Effect of Pyrolysis Temperature on Copper Aqueous Removal Capability of Biochar Derived from the Kelp Macrocystis pyrifera
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Collection and Pyrolysis Conditions
2.3. Physicochemical Characterization of Biochar
2.4. Adsorption Studies of Cu2+
2.5. Statistical Analyses
3. Results and Discussion
3.1. Effect of Pyrolysis Temperature on Biochar Yield and Physicochemical Characteristics
3.1.1. Yield and Ash Content of the Biochar Samples
3.1.2. pH, pHPZC, EC, and CEC of the Biochar Samples
3.1.3. Elemental Composition of Biochar Samples
3.1.4. Surface Morphology of Biochar Samples
3.1.5. Identification of Functional Groups of Biochar Samples
3.2. Adsorption Isotherms of Biochar Samples
3.3. Cu2+ Removal Performance of the Biochar Sample
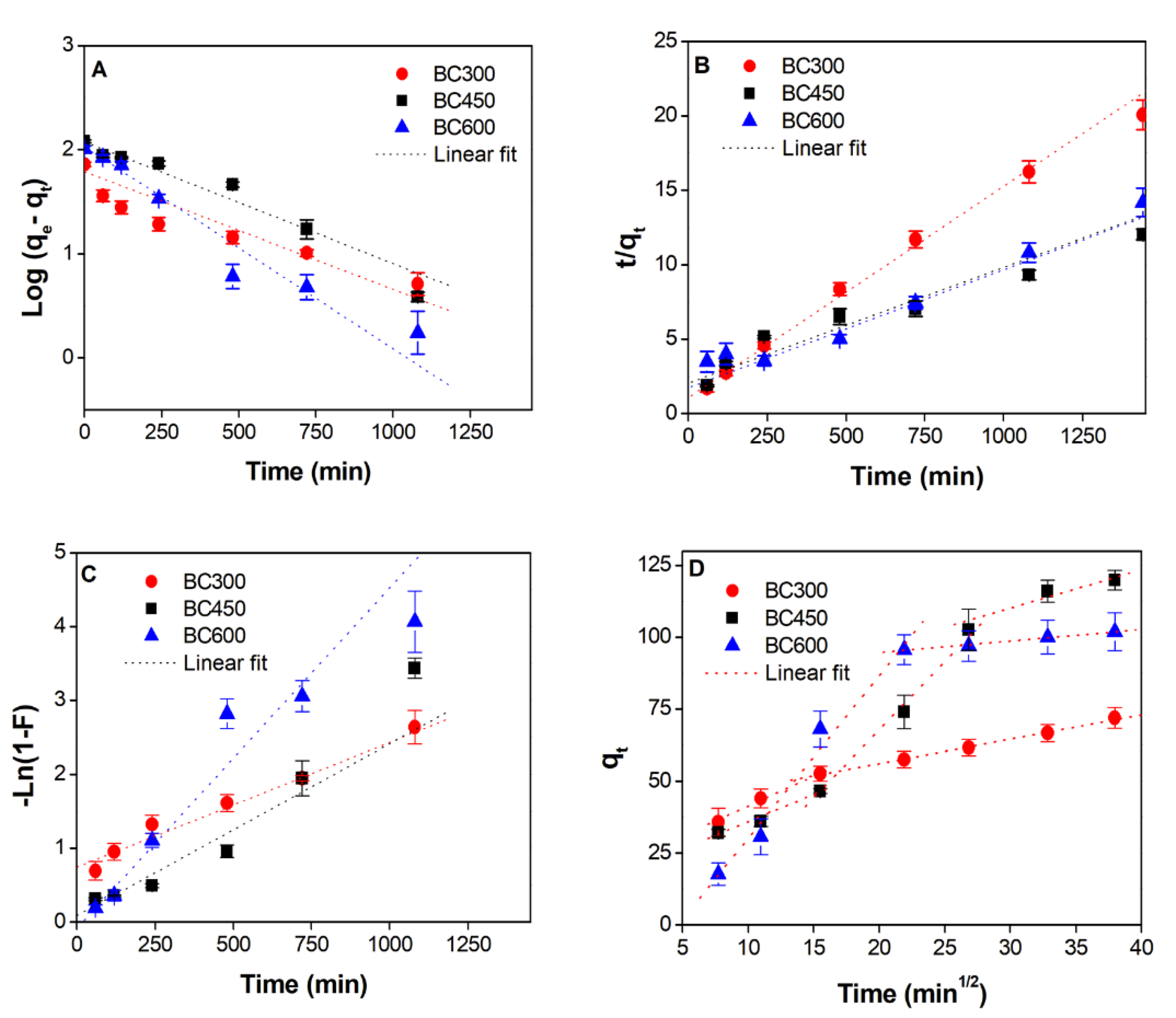
Kinetics of Cu2+ Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, G.; Lu, Q.; Ye, S.; Yuan, H.; Ding, X.; Wang, J. Assessment of heavy metal contamination in surface sediments of the west Guangdong coastal region, China. Mar. Pollut. Bull. 2016, 108, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.H.; Sibly, R.M.; Hopkin, S.P.; Peakal, D.B. Pollutants and their fate in ecosystems. In Principles of Ecotoxicology, 4th ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 3–6. [Google Scholar]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Masindi, V.; Muedi, K. Environmental contamination by heavy metals. In Heavy Metals; Saleh, H., Aglan, E.S., Eds.; IntechOpen: London, UK, 2018; pp. 117–130. [Google Scholar]
- Uriu-Adams, J.; Keen, C.L. Copper, oxidative stress, and human health. Mol. Aspects. Med. 2005, 26, 268–298. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.S.; Bhattacharyya, K.G. Kinetics of adsorption of metal ions on inorganic materials: A review. Adv. Colloid Interface Sci. 2011, 162, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.S.; Park, S.H.; Jung, S.-C.; Ryu, C.; Jeon, J.-K.; Shin, M.-C.; Park, Y.-K. Production and utilization of biochar: A review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.; Chen, L.; Chen, Y.; Lehmann, J.; McBride, M.B.; Hay, A.G. Adsorption of copper and zinc by biochars produced from pyrolysis of hardwood and corn straw in aqueous solution. Bioresour. Technol. 2011, 102, 8877–8884. [Google Scholar] [CrossRef]
- Park, S.H.; Cho, H.J.; Ryu, C.; Park, Y.-N. Removal of copper(II) in aqueous solution using pyrolytic biochars derived from red macroalga Porphyra tenera. J. Ind. Eng. Chem. 2016, 36, 314–319. [Google Scholar] [CrossRef]
- Contreras-Porcia, L.; Araya, M.; Garrido-Ramírez, E.; Bulboa, C.; Remonsellez, J.P.; Zapata, J.; Espinoza, C.; Rivas, J. Biochar production from seaweeds. In Protocols for Macroalgae Research; Charrier, B., Wichard, T., Reddy, C.R.K., Eds.; CRS Press, Taylor & Francis Group: Boca Raton, FL, USA, 2018; pp. 175–185. [Google Scholar]
- Ronsse, F.; van Hecke, S.; Dickinson, D.; Prins, W. Production and characterization of slow pyrolysis biochar: Influence of feedstock type and pyrolysis conditions. GCB Bioenergy 2013, 5, 104–115. [Google Scholar] [CrossRef]
- Tag, A.T.; Duman, G.; Ucar, S.; Yanik, J. Effects of feedstock type and pyrolysis temperature on potential applications of biochar. J. Anal. Appl. Pyrol. 2016, 120, 200–206. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, D.; Luo, G.; Zhang, S.; Chen, J. Macroalgae for biofuels production: Progress and perspectives. Renew. Sustain. Energy Rev. 2015, 47, 427–437. [Google Scholar] [CrossRef]
- Gouvêa, L.P.; Assis, J.; Gurgel, C.F.D.; Serrao, E.A.; Silveira, T.C.L.; Santos, R.; Duarte, C.M.; Peres, L.M.C.; Carvalho, V.F.; Batista, M.; et al. Golden carbon of Sargassum forests revealed as an opportunity for climate change mitigation. Sci. Total Environ. 2020, 729, 138745. [Google Scholar] [CrossRef]
- Jung, K.-W.; Kim, K.; Jeong, T.-U.; Ahn, K.-H. Influence of pyrolysis temperature on characteristics and phosphate adsorption capability of biochar derived from waste-marine macroalgae (Undaria pinnatifida roots). Bioresour. Technol. 2016, 200, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochar: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef]
- Poo, K.-M.; Son, E.-B.; Chang, J.-S.; Ren, X.; Choi, Y.-J.; Chae, K.-J. Biochars derived from wasted macro-algae (Saccharina japonica and Sargassum fusiforme) and their potential for heavy metal removal in aqueous solution. J. Environ. Manage. 2018, 206, 364–372. [Google Scholar] [CrossRef]
- Kim, B.S.; Lee, H.W.; Park, S.H.; Baek, K.; Jeon, J.-K.; Cho, H.J.; Jung, S.-C.; Kim, S.C.; Park, Y.-K. Removal of Cu2+ by biochars derived from green macroalgae. Environ. Sci. Pollut. Res. 2016, 23, 985–994. [Google Scholar] [CrossRef]
- Schiel, D.R.; Foster, M.S. The Biology and Ecology of Giant Kelp Forest, 1st ed.; University of California Press: Berkeley, CA, USA, 2015; pp. 23–40. [Google Scholar]
- Davis, T.A.; Volesky, B.; Mucci, A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 2003, 37, 4311–4330. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barret, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computation from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 16th ed.; Method 973.04; AOAC International: Rockville, MD, USA, 1995; Volume 1. [Google Scholar]
- Houshang, F.; Fatemeh, H.; Rahmatollah, R.; Ali, R. Surfactant-free hydrothermal synthesis of mesoporous niobia samples and their photoinduced decomposition of terephthalic acid (TPA). J. Clust. Sci. 2014, 25, 651–666. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2018. Available online: https://www.r-project.org (accessed on 15 September 2020).
- Yu, K.L.; Lau, B.F.; Show, P.L.; Ong, H.C.; Ling, T.C.; Chen, W.-H.; Ng, E.P.; Chang, J.-S. Recent developments on algal biochar production and characterization. Bioresour. Technol. 2017, 246, 2–11. [Google Scholar] [CrossRef]
- Ross, A.B.; Jones, J.M.; Kubacki, M.L.; Bridgeman, T. Classification of macroalgae as fuel and its thermochemical behaviour. Bioresour. Technol. 2008, 99, 6494–6504. [Google Scholar] [CrossRef]
- Zhao, H.; Yan, H.; Dong, S.; Zhang, Y.; Sun, B.; Zhang, C.; Ai, Y.; Chen, B.; Liu, Q.; Sui, T.; et al. Thermogravimetry study of the pyrolytic characteristics and kinetics of macro-algae Macrocystis pyrifera residue. J. Therm. Anal. Calorim. 2013, 111, 1685–1690. [Google Scholar] [CrossRef]
- Li, D.; Chen, L.; Yi, X.; Zhang, X.; Ye, N. Pyrolytic characteristics and kinetics of two brown algae and sodium alginate. Bioresour. Technol. 2010, 101, 7131–7136. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.B.; Anastasakis, K.; Kubacki, M.; Jones, J.M. Investigation of the pyrolysis behavior of brown algae before and after pre-treatment using PY-GC/MS and TGA. J. Anal. Appl. Pyrol. 2009, 85, 3–10. [Google Scholar] [CrossRef]
- Yuan, J.-H.; Xu, R.-K.; Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Huang, Y.; Li, Y.; Huang, L.; Mar, N.N.; Huang, Q.; Liu, Z. Biochar characteristics produced from rice husks and their sorption properties for the acetanilide herbicide metolachlor. Environ. Sci. Pollut. Res. 2017, 24, 4552–4561. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Guo, M. Quality variations of poultry litter biochar generated at different pyrolysis temperatures. J. Anal. Appl. Pyrol. 2012, 94, 138–145. [Google Scholar] [CrossRef]
- Mays, T.J. A New Classification of Pore Sizes. In Studies in Surface Science and Catalysis; Llewellyn, P.L., Rodriguez-Reinoso, F., Rouqerol, J., Seaton, N., Eds.; Elsevier B.V.: Bath, UK, 2007; pp. 57–62. [Google Scholar]
- Cao, X.; Harris, W. Properties of dairy-manure biochar pertinent to its potential use in remediation. Bioresour. Technol. 2010, 101, 5222–5228. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef]
- Murdock, J.N.; Wetzel, D.L. FT-IR Microspestrocopy enhances biological and ecological analysis of algae. Appl. Spesctrosc. Rev. 2009, 44, 335–361. [Google Scholar] [CrossRef]
- Plaza, J.; Viera, M.; Donati, E.; Guibal, E. Biosorption of mercury by Macrocystis pyrifera and Undaria pinnatifida: Influence of zinc, cadmium and nickel. J. Environ. Sci. 2011, 23, 1778–1786. [Google Scholar] [CrossRef]
- Miller, F.A.; Wilkins, C.H. Infrared spectra and characteristics frequencies of inorganic ions. Anal. Chem. 1952, 24, 1253–1294. [Google Scholar] [CrossRef]
- Pongkua, W.; Dolphen, R.; Thiravetyan, P. Effect of functional groups of biochar and their ash content on gaseous methyl tert-butyl ether removal. Colloids Surf. A. 2018, 558, 531–537. [Google Scholar] [CrossRef]
- Uchimiya, M.; Ohno, T.; He, Z. Pyrolysis temperature-dependent release of dissolved organic carbon from plant, manure, and biorefinery wastes. J. Anal. Appl. Pyrol. 2013, 104, 84–94. [Google Scholar] [CrossRef]
- Das, S.K.; Ghosh, G.K.; Avasthe, R.K.; Sinha, K. Compositional heterogeneity of different biochar: Effect of pyrolysis temperature and feedstocks. J. Environ. Manage. 2021, 278, 111501. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Moreira, V.R.; Lebron, Y.A.R.; Freire, S.J.; Santos, L.V.S.; Palladino, F.; Jacob, R.S. Biosorption of copper ions from solution using Chlorella pyrenoidosa: Optimization, equilibrium, and kinetics studies. Microchem. J. 2019, 145, 119–129. [Google Scholar] [CrossRef]
- Koyuncu, H.; Kul, A.R. Biosorption study for removal of methylene blue dye from aqueous solution using a novel activated carbon obtained from nonliving lichen (Pseudevernia furfuracea (L.) Zopf.). Surf. Interfaces. 2020, 19, 100527. [Google Scholar] [CrossRef]
- Mondal, S.; Majumder, S.K. Honeycomb-like porous activated carbon for efficient copper (II) adsorption synthesized from natural source: Kinetic study and equilibrium isotherm analysis. J. Environ. Chem. Eng. 2019, 7, 103236. [Google Scholar] [CrossRef]
- Daneshvar, E.; Kousha, M.; Jokar, M.; Koutahzadeh, N.; Guibal, E. Acidic dye biosorption onto marine brown macroalgae: Isotherm, kinetic and thermodynamic studies. Chem. Eng. J. 2012, 204–206, 225–234. [Google Scholar] [CrossRef]
- Rodríguez-Vila, A.; Selwyn-Smith, H.; Enunwa, L.; Smail, I.; Covelo, E.F.; Sizmur, T. Predicting Cu and Zn sorption capacity of biochar from feedstock C/N ratio and pyrolysis temperature. Environ. Sci. Pollut. R. 2018, 25, 7730–7739. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.J.; Baek, K.; Jeon, J.-K.; Park, S.H.; Suh, D.J.; Park, Y.-K. Removal characteristics of copper by marine macro-algae-derived chars. Chem. Eng. J. 2013, 217, 205–211. [Google Scholar] [CrossRef]
- Katiyar, R.; Patel, A.K.; Nguyen, T.-B.; Singhania, R.R.; Chen, C.-W.; Dong, C.-D. Adsorption of copper (II) in aqueous solution using biochars derived from Ascophyllum nodosum seaweed. Bioresour. Technol. 2021, 328, 124829. [Google Scholar] [CrossRef]
- Wang, M.; Liu, Y.; Yao, Y.; Han, L.; Liu, X. Comparative evaluation of bone chars derived from bovine parts: Physicochemical properties and copper sorption behavior. Sci. Total. Environ. 2020, 700, 134470. [Google Scholar] [CrossRef]
- Nath, H.; Saikia, A.; Goutam, P.J.; Saikia, B.K.; Saiki, N. Removal of methylene blue from water using okra (Abelmoschus esculentus L.) mucilage modified biochar. Bioresour. Technol. Rep. 2021, 14, 100689. [Google Scholar] [CrossRef]
- Qiu, H.; Lv, L.; Pan, B.-C.; Zhang, Q.-J.; Zhang, W.-M.; Zhang, Q.-X. Critical review in adsorption kinetic models. J. Zhejiang Univ. Sci. A 2009, 10, 716–724. [Google Scholar] [CrossRef]
- Nishikawa, E.; da Silva, M.G.C.; Vieira, M.G.A. Cadmium biosorption by alginate extraction waste and process overview in Life Cycle Assessment context. J. Clean. Prod. 2018, 178, 166–175. [Google Scholar] [CrossRef]
- Suteu, D.; Zaharia, C.; Badeanu, M. Kinetic modeling of dye sorption from aqueous solutions onto apple seed powder. Cellul. Chem. Technol. 2016, 50, 1085–1091. [Google Scholar]
- Plazinski, W. Binding of heavy metals by algal biosorbents. Theoretical models of kinetics, equilibria and thermodynamics. Adv. Colloid Interface Sci. 2013, 197–198, 58–67. [Google Scholar] [CrossRef]
- Boyd, G.E.; Adamson, A.W.; Myers, L.S., Jr. The exchange adsorption of ions from aqueous solutions by organic zeolites II. Kinetics. J. Am. Chem. Soc. 1947, 69, 2836–2848. [Google Scholar] [CrossRef]
- Plaza Cazón, J.; Bernardelli, C.; Viera, M.; Donati, E.; Guibal, E. Zinc and cadmium biosorption by untreated and calcium-treated Macrocystis pyrifera in a batch system. Bioresour. Technol. 2012, 116, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Arancibia-Miranda, N.; Baltazar, S.E.; García, A.; Muñoz-Lira, D.; Sepúlveda, P.; Rubio, M.A.; Altbir, D. Nanoscale zero valent supported by Zeolite and Montmorillonite: Template effect of the removal of lead ion from an aqueous solution. J. Hazard. Mater. 2016, 301, 371–380. [Google Scholar] [CrossRef] [PubMed]
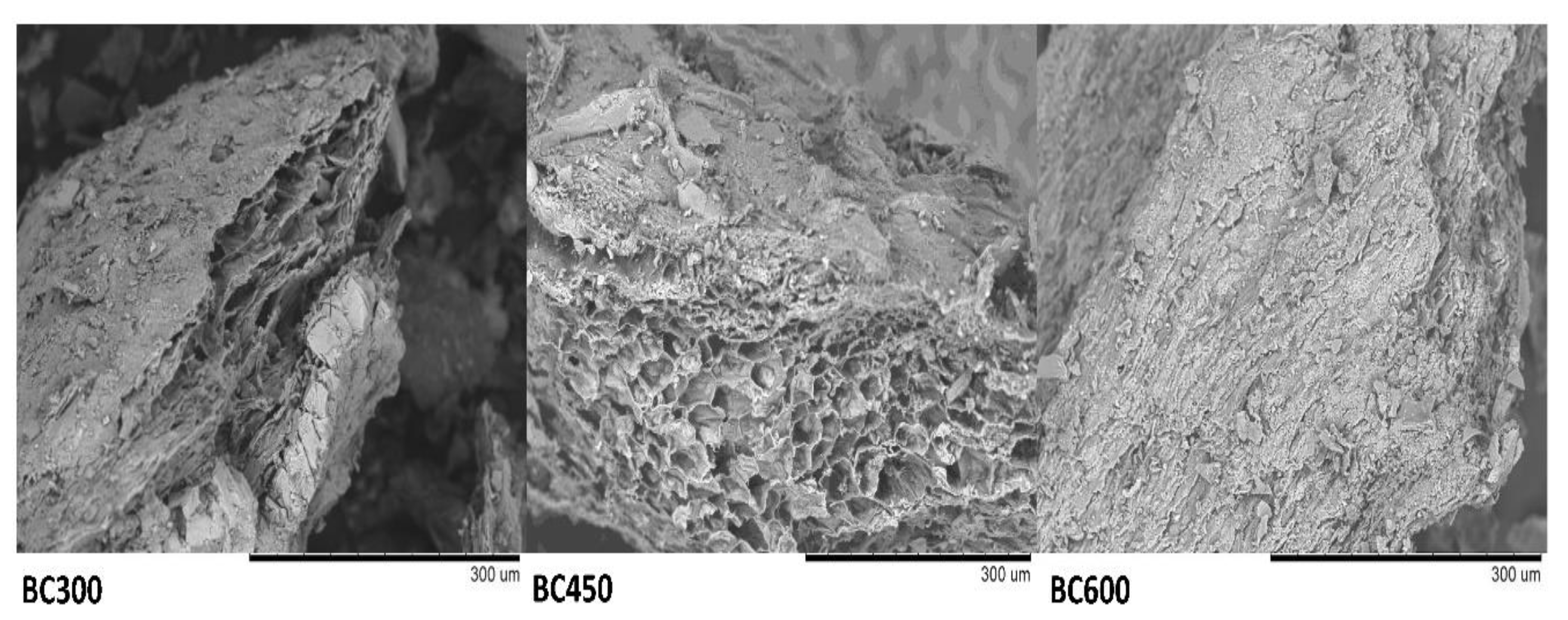



| Parameter * | Unit | BC300 | BC450 | BC600 |
|---|---|---|---|---|
| Yield | % | 62.10 a | 53.30 b | 48.80 b |
| Ash | % | 57.50 a | 69.90 b | 71.10 b |
| pH | 9.80 a | 11.00 b | 11.80 b | |
| pHpzc | 9.13 a | 10.70 b | 11.30 c | |
| EC | dS m−1 | 25.70 a | 27.40 b | 28.10 b |
| CEC | cmol kg−1 | 34.70 a | 40.20 b | 35.00 a |
| C | % | 28.20 a | 23.90 a | 25.50 a |
| H | % | 1.95 a | 3.01 b | 0.87 c |
| N | % | 3.15 a | 2.00 b | 1.58 c |
| H:C | 0.82 a | 1.51 b | 0.41 c | |
| OM | % | 40.00 a | 29.50 b | 28.00 b |
| Unit | BC300 | BC450 | BC600 | |
|---|---|---|---|---|
| BET surface area | m2 g−1 | 2.0 | 4.0 | 4.0 |
| Pore volume | cm3 g−1 | 0.009211 | 0.012871 | 0.018898 |
| Pore diameter | nm | 18.1 | 12.4 | 19.9 |
| Wave Number | Signal Assignment | Reference |
|---|---|---|
| 3400 | –OH stretching (water, hydrogen bonded hydroxyl); N–H | [36] |
| 2920–2880 | Aliphatic –CH2; asymmetric C–H stretching band; Aliphatic –CH2; symmetric C–H stretching band; CH3 | [36,37] |
| 1617 | N–H bending | [38] |
| 1558 | C=C of secondary aromatics amines | [37] |
| 1521 | Amide N–H bending | [37] |
| 1444–1414 | –CH2; –CO32−; aromatic C=C stretching; in-plane bending of carbonyl (-COH); Symmetry C=O stretch | [16,31,37,39] |
| 1234 | C–O alcohol or carboxylic acid stretching | [38] |
| 1103–1093 | C–O or C–O–C bonds stretching or bending | [40] |
| 1034 | Symmetric stretching C–O bonds | [37] |
| 873–853 | –CO32−; Si–H2 or metallic oxides | [39,40] |
| 711 | –CO32− | [39] |
| 620–616 | FeSO4 | [39] |
| Linear Parameters | BC300 | BC450 | BC600 |
|---|---|---|---|
| Langmuir | |||
| qm (mg g−1) | 49.24 | 58.00 | 93.55 |
| kL (L mg−1) | 23.50 | 10.91 | 0.372 |
| RL | 0.0003978 | 0.0003377 | 0.0002094 |
| R2 | 0.994 | 0.999 | 0.998 |
| Freundlich | |||
| kF (mg g−1) | 24.78 | 24.89 | 23.20 |
| n−1 (mg L−1) | 0.2364 | 0.1933 | 0.4417 |
| R2 | 0.963 | 0.919 | 0.952 |
| Dubinin–Radushkevich C | |||
| qm (mg g−1) | 139.22 | 222.20 | 637.08 |
| K (×10−9 mol2/kJ2) | 1.25 | 1.57 | 2.41 |
| E (kJ mol−1) | 19.99 | 17.83 | 14.4 |
| R2 | 0.967 | 0.938 | 0.970 |
| Tempkin | |||
| bT (J g mol−1 mg−1) | 575.0 | 333.0 | 124.2 |
| aT (L mg−1) | 479.0 | 92.99 | 3.705 |
| R2 | 0.850 | 0.934 | 0.991 |
| Linear Parameters | BC300 | BC450 | BC600 |
|---|---|---|---|
| qe, expa (mg g−1) | 71.91 | 119.9 | 101.9 |
| Pseudo-first-order | |||
| qe (mg g−1) | 61.24 | 118.7 | 106.67 |
| k1 (min−1) | 0.00260 | 0.00269 | 0.00447 |
| R2 | 0.917 | 0.933 | 0.960 |
| Pseudo-second-order | |||
| qe (mg g−1) | 70.37 | 128.2 | 125.9 |
| k2 (g mg−1 min−1) | 0.00019 | 0.000030 | 0.000037 |
| R2 | 0.995 | 0.844 | 0.958 |
| Liquid-film diffusion | |||
| kfd (min−1) | 0.00169 | 0.00233 | 0.00461 |
| R2 | 0.979 | 0.854 | 0.979 |
| Intra-particle diffusion | |||
| ki1 (mg g−1 min1/2) | 2.081 | 1.906 | 5.646 |
| C1 (mg g−1) | 20.560 | 16.783 | 26.285 |
| R2 | 0.992 | 0.990 | 0.988 |
| ki2 (mg g−1 min1/2) | 0.844 | 4.7234 | 0.400 |
| C2 (mg g−1) | 39.2315 | 26.639 | 86.671 |
| R2 | 0.997 | 0.995 | 0.987 |
| ki3 (mg g−1 min1/2) | - | 1.342 | - |
| C3 | 69.843 | ||
| R2 | - | 0.880 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araya, M.; Rivas, J.; Sepúlveda, G.; Espinoza-González, C.; Lira, S.; Meynard, A.; Blanco, E.; Escalona, N.; Ginocchio, R.; Garrido-Ramírez, E.; et al. Effect of Pyrolysis Temperature on Copper Aqueous Removal Capability of Biochar Derived from the Kelp Macrocystis pyrifera. Appl. Sci. 2021, 11, 9223. https://doi.org/10.3390/app11199223
Araya M, Rivas J, Sepúlveda G, Espinoza-González C, Lira S, Meynard A, Blanco E, Escalona N, Ginocchio R, Garrido-Ramírez E, et al. Effect of Pyrolysis Temperature on Copper Aqueous Removal Capability of Biochar Derived from the Kelp Macrocystis pyrifera. Applied Sciences. 2021; 11(19):9223. https://doi.org/10.3390/app11199223
Chicago/Turabian StyleAraya, Matías, Jorge Rivas, Graciela Sepúlveda, Camila Espinoza-González, Sebastián Lira, Andrés Meynard, Elodie Blanco, Nestor Escalona, Rosanna Ginocchio, Elizabeth Garrido-Ramírez, and et al. 2021. "Effect of Pyrolysis Temperature on Copper Aqueous Removal Capability of Biochar Derived from the Kelp Macrocystis pyrifera" Applied Sciences 11, no. 19: 9223. https://doi.org/10.3390/app11199223
APA StyleAraya, M., Rivas, J., Sepúlveda, G., Espinoza-González, C., Lira, S., Meynard, A., Blanco, E., Escalona, N., Ginocchio, R., Garrido-Ramírez, E., & Contreras-Porcia, L. (2021). Effect of Pyrolysis Temperature on Copper Aqueous Removal Capability of Biochar Derived from the Kelp Macrocystis pyrifera. Applied Sciences, 11(19), 9223. https://doi.org/10.3390/app11199223








