve, with a bent girdle view…………………..........................Achnantes
- 13b
- elliptic and almost circular valve, straight girdle view ………….……….…...Cocconeis
- 14a
- needle-shaped valves with parallel and thin striations, lacking septa (a silica internal sheet, occluding a portion of the frustula)..................................................Synedra (Ulnaria)
- 14b
- the frustules present septa, observed in girdle view, forming filaments........Tabellaria
4. Discussion
4.1. Fountains’ Phototrophic Biodiversity
Fountains are ideal environments for the rapid development of microorganisms, which induce the occurrence of biodeterioration processes and algal blooms that should be controlled in the case of artistic structures. Besides the bioreceptivity of the material and the characteristics of the environmental factors, the water origin is another element that highly influences the biodiversity of the colonizers on monumental fountains. The water source used for their alimentation can be driven from a public water line (e.g., mainly in the city centers) or can be directly supplied from a nearby river (e.g., in some gardens) [4,7,8,21,23,24,27]. The latter type of water source induces a greater biodeterioration risk for the monumental fountains.
A review of the main phototrophic microorganisms detected by various authors on this kind of artefact (Table 1) is presented in the Table 2, Table 3 and Table 4. Table 1 contains the legend of Table 1, Table 2 and Table 3. The microorganisms are divided in the following three groups: cyanobacteria (Table 2), green algae (Table 3) and diatoms (Table 4) and most of them are included in the identification keys. Very few studies have been undertaken on the molecular identification of the phototrophs dwelling on cultural fountain structures [23,27]. The identification to the species level needs axenic cultures of the isolated organisms, increasing the complexity of these types of analyses. Moreover, many of these phototrophs (mainly cyanobacteria) have a slow growth and their isolation is very time consuming. Thanks to the recent developments in the molecular biology domain, new techniques such as NGS (next-generation sequencing, called also high throughput sequencing—HTS) and metagenomics approaches give now valid and cost-effective solutions in the cultural heritage field [39,40]. The latter technique, called also community genomics, contributes with new insights for a better understanding of both the role and the structure of the microbial communities colonizing stone artefacts. However, these studies [23,27] confirmed the presence of various cosmopolitan phototrophic microorganisms with wide tolerance ranges concerning, for example, pH and temperatures. Many of these common genera (such as Calothrix, Phormidium, Oscillatoria, Cosmarium, Scenedesmus, Ulothrix, Navicula, Nitzchia, etc.) can be easily identified by their morphological characteristics by using fresh samples, a transmission microscope and an easy to use identification key. It should be noted that some genera can be easily confounded in between [41,42] and therefore it is important to pay attention to the minimal morphological differences (e.g., Gloeocapsa with Aphanocapsa, when the Gloeocapsa individual sheath cannot be distinguished in a colony; Aphanocapsa with Microcystis, as Aphanocapsa can be round-shaped as well; Cyanosarcina with Myxossarcina as the shapes of the single colonies in the agglomeration of cells are not always very clear; some small sized diatoms can be confused with Navicula or Nitzschia).
The phototrophic microorganisms form different types of biocenosis (biotic communities) and it can be stated that, almost always, the phototrophic growth will be a mix of different genera as can be usually observed when analyzing fresh samples (Figures S1–S5 Supplementary Materials), containing even other types of organisms such as bacteria or fungi. The stone support induces a certain bioreceptivity for the phototrophic colonization. Stone materials with higher porosity (travertine—fountain no. 7; sandstones—fountain no.3; cementitious materials—fountains no.4 and no.6; volcanic rocks—fountain no.1, see Table 1) are easily colonized by the phototrophic organisms with respect to the more compact stone materials (granite—fountain no. 5; marble—fountains no.5, no. 6, no.7 and no.15). The limestones and sandstones are more sensitive to biodeterioration with respect to granitic stones [43]. The common genera observed in fountains worldwide can have an epilithic growth forming mucous (Apatococcus, Phormidium) or fibrous mats (Cladophora, Gongrosira, Melosira, Pleurastrum). The existing fissures and cracks are colonized by chasmo-endoliths (mainly simple cyanobacteria and green algae) while the internal pores are colonized by cryptoendoliths (Chroococcidiopsis). Some genera (Chroococcidiopsis, Chroococcus, Gloeocapsa, Klebsormidium, Schizothrix, Synechococcus) can have an endolithic growth, actively penetrating inside the material, being therefore more harmful for the stone support [44,45].
Green algae and cyanobacteria present a greater diversity compared to diatoms when the number of most frequent genera in the three groups is analyzed (Table 2, Table 3 and Table 4). On the studied fountains, Navicula spp. and Nitzschia spp. are the most prevalent. Among cyanobacteria, the filamentous type, such as Calothrix spp., Leptolyngbya spp. and Phormidium spp., is the most common. These blue-green algae are able to form mats on their own or to be part of more diverse communities. The most common genera of green algae seem to vary more between countries than other groups of algae. Nevertheless, they are also cosmopolitan. Apatococcus, Chlorella, Chlorosarcinopsis, Stichococcus and Scenedesmus are the most frequent genera among all the countries. However, putting some differences in the distribution of the genera aside, it is apparent that the same types of microalgae colonize fountains all around the world. Therefore, a key that allows to identify them could be used internationally by restorers from any country.
Knowing the main types of phototrophic groups present on the monumental fountains helps to better understand the risk related to the biologically induced biodeterioration phenomena.
4.2. Phototrophic Control on the Fountains
Fountains need regular maintenance and restoration work [46] due both to material alterations and to the rapid colonization by phototrophic microorganisms that affects their esthetic value and induces biodeterioration processes.
Parameters such as the pH (an alkaline pH accelerates the algal growth, while an acidic one dissolves carbonatic stones and induces copper staining on stone), the calcium hardness (induces crust formations), the dissolved solids (organic and inorganic, favor biological growth and the clogging of pipes) and the temperature (air and water) are key elements in promoting or not the biodeterioration phenomena. Often, the water is recycled and chemically treated to avoid biological growth [47], but this only helps to postpone the biological development.
Different other strategies [48] can be used in controlling the biological development in the monumental fountains. One is related with the use of antifouling agents aiming to deter the biofilm formation [49,50,51]. They should be applied on cleaned surfaces in order to postpone biological colonization. These methods are still under investigations for defining practical effectiveness. Another strategy is related to the elimination of the biological growth with the help of biocides [52,53] and other chemical cleaners, such as chlorine. A new trend in controlling the unwanted phototrophic growth is the use of viruses [54]. However, the implementation of a management/maintenance plan (regular checks, periodic tests of water quality, protection from freeze–thaw damage, use of inert control treatments) is preferred, while knowing the phototrophic biodiversity (identified to the genera level by using these keys) helps in monitoring changes in the quality of water and in programming the interventions.
5. Conclusions
These identification keys could be a useful instrument for a better understanding of the phototrophic biodeteriogens present on the artistic fountains and other wet stone surfaces. These keys are indicative, and it is highly recommended to not assign a genera name to the observed microorganisms if the specific features are not observed, keeping the identification to the upper level (e.g., filamentous cyanobacteria with no clearly developed trichomes).
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/app11188787/s1, Figure S1: Microscopic observations of different samples taken from Sultana Fountain and North “Guitar” Fountain of the Court of the Myrtles, both in the Alhambra complex, Spain, Figure S2: Microscopic observations of different samples taken from Sultana Fountain and the Lions Fountain in the Alhambra complex, Spain, and from Villa la Pietra Fountain from Florence, Italy, Figure S3: Microscopic observations of different samples taken from Villa la Pietra Fountain from Florence and from different fountains from the Alhambra complex, Spain, Figure S4: Microscopic observations of different samples taken from Tacca Fountain from Florence, Italy, and from two fountains from the Alhambra complex in Spain, Figure S5: Microscopic observations of different samples taken from different fountains from the Alhambra complex, Spain, Figure S6: Microscopic observations of some isolated cyanobacteria from monumental fountains: Aphanocapsa (a), Aphanothece (b), Synechococcus (c), Gloeobacter (d), Chroococcus (e), and Gloeocapsa (f). Figure S7: Microscopic observations of some isolated cyanobacteria from monumental fountains: Leptolyngbya, that is thinner than Oscillatoria, presents sheath and lacks motility (a,b), Pseudoanabaena (c), Pseudophormidium (d), Calothrix (e,f). Figure S8: Microscopic observations of some isolated cyanobacteria from monumental fountains: Nostoc (a,b), Pleurocapsa (c), Rivularia (d). Figure S9: Microscopic observations of some isolated cyanobacteria from monumental fountains: Chlorella (a,b), Scenedesmus (c), Bracteococcus (d), Monoraphidium (e), Dilabifilum (f). Figure S10: Microscopic observations of some isolated cyanobacteria from monumental fountains: Diatoma (a), Nitzchia (b), Achnantes (c), Surirella (d).
Author Contributions
Conceptualization, F.B.-G., P.S.-C. and O.A.C.; methodology, F.B.-G., P.S.-C., O.A.C.; investigation, F.B.-G., P.S.-C., O.A.C., C.A.-R.; writing—review and editing, O.A.C., F.B.-G., P.S.-C. and C.A.-R.; supervision, O.A.C., F.B.-G. and P.S.-C.; project administration, F.B.-G.; funding acquisition, F.B.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the GOVERNMENT OF ANDALUSIA AND EUROPEAN REGIONAL DEVELOPMENT FUND, grant number A-HUM-279-UGR18 and P18-FR-4477 (FICOARTE regional project), and by the SPANISH MINISTRY OF ECONOMY AND COMPETITIVENESS, grant number PID2019.109713RB.100 (VIRARTE national project).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors would like to thank the Alhambra and Generalife Patronate (Granada, Spain), the Real Alcázar de Sevilla (Seville, Spain), the Smithsonian Institution (Washington DC, USA) and the Florence Municipality and Villa la Pietra (Florence, Italy) for their assistance in collecting samples, the Ligalismo Foundation for its ongoing dissemination activity, and the Vice-Rectorate for Research and Knowledge Transfer of the University of Granada for their support and the creation of the unit of excellence Ciencia en la Alhambra.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Juuti, P.S.; Antoniou, G.P.; Dragoni, W.; El-Gohary, F.; De Feo, G.; Katko, T.S.; Rajala, R.P.; Zheng, X.Y.; Drusiani, R.; Angelakis, A.N. Short Global History of Fountains. Water 2015, 7, 2314–2348. [Google Scholar] [CrossRef]
- Tomaselli, L.; Pietrini, A.M. Alghe e cianobatteri. In La Biologia Vegetale per i Beni Culturali. Biodeterioramento e Conservazione; Caneva, G., Nugari, M.P., Salvadori, O., Eds.; Nardini Editore: Firenze, Italy, 2007; Volume 1, pp. 71–77. [Google Scholar]
- Gattuso, C.; Cozza, R.; Gattuso, P.; Villella, F. La Conoscenza Per il Restauro e la Conservazione. Il Ninfeo di Vadue a Carolei e la Fontana Nuova di Lamezia Terme; Franco Angeli S.R.L.: Milano, Italy, 2012; p. 75. [Google Scholar]
- Altieri, A.; Pietrini, A.M.; Ricci, S.; Roccardi, A. Il Controllo del Biodeterioramento. In Il Restauro della Fontana del Fuga nell’Orto Botanico di Roma; Micheli, M.P., Tammeo, G., Eds.; Gangemi Editore Spa: Roma, Italy, 2011; pp. 111–117. [Google Scholar]
- Villalba Corredor, L.S.; Malagón Forero, A. Biodeterioro de la Fuente de Lavapatas, parque arqueológico de San Agustín-Huila. Colombia. Ge-Conserv. 2011, 2, 65–80. [Google Scholar] [CrossRef][Green Version]
- Sánchez-Castillo, P.M.; Bolivar-Galiano, F.C. Characterización de comunidades algales epilìticas en fuentes monumentales y aplicacion a la diagnosis del biodeterioro. Limnetica 1997, 13, 31–46. [Google Scholar]
- Not, R.; Miceli, G.; Terranova, F.; Catalisano, A.; Lo Campo, P. Indagini sulla natura biotica delle alterazioni. In Fontana Pretoria—Studi Per un Progetto di Restauro. Regione Siciliana—Assessorato dei Beni Culturali e Ambientali e della Pubblica Istruzione, Centro Regionale Per la Progettazione e il Restauro e Per le Scienze Naturali ed Applicate ai Beni Culturali, Palermo; Catalisano, A., Di Natale, R., Gallo, E., Vergara, F., Eds.; Arti Grafiche Siciliane: Palermo, Italy, 1996; pp. 51–64. [Google Scholar]
- Hamadeh, S. Splash and Spectacle: The Obsession with Fountains in Eighteenth-Century Istanbul. Muqarnas Online 2002, 19, 123. [Google Scholar] [CrossRef]
- Guillaud, H. Socio-Cultural Sustainability in Vernacular Architecture. Versus: Heritage for Tomorrow; Lotti, L., Ed.; Firenze University Press: Firenze, Italy, 2014; pp. 48–55. [Google Scholar]
- Delgado-Rodrigues, J. Stone patina. A controversial concept of relevant importance in conservation. In Proceedings of International Seminar Theory and Practice in Conservation-A Tribute to Cesare Brandi, Lisbon, Portugal, 4–5 May 2006; Delgado Rodrigues, J., Mimoso, J.-M., Eds.; LNEC Editor: Lisbon, Portugal, 2006; pp. 163–174. [Google Scholar]
- Bolivar-Galiano, F.C.; Sánchez-Castillo, P.M. Claves de identificación de microalgas frecuentes en monumentos. PH: Boletín Del Inst. Andal. Del Patrim. Histórico 1999, 7, 93–98. [Google Scholar] [CrossRef]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota Chroococcales. In Süsswasserflora von Mitteleuropa; Ettl, H., Gärtner, G., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer Verlag, JenaStuttgart-Lübeck-Ulm: Jena, Germany, 1998; p. 548. [Google Scholar]
- Biggs, B.J.F.; Kilroy, C. Identification guide to common periphyton in New Zealand streams and rivers. In Stream Periphyton Monitoring Manual, Prepared for The New Zealand Ministry for the Environment; NIWA: Christchurch, New Zealand, 2000; pp. 121–210. [Google Scholar]
- Komárek, J. Coccoid and Colonial Cyanobacteria. In Freshwater Algae of North America: Ecology and Classification; Wehr, J.D., Sheath, R.G., Eds.; Academic Press: Cambridge, MA, USA, 2003; pp. 59–116. [Google Scholar]
- B-Komárek, J.; Komárková, J.; Kling, H. Filamentous cyanobacteria. Freshwater Algae of North America: Ecology and Classification, Wehr , J.D., Sheath, R.G., Eds.; Academic Press: Cambridge, MA, USA, 2003; 117–196. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota. 2. Teil: Oscillatoriales. In Süsswasserflora von Mitteleuropa; Büdel, B., Krienitz, L., Gärtner, G., Schagerl, M., Eds.; Springer Spektrum: Heidelberg, Germany, 2005; pp. 1–759. [Google Scholar]
- Komárek, J. Cyanoprokaryota. Heterocytous genera. In Süswasserflora von Mitteleuropa/Freshwater Flora of Central Europe; Büdel, B., Gärtner, G., Krienitz, L., Schagerl, M., Eds.; Springer Spektrum: Berlin/Heidelberg, Germany, 2013; p. 1130. [Google Scholar]
- Komárek, J.; Johansen, J.R. Filamentous Cyanobacteria. In Freshwater Algae of North America, 2nd ed; Academic Press: Cambridge, MA, USA, 2015; pp. 135–235. [Google Scholar] [CrossRef]
- Komárek, J.; Johansen, J. Coccoid Cyanobacteria. In Freshwater Algae of North America, 2nd ed; Academic Press: Cambridge, MA, USA, 2015; pp. 75–133. [Google Scholar] [CrossRef]
- Bourrelly, P. Diatomophycées. In Les Alguesd’eaudouce. Initiation à la Systématique. Tome II: Les Algues Jaunes et Brunes-Chrysophyycées, Phéophycées, Xanthophycées et Diatomées; Boubée, N., Ed.; Cie: Paris, France, 1968; pp. 259–399. [Google Scholar]
- Bolívar-Galiano, F.; Abad-Ruiz, C.; Sánchez-Castillo, P.; Toscano, M.; Romero-Noguera, J. Frequent Microalgae in the Fountains of the Alhambra and Generalife: Identification and Creation of a Culture Collection. Appl. Sci. 2020, 10, 6603. [Google Scholar] [CrossRef]
- Bolívar-Galiano, F.C.; Abad-Ruiz, C.; Yebra, A.; Romero-Noguera, J.; Sánchez-Castillo, P. Chromatic alterations by microalgae at National Mall fountains in Washington DC (USA). In Proceedings of the 6th International Conference on Heritage and Sustainable Development, Granada, Spain, 12–15 June 2018, Granada, Spain; EUG Green Lines Institute for Sustainable Development: Barcelos, Portugal, 2018; Volume 2, pp. 1211–1218. [Google Scholar]
- Cuzman, O.A.; Ventura, S.; Sili, C.; Mascalchi, C.; Turchetti, T.; D’Acqui, L.P.; Tiano, P. Biodiversity of Phototrophic Biofilms Dwelling on Monumental Fountains. Microb. Ecol. 2010, 60, 81–95. [Google Scholar] [CrossRef]
- Zurita, Y.P.; Cultrone, G.; Castillo, P.S.; Sebastián, E.; Bolívar, F. Microalgae associated with deteriorated stonework of the fountain of Bibatauín in Granada, Spain. Int. Biodeterior. Biodegrad. 2005, 55, 55–61. [Google Scholar] [CrossRef]
- Peraza Zurita, Y. Biodeterioro por Microalgas en Fuentes de Mármol. Ph.D. Thesis, University of Granada Departamento de Pintura, Granada, Spain, 2004. [Google Scholar]
- Bolívar-Galiano, F.C.; Sánchez-Castillo, P.M. Biodeterioro del patrimonio artístico por cianobacterias, algas verdes y diatomeas. Idea PH Boletìn 1998, 24, 52–63. [Google Scholar] [CrossRef]
- De Natale, A.; Mele, B.H.; Cennamo, P.; Del Mondo, A.; Petraretti, M.; Pollio, A. Microbial biofilm community structure and composition on the lithic substrates of Herculaneum Suburban Baths. PLoS ONE 2020, 15, e0232512. [Google Scholar] [CrossRef] [PubMed]
- Pietrini, A.M. La microflora fotosintetica. In Il restauro della Fontana del Fuga nell’Orto Botanico di Roma; Micheli, M.P., Tammeo, G., Eds.; Gangemi Editore Spa: Roma, Italy, 2011; pp. 107–111. [Google Scholar]
- Sarró, M.I.; Garcia, A.M.; Rivalta, V.M.; Moreno, D.A.; Arroyo, I. Biodeterioration of the Lions Fountain at the Alhambra Palace, Granada (Spain). Build. Environ. 2006, 41, 1811–1820. [Google Scholar] [CrossRef]
- Hindáková, A.; Hindác, F. Green algae of five city fountains in Bratislava (Slovakia). Biol. Bratisl. 1998, 53, 481–493. [Google Scholar]
- Nugari, M.P.; Pietrini, A.M. Trevi Fountain: An evaluation of inhibition effect of water-repellents on cyanobacteria and algae. Int. Biodeterior. Biodegrad. 1997, 40, 247–253. [Google Scholar] [CrossRef]
- Gandhi, H.P. Notes on the diatomaceae from Ahmedabad and its environs ? VI. On some diatoms from fountain reservoir of Seth Sarabhai’s garden. Hydrobiology 1967, 30, 248–272. [Google Scholar] [CrossRef]
- Chorus, I.; Cavalieri, M. Cyanobacteria and algae. In Monitoring Bathing Waters—A practical Guide to the Design and Implementation of Assessments and Monitoring Programme; Bartram, J., Rees, G., Eds.; CRC Press: London, UK, 2000; pp. 219–272. Available online: https://www.who.int/water_sanitation_health/bathing/bathwatchap10.pdf (accessed on 28 August 2021).
- Trobajo, R.; Mann, D.G. A rapid cleaning method for diatoms. Diatom Res. 2019, 34, 115–124. [Google Scholar] [CrossRef]
- Franchini, W. The Collecting, Cleaning, and Mounting of Diatoms. Available online: https://www.mccrone.com/mm/the-collecting-cleaning-and-mounting-of-diatoms/ (accessed on 30 August 2021).
- Popović, S.; Nikolić, N.; Jovanović, J.; Predojević, D.; Trbojević, I.; Manić, L.; Subakov Simić, G. Cyanobacterial and algal abundance and biomass in cave biofilms and relation to environmental and biofilm parameters. Int. J. Speleol. 2019, 48, 49–61. [Google Scholar] [CrossRef]
- Bolívar-Galiano, F.C. Diagnosis y Tratamiento del Deterioro por Microalgas en los Palacios Nazaries de la Alhambra. Ph.D. Thesis, Universidad de Granada, Granada, Spain, 1994; pp. 193–218. Available online: https://digibug.ugr.es/ (accessed on 1 September 2021).
- Phyco Key, A List of Helpful Texts and Websites with Excellent Images; Baker, A.L., Ed.; University of Hampshire: Hampshire, UK; Available online: http://cfb.unh.edu/phycokey/Choices/Text_html/groups/genera.htm (accessed on 1 September 2021).
- Mihajlovski, A.; Seyer, D.; Benamara, H.; Bousta, F.; Di Martino, P. An overview of techniques for the characterization and quantification of microbial colonization on stone monuments. Ann. Microbiol. 2015, 65, 1243–1255. [Google Scholar] [CrossRef]
- Piñar, G.; Sterflinger, K. Natural sciences at the service of art and cultural heritage: An interdisciplinary area in development and important challenges. Microb. Biotechnol. 2021, 14, 806–809. [Google Scholar] [CrossRef]
- Pandey, D.C.; Mitra, A.K. On the morphology and life-history of a form of gloeocapsa showing some new stages. Hydrobiologia 1966, 27, 379–384. [Google Scholar] [CrossRef]
- Absalón, I.B.; Muñoz-Martín, M.A.; Montejano, G.; Mateo, P. Differences in the Cyanobacterial Community Composition of Biocrusts from the Drylands of Central Mexico. Are There Endemic Species? Front. Microbiol. 2019, 10, 937. [Google Scholar] [CrossRef]
- Charola, A.E.; Wendler, E. An Overview of the Water-Porous Building Materials Interactions. Restor. Build. Monum. 2015, 21, 55–65. [Google Scholar] [CrossRef]
- Gaylarde, C.; Gaylarde, P.M.; Neilan, B. Endolithic Phototrophs in Built and Natural Stone. Curr. Microbiol. 2012, 65, 183–188. [Google Scholar] [CrossRef]
- Cuzman O., A.; Tiano, P.; Ventura, S.; Frediani, P. Biodiversity on Stone Artifacts. In The Importance of Biological Interactions in the Study of Biodiversity; IntechOpen: Rijeka, Croatia, 2011; pp. 367–390. [Google Scholar]
- Conference on Conservation of Fountains, organized by The National Center for Preservation Technology and Training, in partnership with The Nelson-Atkins Museum of Art and conservator Martin Burke. Kansas City, MO, USA, 10–11 July 2013; Available online: https://www.ncptt.nps.gov/blog/fountain-fundamentals/ (accessed on 10 July 2021).
- Krueger, R. A new approach to maintaining water features and reducing biological growth. In Objects Specialty Group Postprints; Riccardelli, C., Del Re, C., Eds.; The American Institute for Conservation of Historic & Artistic Works: Washington, DC, USA, 2010; Volume 17, pp. 33–39. [Google Scholar]
- Salvadori, O.; Charola, A.E. Methods to Prevent Biocolonization and recolonization: An overview of current research for architectural and archaeological heritage. In Biocolonization of Stone: Control and Preventive Methods Proceedings from the MCI Workshop Series; Charola, A.E., McNamara, C., Koestler, R.J., Eds.; Smithsonian Institution Scholarly Press: Washington, DC, USA, 2009; pp. 37–50. [Google Scholar]
- Lo Schiavo, S.; De Leo, F.; Urzì, C. Present and Future Perspectives for Biocides and Antifouling Products for Stone-Built Cultural Heritage: Ionic Liquids as a Challenging Alternative. Appl. Sci. 2020, 10, 6568. [Google Scholar] [CrossRef]
- Cuzman, O.A.; Camaiti, M.; Sacchi, B.; Tiano, P. Natural antibiofouling agents as new control method for phototrophic biofilms dwelling on monumental stone surfaces. Int. J. Conserv. Sci. 2011, 2, 3–16. [Google Scholar]
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology—past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Pandolfi, A.; Capponi, G.; Fazio, G. The restoration of historical fountains. In Restoring in Italy—Art and Technology in the Activities of the Istituto Superiore per la Conservazione ed Il Restauro; Gangemi Editore: Rome, Italy, 2012; pp. 87–94. [Google Scholar]
- Gaylarde, C.; Morton, L.H.G. Deteriogenic biofilms on buildings and their control: A review. Biofouling 1999, 14, 59–74. [Google Scholar] [CrossRef]
- May, E.; Zamarreño, D.; Hotchkiss, S.; Mitchell, J.; Inkpen, R. Bioremediation of Algal Contamination on Stone. In Biocolonization of Stone: Control and Preventive Methods Proceedings from the MCI Workshop Series; Charola, A.E., McNamara, C., Koestler, R.J., Eds.; Smithsonian Institution Scholarly Press: Washington, DC, USA, 2009; pp. 59–70. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).