The Role and Molecular Mechanism of P2Y12 Receptors in the Pathogenesis of Atherosclerotic Cardiovascular Diseases
Abstract
:1. Introduction
2. P2Y12 Receptor and Fluid Shear Stress
3. P2Y12 and Thrombosis
- Inhibiting adenylate cyclase, reducing the concentration of cyclic adenosine monophosphate (cAMP), thereby increasing the free Ca2+ concentration, inactivating the cyclic adenosine monophosphate-dependent protein kinase and promoting platelet aggregation (first-phase gathering).
- The first-phase aggregation reaction can activate PI3K kinase and promote the release of related substances (ADP, serotonin, etc.) in platelets, resulting in continuous and stable platelet aggregation (second-phase aggregation).
- Activating phosphatidylinositol-3 kinase, serine-threonine protein kinase B and rapid guanosine triphosphate (GTP) binding protein, promote the exposure of the active site of platelet GPIIb/IIIa receptor binding to fibrinogen and promote platelet fibrin cross-linking and aggregation between platelets [43,44].These interactions are facilitated by the release of the intracellular tether of GP IIb/IIIa, possibly via release from cytoskeletal actin components (the so-called inside-out signaling), which allows the extracellular domains of the GP IIb/IIIa receptor complex to expose multiple binding sites for fibrinogen and, also, for the von Willebrand factor [45]. Therefore, the P2Y12 receptor plays a vital role in platelet activation and aggregation. Furthermore, platelet aggregation may affect the infiltration of monocytes into the infarcted myocardium and influence the prognosis, which is contributed by P2Y12 [46]. The unique feature of P2Y12 may mediate platelet independent responses, especially in enhanced thrombin formation, such as local vascular injury and the rupture of atherosclerotic plaques [47]. Although this new biological signal is associated with long-term functional outcomes, the corresponding cellular substrates remain unclear.
4. P2Y12 Receptor and Atherosclerosis
5. Discussion and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wang, Y.X.; Shi, Y.H.; Gong, L.H.; Li, Y.; Heng, W.J.; You, J.F.; Zhong, H.H.; Fang, W.G. P2Y purinergic receptor activated PI-3K/Akt signaling pathway in regulation of growth and invasion of prostatic cancer. Zhonghua Bing Li Xue Za Zhi 2007, 36, 681–686. [Google Scholar] [PubMed]
- Von Kügelgen, I. Molecular pharmacology of P2Y receptor subtypes. Biochem. Pharmacol. 2021, 187, 114361. [Google Scholar] [CrossRef] [PubMed]
- Chiarito, M.; Sanz-Sánchez, J.; Cannata, F.; Cao, D.; Stefanini, G.G. Monotherapy with a P2Y12 inhibitor or aspirin for secondary prevention in patients with established atherosclerosis: A systematic review and meta-analysis. Lancet 2020, 395, 1487–1495. [Google Scholar] [CrossRef]
- Pokorski, M. Rehabilitation Science in Context Volume 1096||Psychological Determinants of Attitude Toward Euthanasia: A Comparative Study of Female Nurses and Female Nonmedical Professionals. Adv. Exp. Med. Biol. 2018, 93–103. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic Signaling in the Cardiovascular System. Circ. Res. 2017, 120, 207–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Xu, D.; Hou, K.; Gou, X.; Li, Y. The role of P2Y12 receptor inhibition in ischemic stroke on microglia, platelets and vascular smooth muscle cells. J. Thromb. Thrombolysis 2020, 50, 874–885. [Google Scholar] [CrossRef]
- Pereira, N.L.; Farkouh, M.E.; So, D.; Lennon, R.; Geller, N.; Mathew, V.; Bell, M.; Bae, J.H.; Jeong, M.; Chavez, H. Effect of Genotype-Guided Oral P2Y12 Inhibitor Selection vs Conventional Clopidogrel Therapy on Ischemic Outcomes After Percutaneous Coronary Intervention: The TAILOR-PCI Randomized Clinical Trial. JAMA 2020, 324, 761–771. [Google Scholar] [CrossRef]
- Kim, H.J.; Oh, J.S.; Park, S.Q.; Yoon, S.M.; Ahn, H.S.; Kim, B.T. The Efficacy of P2Y12 Reactive Unit to Predict the Periprocedural Thromboembolic and Hemorrhagic Complications According to Clopidogrel Responsiveness and Safety of Modification of Dual Antiplatelet Therapy: A Meta-Analysis. J. Korean Neurosurg. Soc. 2019, 63, 539–549. [Google Scholar] [CrossRef]
- Baldetti, L.; Melillo, F.; Moroni, F.; Gallone, G.; Pagnesi, M.; Venuti, A.; Beneduce, A.; Calvo, F.; Gramegna, M.; Godino, C.; et al. Meta-Analysis Comparing P2Y12 Inhibitors in Acute Coronary Syndrome. Am. J. Cardiol. 2020, 125, 1815–1822. [Google Scholar] [CrossRef]
- Roka-Moiia, Y.; Walk, R.; Palomares, D.E.; Ammann, K.R.; Slepian, M.J. Platelet activation via shear stress exposure induces a differing pattern of biomarkers of activation versus biochemical agonists. Thromb. Haemost. 2020, 120, 776–792. [Google Scholar] [CrossRef]
- Nesbitt, W.S.; Westein, E.; Tovar-Lopez, F.J.; Tolouei, E.; Mitchell, A.; Fu, J.; Carberry, J.; Fouras, A.; Jackson, S.P. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat. Med. 2009, 15, 665–673. [Google Scholar] [CrossRef]
- Maxwell, M.J.; Westein, E.; Nesbitt, W.S.; Giuliano, S.; Jackson, S.P.J.B. Identification of a 2-stage platelet aggregation process mediating shear-dependent thrombus formation. Blood 2007, 109, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, Z.M.; Orje, J.N.; Habermann, R.; Federici, A.B.; Reininger, A.J. Activation-independent platelet adhesion and aggregation under elevated shear stress. Blood 2006, 108, 1903–1910. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Topol, E.J. Scientific and therapeutic advances in antiplatelet therapy. Nat. Rev. Drug Discov. 2003, 2, 15–28. [Google Scholar] [CrossRef]
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002, 324, 71–86. [Google Scholar] [CrossRef] [Green Version]
- Muniz-Lozano, A.; Rollini, F.; Franchi, F.; Angiolillo, D.J. Update on platelet glycoprotein IIb/IIIa inhibitors: Recommendations for clinical practice. Ther. Adv. Cardiovasc. Dis. 2013, 7, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Storey, R.F.; Newby, L.J.; Heptinstall, S. Effects of P2Y(1) and P2Y(12) receptor antagonists on platelet aggregation induced by different agonists in human whole blood. Platelets 2001, 12, 443–447. [Google Scholar] [CrossRef]
- Franchi, F.; Angiolillo, D.J. Novel antiplatelet agents in acute coronary syndrome. Nat. Rev. Cardiol. 2015, 12, 30–47. [Google Scholar] [CrossRef]
- Pi, S.; Mao, L.; Chen, J.; Shi, H.; Liu, Y.; Guo, X.; Li, Y.; Zhou, L.; He, H.; Yu, C.; et al. The p2ry12 receptor promotes vsmc-derived foam cell formation by inhibiting autophagy in advanced atherosclerosis. Autophagy 2021, 17, 980–1000. [Google Scholar] [CrossRef]
- Griffin, G.K.; Newton, G.; Tarrio, M.L.; Bu, D.X.; Maganto-Garcia, E.; Azcutia, V.; Alcaide, P.; Grabie, N.; Luscinskas, F.W.; Croce, K.J.; et al. IL-17 and TNF-alpha sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J. Immunol. 2012, 188, 6287–6299. [Google Scholar] [CrossRef]
- Giachini, F.R.; Leite, R.; Osmond, D.A.; Lima, V.V.; Inscho, E.W.; Webb, R.C.; Tostes, R.C. Anti-platelet therapy with clopidogrel prevents endothelial dysfunction and vascular remodeling in aortas from hypertensive rats. PLoS ONE 2014, 9, e91890. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, N.; Kawasaki, K.; Nejime, N.; Kubota, Y.; Nakamura, K.; Kunitomo, M.; Takahashi, K.; Hashimoto, M.; Shinozuka, K. P2Y receptor-mediated Ca2+ signaling increases human vascular endothelial cell permeability. J. Pharmacol. Sci. 2004, 95, 174–180. [Google Scholar] [CrossRef] [Green Version]
- Barnhill, R.L.; Ryan, T.J. Biochemical modulation of angiogenesis in the chorioallantoic membrane of the chick embryo. J. Investig. Dermatol. 1983, 81, 485–488. [Google Scholar] [CrossRef] [Green Version]
- Teuscher, E.; Weidlich, V. Adenosine nucleotides, adenosine and adenine as angiogenesis factors. Biomed. Biochim. Acta 1985, 44, 493–495. [Google Scholar]
- Shen, J.; DiCorleto, P.E. ADP stimulates human endothelial cell migration via P2Y1 nucleotide receptor-mediated mitogen-activated protein kinase pathways. Circ. Res. 2008, 102, 448–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maloney, J.P.; Silliman, C.C.; Ambruso, D.R.; Wang, J.; Tuder, R.M.; Voelkel, N.F. In vitro release of vascular endothelial growth factor during platelet aggregation. Am. J. Physiol. 1998, 275, H1054–H1061. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Elliott, S.N.; Cirino, G.; Buret, A.; Ignarro, L.J.; Wallace, J.L. Platelets modulate gastric ulcer healing: Role of endostatin and vascular endothelial growth factor release. Proc. Natl. Acad. Sci. USA 2001, 98, 6470–6475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, J.; Arthur, J.F.; Gardiner, E.E.; Andrews, R.K.; Zeng, L.; Xu, K. Regulation of platelet activation and thrombus formation by reactive oxygen species. Redox. Biol. 2018, 14, 126–130. [Google Scholar] [CrossRef]
- Chen, J.; Chung, D.W. Inflammation, von Willebrand factor, and ADAMTS13. Blood 2018, 132, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, J.; Ishihara, A.; Starke, R.D.; Peghaire, C.R.; Smith, K.E.; McKinnon, T.A.; Tabata, Y.; Sasaki, K.; White, M.J.V.; Fukunaga, K.; et al. The heparin binding domain of von Willebrand factor binds to growth factors and promotes angiogenesis in wound healing. Blood 2019, 133, 2559–2569. [Google Scholar] [CrossRef]
- Malehmir, M.; Pfister, D.; Gallage, S.; Szydlowska, M.; Inverso, D.; Kotsiliti, E.; Leone, V.; Peiseler, M.; Surewaard, B.G.J.; Rath, D.; et al. Platelet GPIbα is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat. Med. 2019, 25, 641–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, L.J.; Stegmeyer, R.I.; Schäfer, K.; Volkery, S.; Currie, S.M.; Kempe, B.; Nottebaum, A.F.; Vestweber, D. Platelets docking to VWF prevent leaks during leukocyte extravasation by stimulating Tie-2. Blood 2020, 136, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Hanke, J.; Ranke, C.; Perego, E.; Köster, S. Human blood platelets contract in perpendicular direction to shear flow. Soft Matter 2019, 15, 2009–2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veyradier, A. A new drug for an old concept: Aptamer to von Willebrand factor for prevention of arterial and microvascular thrombosis. Haematologica 2020, 105, 2512–2515. [Google Scholar] [CrossRef]
- Wu, L.; Zhao, F.; Dai, M.; Li, H.; Chen, C.; Nie, J.; Wang, P.; Wang, D.W. P2Y12 Receptor Promotes Pressure Overload-Induced Cardiac Remodeling via Platelet-Driven Inflammation in Mice. Hypertension 2017, 70, 759–769. [Google Scholar] [CrossRef]
- Sokol, J.; Skerenova, M.; Ivankova, J.; Simurda, T.; Stasko, J. Association of Genetic Variability in Selected Genes in Patients With Deep Vein Thrombosis and Platelet Hyperaggregability. Clin. Appl. Thromb. Hemost. 2018, 24, 1027–1032. [Google Scholar] [CrossRef] [Green Version]
- Ni, H.; Denis, C.V.; Subbarao, S.; Degen, J.L.; Sato, T.N.; Hynes, R.O.; Wagner, D.D. Persistence of platelet thrombus formation in arterioles of mice lacking both von Willebrand factor and fibrinogen. J. Clin. Investig. 2000, 106, 385–392. [Google Scholar] [CrossRef] [Green Version]
- Becker, R.C.; Gurbel, P.A. Platelet P2Y12 receptor antagonist pharmacokinetics and pharmacodynamics: A foundation for distinguishing mechanisms of bleeding and anticipated risk for platelet-directed therapies. Thromb. Haemost. 2010, 103, 535–544. [Google Scholar] [CrossRef] [Green Version]
- Yagmur, E.; Bast, E.; Mühlfeld, A.S.; Koch, A.; Weiskirchen, R.; Tacke, F.; Neulen, J. High Prevalence of Sticky Platelet Syndrome in Patients with Infertility and Pregnancy Loss. J. Clin. Med. 2019, 8, 1328. [Google Scholar] [CrossRef] [Green Version]
- Angiolillo, D.J.; Rollini, F.; Storey, R.F.; Bhatt, D.L.; James, S.; Schneider, D.J.; Sibbing, D.; So, D.Y.F.; Trenk, D.; Alexopoulos, D.; et al. International Expert Consensus on Switching Platelet P2Y12 Receptor-Inhibiting Therapies. Circulation 2017, 136, 1955–1975. [Google Scholar] [CrossRef]
- Rollini, F.; Franchi, F.; Angiolillo, D.J. Switching P2Y12-receptor inhibitors in patients with coronary artery disease. Nat. Rev. Cardiol. 2016, 13, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Grosdidier, C.; Blanz, K.D.; Deharo, P.; Bernot, D.; Poggi, M.; Bastelica, D.; Wolf, D.; Duerschmied, D.; Grino, M.; Cuisset, T.; et al. Platelet CD40 ligand and bleeding during P2Y12 inhibitor treatment in acute coronary syndrome. Res. Pract. Thromb. Haemost. 2019, 3, 684–694. [Google Scholar] [CrossRef] [Green Version]
- Kossmann, S.; Lagrange, J.; Jackel, S.; Jurk, K.; Ehlken, M.; Schonfelder, T.; Weihert, Y.; Knorr, M.; Brandt, M.; Xia, N.; et al. Platelet-localized FXI promotes a vascular coagulation-inflammatory circuit in arterial hypertension. Sci. Transl. Med. 2017, 9, eaah4923. [Google Scholar] [CrossRef] [PubMed]
- Amelirad, A.; Shamsasenjan, K.; Akbarzadehlaleh, P.; Pashoutan Sarvar, D. Signaling Pathways of Receptors Involved in Platelet Activation and Shedding of These Receptors in Stored Platelets. Adv. Pharm. Bull. 2019, 9, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Simurda, T.; Brunclikova, M.; Asselta, R.; Caccia, S.; Zolkova, J.; Kolkova, Z.; Loderer, D.; Skornova, I.; Hudecek, J.; Lasabova, Z.; et al. Genetic Variants in the FGB and FGG Genes Mapping in the Beta and Gamma Nodules of the Fibrinogen Molecule in Congenital Quantitative Fibrinogen Disorders Associated with a Thrombotic Phenotype. Int. J. Mol. Sci. 2020, 21, 4616. [Google Scholar] [CrossRef]
- Wrigley, B.J.; Shantsila, E.; Tapp, L.D.; Lip, G.Y. Increased formation of monocyte-platelet aggregates in ischemic heart failure. Circ. Heart Fail. 2013, 6, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Rauch, B.H.; Rosenkranz, A.C.; Ermler, S.; Bohm, A.; Driessen, J.; Fischer, J.W.; Sugidachi, A.; Jakubowski, J.A.; Schror, K.J.A.T.V.B. Regulation of Functionally Active P2Y12 ADP Receptors by Thrombin in Human Smooth Muscle Cells and the Presence of P2Y12 in Carotid Artery Lesions. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2434–2442. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.M.; Moroi, M. Platelet collagen receptor integrin alpha2beta1 activation involves differential participation of ADP-receptor subtypes P2Y1 and P2Y12 but not intracellular calcium change. Eur. J. Biochem. 2001, 268, 3513–3522. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, J.; Gao, Z.G.; Zhang, D.; Zhu, L.; Han, G.W.; Moss, S.M.; Paoletta, S.; Kiselev, E.; Lu, W.; et al. Structure of the human P2Y12 receptor in complex with an antithrombotic drug. Nature 2014, 509, 115–118. [Google Scholar] [CrossRef] [Green Version]
- Isfort, K.; Ebert, F.; Bornhorst, J.; Sargin, S.; Kardakaris, R.; Pasparakis, M.; Bahler, M.; Schwerdtle, T.; Schwab, A.; Hanley, P.J. Real-time imaging reveals that P2Y2 and P2Y12 receptor agonists are not chemoattractants and macrophage chemotaxis to complement C5a is phosphatidylinositol 3-kinase (PI3K)- and p38 mitogen-activated protein kinase (MAPK)-independent. J. Biol. Chem. 2011, 286, 44776–44787. [Google Scholar] [CrossRef] [Green Version]
- Shan, J.; Zhang, B.; Zhu, Y.; Jiao, B.; Zheng, W.; Qi, X.; Gong, Y.; Yuan, F.; Lv, F.; Sun, H. Overcoming clopidogrel resistance: Discovery of vicagrel as a highly potent and orally bioavailable antiplatelet agent. J. Med. Chem. 2012, 55, 3342–3352. [Google Scholar] [CrossRef]
- Pereira, N.L.; Rihal, C.S.; So, D.Y.F.; Rosenberg, Y.; Lennon, R.J.; Mathew, V.; Goodman, S.G.; Weinshilboum, R.M.; Wang, L.; Baudhuin, L.M.; et al. Clopidogrel Pharmacogenetics. Circ. Cardiovasc. Interv. 2019, 12, e007811. [Google Scholar] [CrossRef]
- Nijenhuis, V.J.; Brouwer, J.; Delewi, R.; Hermanides, R.S.; Holvoet, W.; Dubois, C.L.F.; Frambach, P.; De Bruyne, B.; van Houwelingen, G.K.; Van Der Heyden, J.A.S.; et al. Anticoagulation with or without Clopidogrel after Transcatheter Aortic-Valve Implantation. N. Engl. J. Med. 2020, 382, 1696–1707. [Google Scholar] [CrossRef]
- Johnston, S.C.; Amarenco, P.; Denison, H.; Evans, S.R.; Himmelmann, A.; James, S.; Knutsson, M.; Ladenvall, P.; Molina, C.A.; Wang, Y.; et al. Ticagrelor and Aspirin or Aspirin Alone in Acute Ischemic Stroke or TIA. N. Engl. J. Med. 2020, 383, 207–217. [Google Scholar] [CrossRef]
- Mehran, R.; Baber, U.; Sharma, S.K.; Cohen, D.J.; Angiolillo, D.J.; Briguori, C.; Cha, J.Y.; Collier, T.; Dangas, G.; Dudek, D.; et al. Ticagrelor with or without Aspirin in High-Risk Patients after PCI. N. Engl. J. Med. 2019, 381, 2032–2042. [Google Scholar] [CrossRef] [PubMed]
- Tomaniak, M.; Chichareon, P.; Onuma, Y.; Deliargyris, E.N.; Takahashi, K.; Kogame, N.; Modolo, R.; Chang, C.C.; Rademaker-Havinga, T.; Storey, R.F.; et al. Benefit and Risks of Aspirin in Addition to Ticagrelor in Acute Coronary Syndromes: A Post Hoc Analysis of the Randomized GLOBAL LEADERS Trial. JAMA Cardiol. 2019, 4, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Schupke, S.; Neumann, F.J.; Menichelli, M.; Mayer, K.; Bernlochner, I.; Wohrle, J.; Richardt, G.; Liebetrau, C.; Witzenbichler, B.; Antoniucci, D.; et al. Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2019, 381, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Gimbel, M.; Qaderdan, K.; Willemsen, L.; Hermanides, R.; Bergmeijer, T.; de Vrey, E.; Heestermans, T.; Tjon Joe Gin, M.; Waalewijn, R.; Hofma, S.; et al. Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome (POPular AGE): The randomised, open-label, non-inferiority trial. Lancet 2020, 395, 1374–1381. [Google Scholar] [CrossRef]
- Cesaro, A.; Taglialatela, V.; Gragnano, F.; Moscarella, E.; Fimiani, F.; Conte, M.; Barletta, V.; Monda, E.; Limongelli, G.; Severino, S.; et al. Low-Dose Ticagrelor in Patients With High Ischemic Risk and Previous Myocardial Infarction: A Multicenter Prospective Real-World Observational Study. J. Cardiovasc. Pharmacol. 2020, 76, 173–180. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Bhatt, D.L.; Cohen, M.; Steg, P.G.; Storey, R.F.; Jensen, E.C.; Magnani, G.; Bansilal, S.; Fish, M.P.; Im, K.; et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N. Engl. J. Med. 2015, 372, 1791–1800. [Google Scholar] [CrossRef] [Green Version]
- Bangalore, S. Prasugrel in the Elderly. Circulation 2018, 137, 2446–2449. [Google Scholar] [CrossRef]
- Kong, D.; Xue, T.; Guo, B.; Cheng, J.; Liu, S.; Wei, J.; Lu, Z.; Liu, H.; Gong, G.; Lan, T.; et al. Optimization of P2Y12 Antagonist Ethyl 6-(4-((Benzylsulfonyl)carbamoyl)piperidin-1-yl)-5-cyano-2-methylnicotinate (AZD1283) Led to the Discovery of an Oral Antiplatelet Agent with Improved Druglike Properties. J. Med. Chem. 2019, 62, 3088–3106. [Google Scholar] [CrossRef]
- Macfarlane, D.E.; Srivastava, P.C.; Mills, D.C. 2-Methylthioadenosine[beta-32P]diphosphate. An agonist and radioligand for the receptor that inhibits the accumulation of cyclic AMP in intact blood platelets. J. Clin. Investig. 1983, 71, 420–428. [Google Scholar] [CrossRef] [Green Version]
- Kimura, M.; Nishi, K.; Higashikawa, A.; Ohyama, S.; Sakurai, K.; Tazaki, M.; Shibukawa, Y. High pH-Sensitive Store-Operated Ca(2+) Entry Mediated by Ca2+ Release-Activated Ca2+ Channels in Rat Odontoblasts. Front. Physiol. 2018, 9, 443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guarracino, J.F.; Cinalli, A.R.; Fernandez, V.; Roquel, L.I.; Losavio, A.S. P2Y13 receptors mediate presynaptic inhibition of acetylcholine release induced by adenine nucleotides at the mouse neuromuscular junction. Neuroscience 2016, 326, 31–44. [Google Scholar] [CrossRef]
- Bhattad, V.B.; Gaddam, S.; Lassiter, M.A.; Jagadish, P.S.; Ardeshna, D.; Cave, B.; Khouzam, R.N. Intravenous cangrelor as a peri-procedural bridge with applied uses in ischemic events. Ann. Transl. Med. 2019, 7, 408. [Google Scholar] [CrossRef] [PubMed]
- Zhan, T.; Wei, T.; Dong, L.; Wang, Q.; Wu, Z.; Yan, Q.; Zhang, W.; Lu, Y.; Wu, M. Cangrelor alleviates bleomycin-induced pulmonary fibrosis by inhibiting platelet activation in mice. Mol. Immunol. 2020, 120, 83–92. [Google Scholar] [CrossRef]
- Beko, K.; Kovanyi, B.; Goloncser, F.; Horvath, G.; Denes, A.; Kornyei, Z.; Botz, B.; Helyes, Z.; Muller, C.E.; Sperlagh, B. Contribution of platelet P2Y12 receptors to chronic Complete Freund’s adjuvant-induced inflammatory pain. J. Thromb. Haemost. 2017, 15, 1223–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libby, P.; Loscalzo, J.; Ridker, P.; Farkouh, M.E.; Hsue, P.Y.; Fuster, V.; Hasan, A.A.; Amar, S. Inflammation, Immunity, and Infection in Atherothrombosis: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2018, 72, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Noels, H.; Weber, C. Fractalkine as an important target of aspirin in the prevention of atherogenesis: Editorial to: “Aspirin inhibits fractalkine expression in atherosclerotic plaques and reduces atherosclerosis in ApoE gene knockout mice” by H. Liu et al. Cardiovasc. Drugs Ther. 2010, 24, 1–3. [Google Scholar] [CrossRef]
- Sim, D.S.; Jeong, M.H.; Kim, H.S.; Gwon, H.C.; Seung, K.B.; Rha, S.W.; Chae, S.C.; Kim, C.J.; Cha, K.S.; Park, J.S.; et al. Association of potent P2Y12 blockers with ischemic and bleeding outcomes in non-ST-segment elevation myocardial infarction. J. Cardiol. 2019, 73, 142–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, T.J.; Schlegel, M.; Zhou, F.; Gorenchtein, M.; Berger, J.S. Platelet regulation of myeloid suppressor of cytokine signaling 3 accelerates atherosclerosis. Sci. Transl. Med. 2019, 11, eaax0481. [Google Scholar] [CrossRef] [PubMed]
- Nergiz-Unal, R.; Cosemans, J.M.; Feijge, M.A.; van der Meijden, P.E.; Storey, R.F.; van Giezen, J.J.; oude Egbrink, M.G.; Heemskerk, J.W.; Kuijpers, M.J. Stabilizing role of platelet P2Y(12) receptors in shear-dependent thrombus formation on ruptured plaques. PLoS ONE 2010, 5, e10130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Held, P.; Himmelmann, A.; Ditmarsch, M. Ticagrelor for the treatment of atherosclerotic disease: Insights from the PARTHENON clinical development program. Future Cardiol. 2016, 12, 405–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- West, L.E.; Steiner, T.; Judge, H.M.; Francis, S.E.; Storey, R.F. Vessel wall, not platelet, P2Y12 potentiates early atherogenesis. Cardiovasc. Res. 2014, 102, 429–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wihlborg, A.K.; Wang, L.; Braun, O.O.; Eyjolfsson, A.; Gustafsson, R.; Gudbjartsson, T.; Erlinge, D. ADP receptor P2Y12 is expressed in vascular smooth muscle cells and stimulates contraction in human blood vessels. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1810–1815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, X.; Pi, S.L.; Baral, S.; Xia, Y.P.; He, Q.W.; Li, Y.N.; Jin, H.J.; Li, M.; Wang, M.D.; Mao, L.; et al. P2Y12 Promotes Migration of Vascular Smooth Muscle Cells Through Cofilin Dephosphorylation During Atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 515–524. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Wang, Y.; Zhang, L.; Luo, X.; Li, J.; Chen, X.; Niu, H.; Wang, K.; Sun, Y.; Wang, X.; et al. Roles of purinergic receptor P2Y, G protein-coupled 12 in the development of atherosclerosis in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2012, 32, e81–e89. [Google Scholar] [CrossRef] [Green Version]
- Cavelier, C.; Ohnsorg, P.M.; Rohrer, L.; von Eckardstein, A. The beta-chain of cell surface F(0)F(1) ATPase modulates apoA-I and HDL transcytosis through aortic endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Burger, P.C.; Wagner, D.D. Platelet P-selectin facilitates atherosclerotic lesion development. Blood 2003, 101, 2661–2666. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.J.; Clare, R.M.; Gao, R.; Held, C.; Himmelmann, A.; James, S.K.; Lim, S.T.; Santoso, A.; Yu, C.M.; Wallentin, L.; et al. Ticagrelor versus clopidogrel in Asian patients with acute coronary syndrome: A retrospective analysis from the Platelet Inhibition and Patient Outcomes (PLATO) Trial. Am. Heart J. 2015, 169, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.; Konrad, I.; Sauer, S.; Orschiedt, L.; Koellnberger, M.; Lorenz, R.; Walter, U.; Massberg, S. Effect of chronic treatment with acetylsalicylic acid and clopidogrel on atheroprogression and atherothrombosis in ApoE-deficient mice in vivo. Thromb. Haemost. 2008, 99, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Jager, B.; Stojkovic, S.; Haller, P.M.; Piackova, E.; Kahl, B.S.; Andric, T.; Vargas, K.G.; Wojta, J.; Huber, K. Course of platelet miRNAs after cessation of P2Y12 antagonists. Eur. J. Clin. Investig. 2019, 49, e13149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.M.; Liu, Y.; Li, T.T.; Piao, C.M.; Liu, O.; Liu, J.L.; Qi, Y.F.; Jia, L.X.; Du, J. Sustained activation of ADP/P2ry12 signaling induces SMC senescence contributing to thoracic aortic aneurysm/dissection. J. Mol. Cell. Cardiol. 2016, 99, 76–86. [Google Scholar] [CrossRef]
- Cicha, I.; Yilmaz, A.; Suzuki, Y.; Maeda, N.; Daniel, W.G.; Goppelt-Struebe, M.; Garlichs, C.D. Connective tissue growth factor is released from platelets under high shear stress and is differentially expressed in endothelium along atherosclerotic plaques. Clin. Hemorheol. Microcirc. 2006, 35, 203–206. [Google Scholar]
- Tabas, I.; Garcia-Cardena, G.; Owens, G.K. Recent insights into the cellular biology of atherosclerosis. J. Cell Biol. 2015, 209, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Gachet, C. P2Y(12) receptors in platelets and other hematopoietic and non-hematopoietic cells. Purinergic Signal 2012, 8, 609–619. [Google Scholar] [CrossRef]
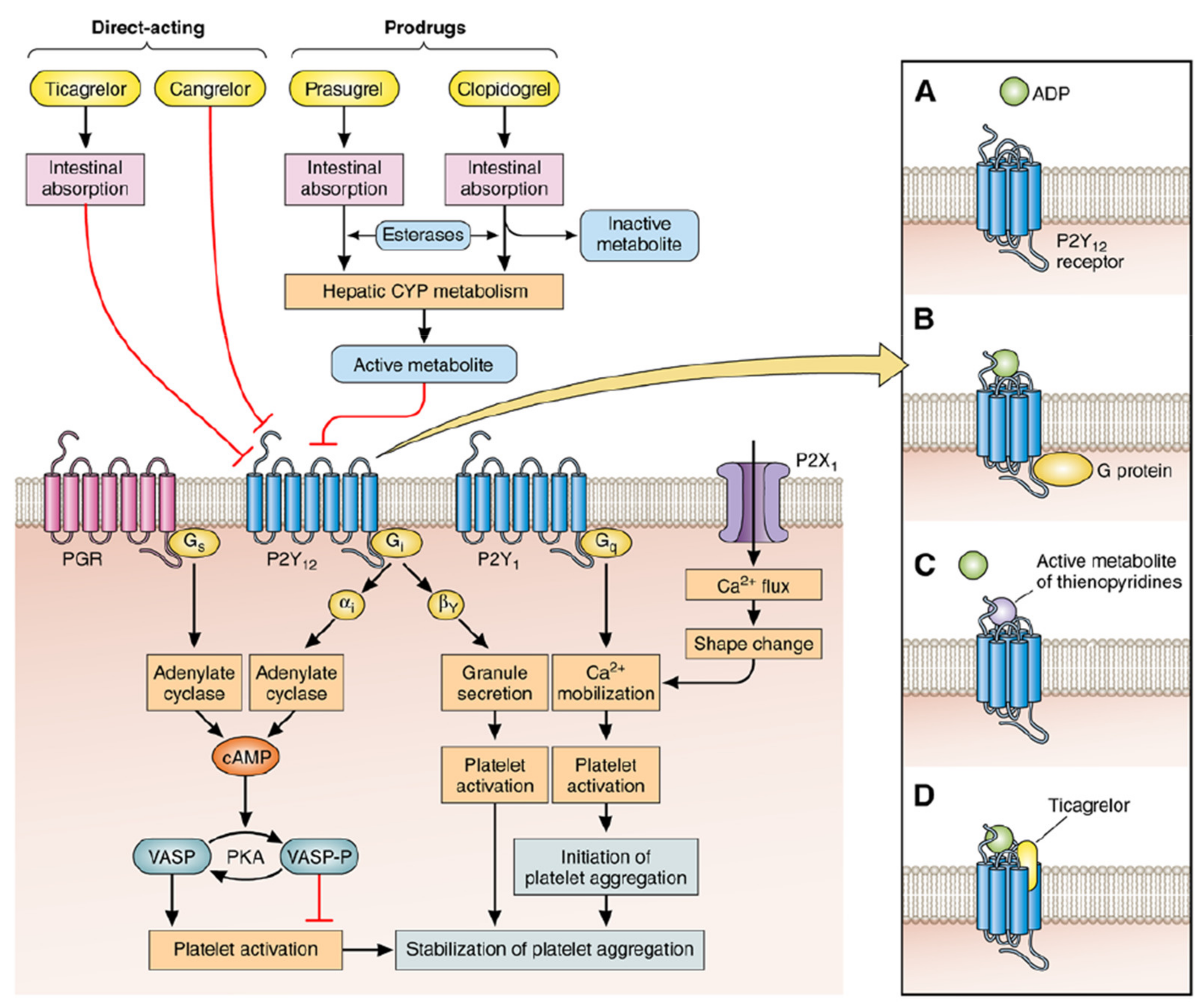
| P2Y12 Inhibitors | The Targets Functions of Drugs | References |
|---|---|---|
| Clopidogrel | Clopidogrel is a P2Y12 receptor inhibitor which selectively inhibits the binding of ADP to platelet receptors, the activation of ADP-mediated glycoprotein GPIIb/IIIa complex, and platelet aggregation. It is used to prevent and treat heart, brain and other arterial circulatory disorders caused by high platelet aggregation, such as recent strokes, myocardial infarctions and confirmed peripheral arterial diseases. | [50,51,52] |
| Prasugrel | Prasugrel is a new orally effective thienopyridine drug. The P2Y12 adenosine diphosphate receptor on platelets can be irreversibly inhibited after the cytochrome P450 enzyme system is metabolized to the active metabolite. Prasugrel has a higher conversion rate of prodrug to active metabolites and higher bioavailability, so it takes effect faster and can reduce the difference in efficacy between individuals, and reduces major ischemic cardiovascular events to a greater extent Incidence rate. | [53,54,55] |
| Ticagrelor | Ticagrelor is an oral P2Y12 receptor antagonist, which is a new type of cyclopentyl triazole pyrimidine oral antiplatelet drug. Ticagrelor promotes a greater inhibition of adenosine 5′-diphosphate (ADP)-induced Ca2+ release in shed platelets compared with other P2Y12R antagonists. Studies have also shown that ticagrelor is significantly better than clopidogrel. In clinical trial, 2 different dosages, 90 twice daily and 60 mg once a day of ticagrelor both showed reduced risk of cardiovascular death. | [56,57,58,59,60] |
| AZD1283 | The P2Y12 receptor inhibitor AZD1283 induces an increasing in blood flow and inhibits ADP-induced platelet aggregation, with antithrombotic ED50 values of 3.0 and 10 μg/kg/min. | [61] |
| 2-Methylthioadenosine diphosphate trisodium | 2-Methylthioadenosine diphosphate trisodium is a potent purinergic P2Y receptor agonist, 2-methylthioadenosine diphosphate trisodium induces platelet aggregation and shape change, and inhibits cyclic AMP accumulation in platelets exposed to prostaglandin E1. | [62,63,64] |
| Cangrelor tetrasodium | An adenosine triphosphate analogue, a reversible and selective platelet P2Y12 antagonist, has a rapid and effective antiplatelet effect. Cangrelor tetrasodium directly blocks adenosine diphosphate (ADP)-induced platelet activation and aggregation. Cangrelor tetrasodium is also a non-specific GPR17 antagonist. | [65,66,67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Wang, J.; Xu, J.; Qin, W.; Wang, Y.; Luo, S.; Wang, G. The Role and Molecular Mechanism of P2Y12 Receptors in the Pathogenesis of Atherosclerotic Cardiovascular Diseases. Appl. Sci. 2021, 11, 9078. https://doi.org/10.3390/app11199078
Wang L, Wang J, Xu J, Qin W, Wang Y, Luo S, Wang G. The Role and Molecular Mechanism of P2Y12 Receptors in the Pathogenesis of Atherosclerotic Cardiovascular Diseases. Applied Sciences. 2021; 11(19):9078. https://doi.org/10.3390/app11199078
Chicago/Turabian StyleWang, Lu, Jinxuan Wang, Jianxiong Xu, Weixi Qin, Yuming Wang, Shisui Luo, and Guixue Wang. 2021. "The Role and Molecular Mechanism of P2Y12 Receptors in the Pathogenesis of Atherosclerotic Cardiovascular Diseases" Applied Sciences 11, no. 19: 9078. https://doi.org/10.3390/app11199078
APA StyleWang, L., Wang, J., Xu, J., Qin, W., Wang, Y., Luo, S., & Wang, G. (2021). The Role and Molecular Mechanism of P2Y12 Receptors in the Pathogenesis of Atherosclerotic Cardiovascular Diseases. Applied Sciences, 11(19), 9078. https://doi.org/10.3390/app11199078





