The Effect of Antagonist Abiotic Stress on Bioactive Compounds from Basil (Ocimum basilicum)
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Photosynthetic Measurements
2.3. Volatile Sampling and GC–MS Analyses
2.4. Chromatographic Analysis of Photosynthetic Pigments
2.5. Flavonoid Content Analysis
2.6. Total Phenolic Content—Folin-Ciocalteu Method
2.7. Statistical Analysis and Data Handling
3. Results
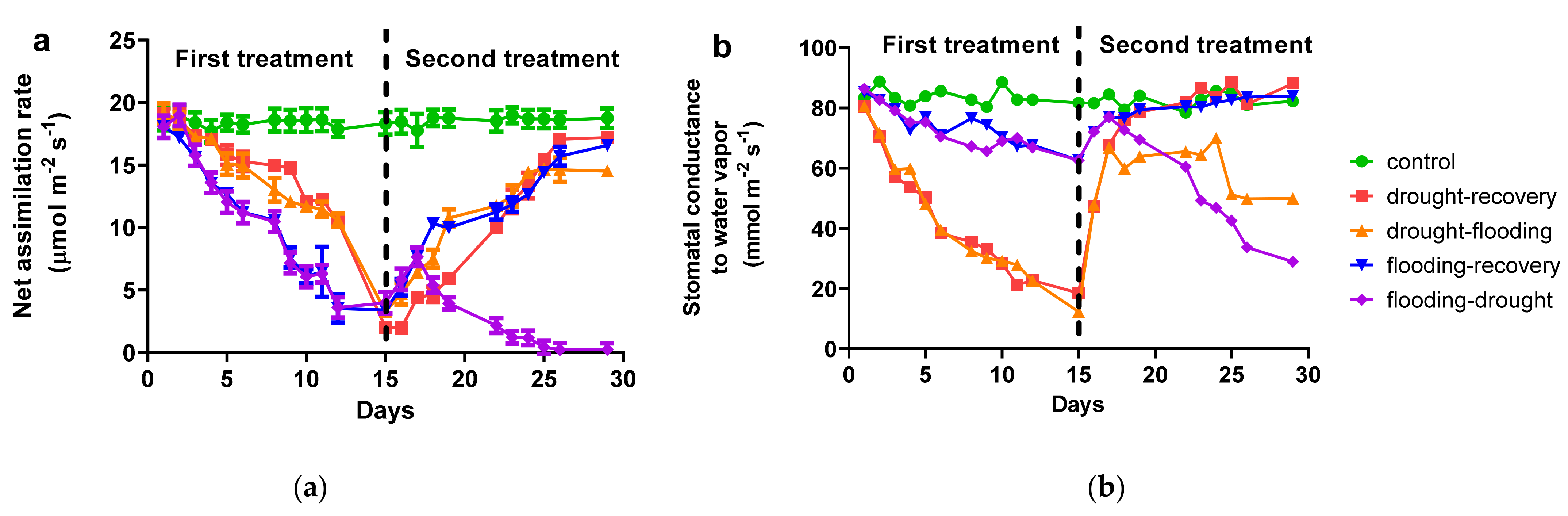
3.1. Effects of Antagonist Stresses on Photosynthetic Characteristics
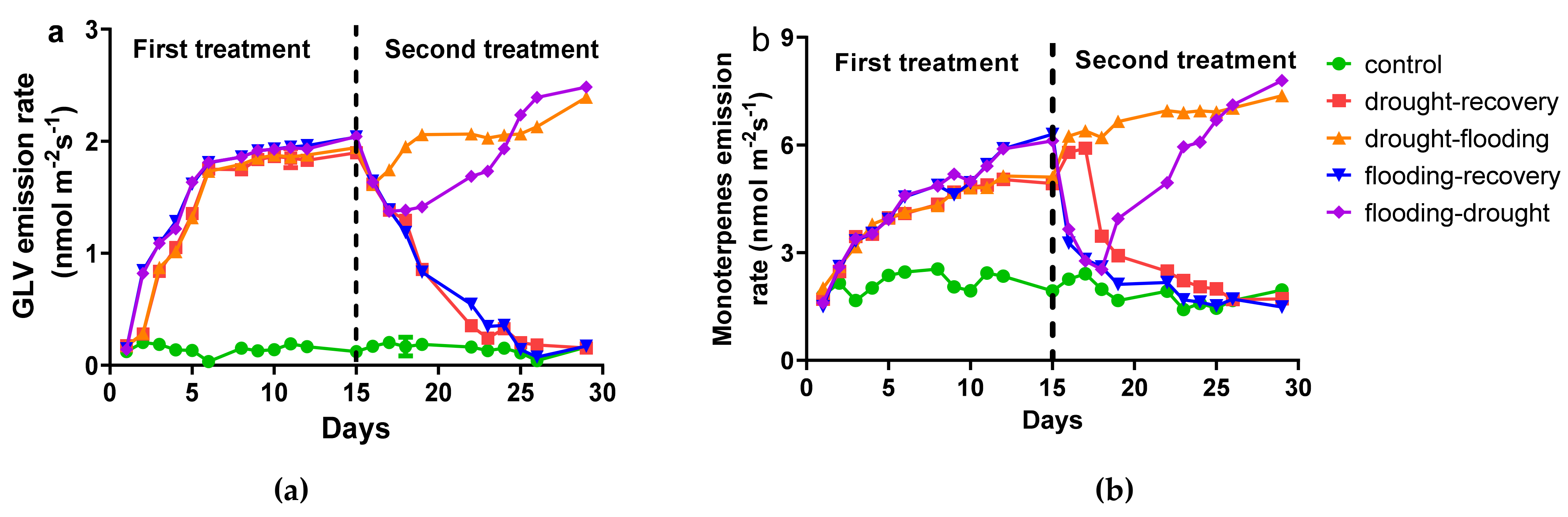
3.2. The Influence of Stress on Volatile Organic Compounds Emission
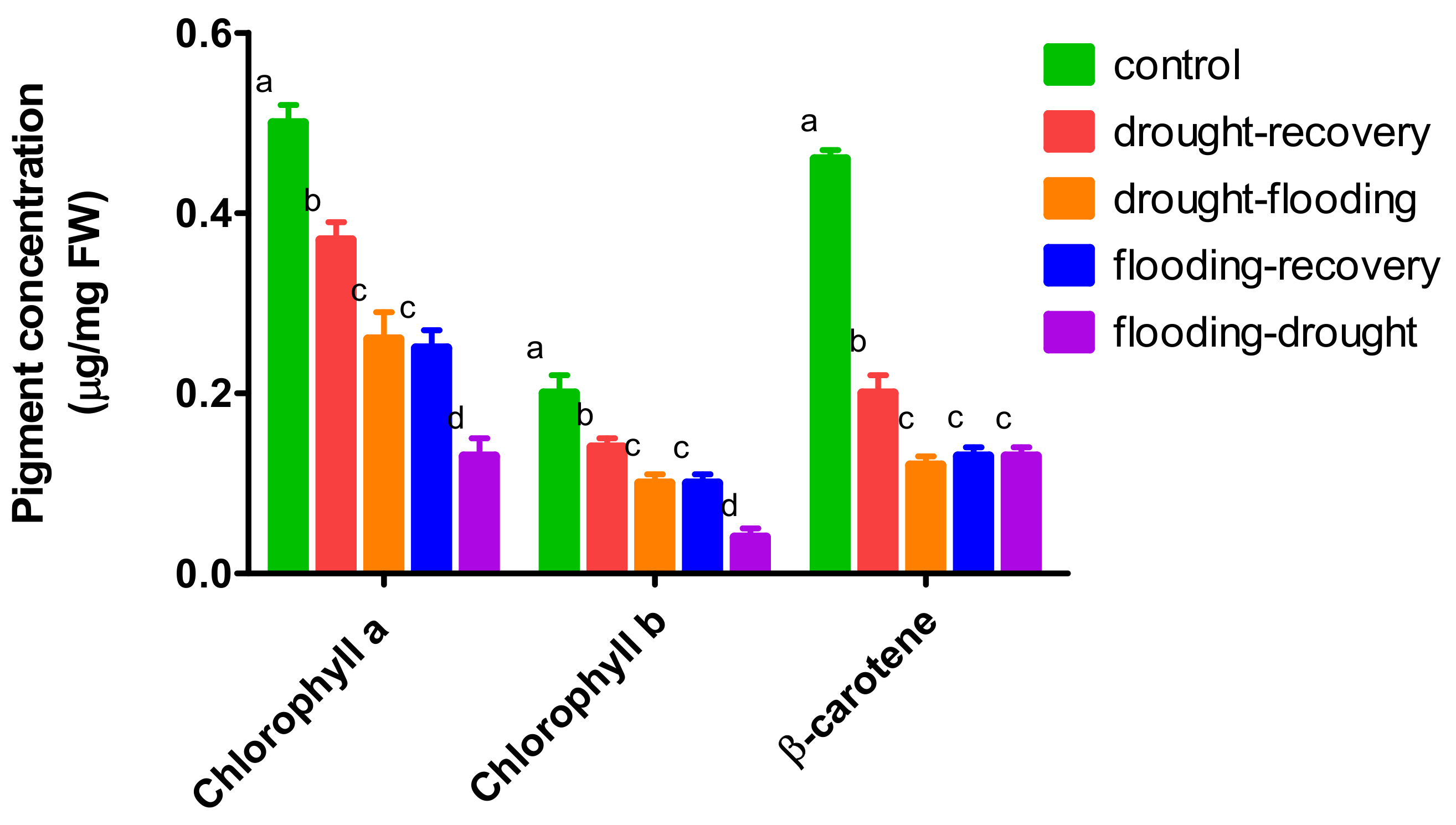
3.3. The Effects of Antagonistic Stress on Photosynthetic Pigments
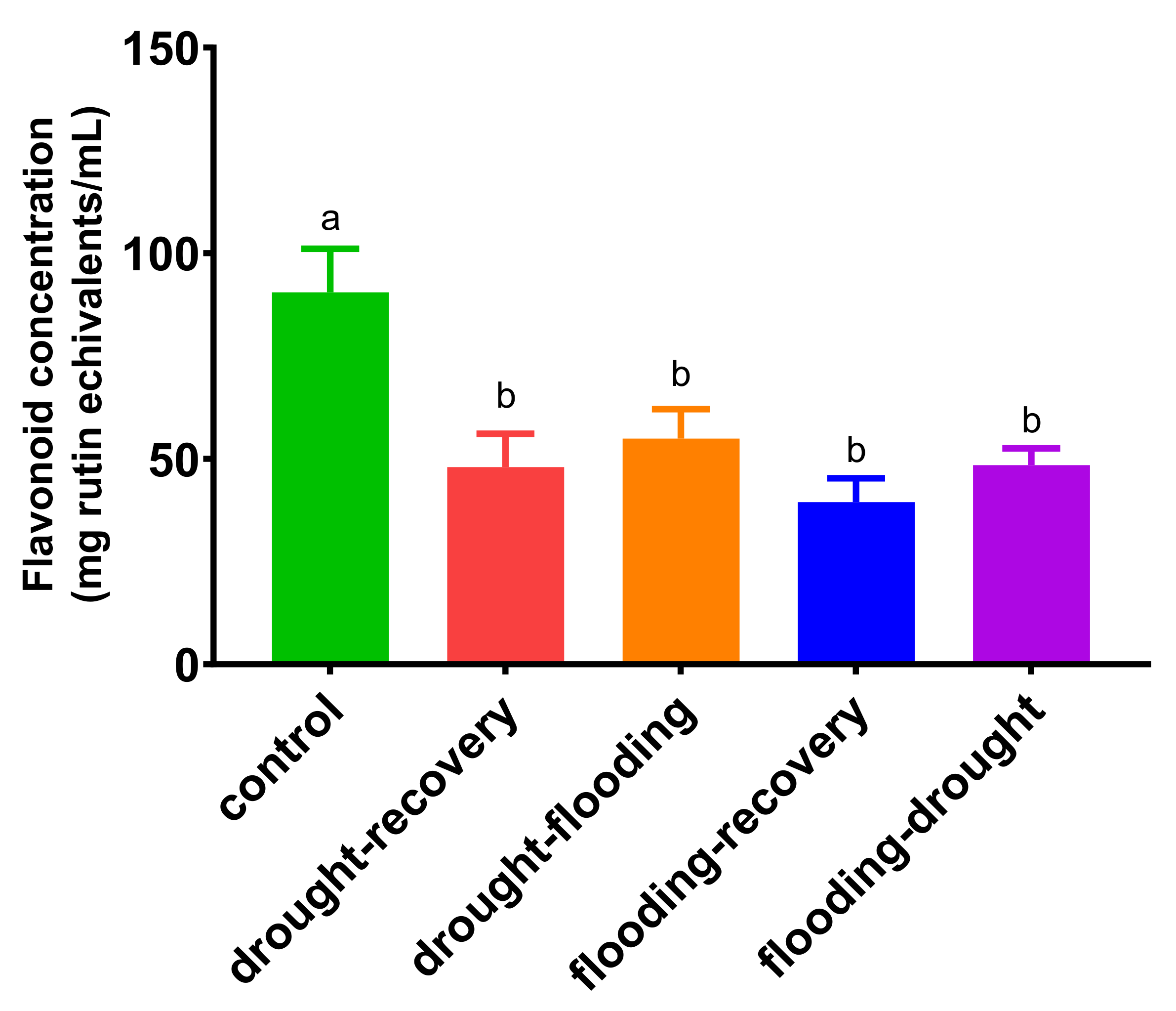
3.4. The Effects of Antagonistic Stress on Total Flavonoid Content
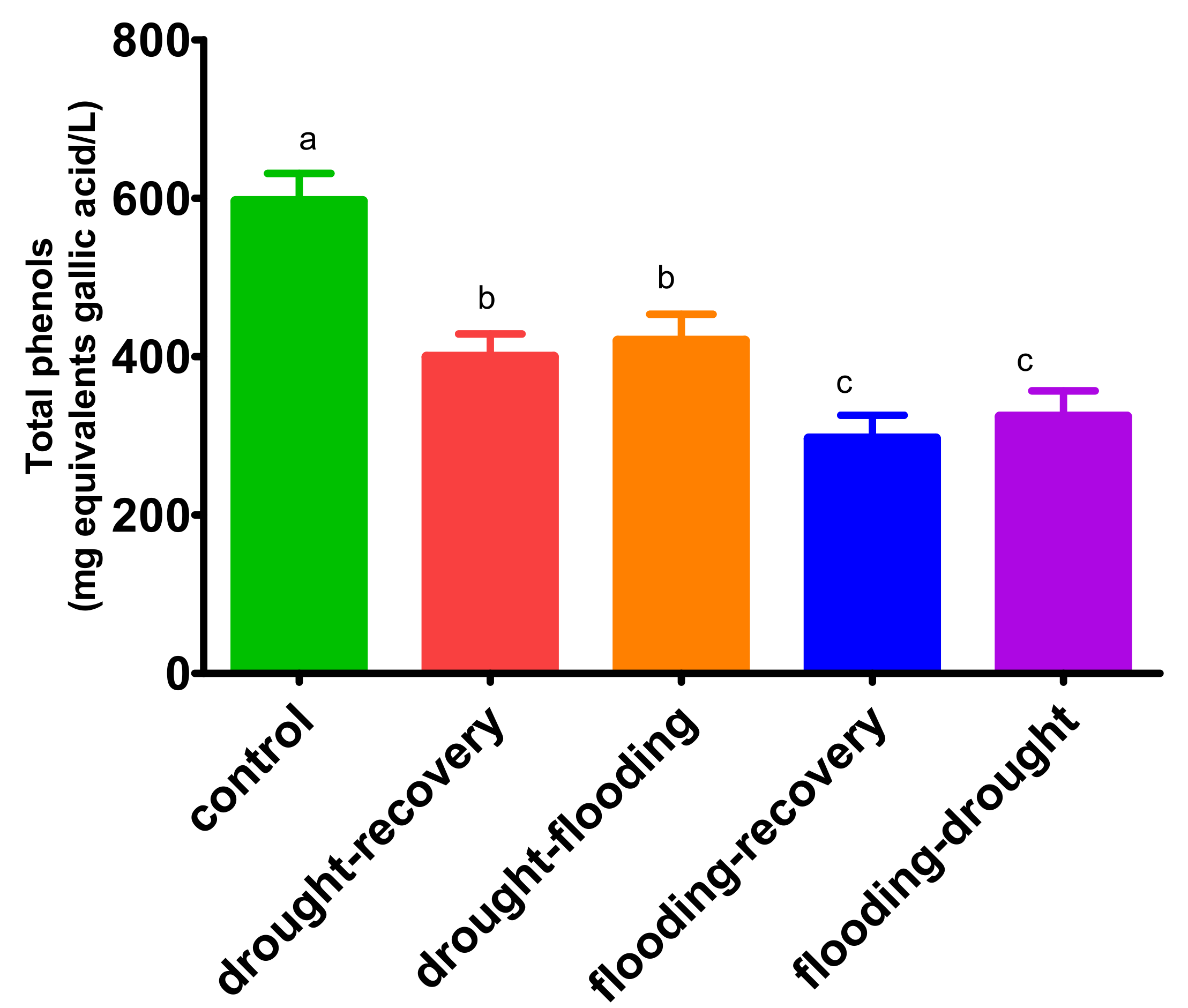
3.5. The Effects of Antagonistic Stress on Total Phenolic Content—Folin-Ciocalteu Method
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, D.; Chaudhuri, P.K. A review on phytochemical and pharmacological properties of Holy basil (Ocimum sanctum L.). Ind. Crops Prod. 2018, 118, 367–382. [Google Scholar] [CrossRef]
- Dafni, A.; Petanidou, T.; Vallianatou, I.; Kozhuharova, E.; Blanché, C.; Pacini, E.; Peyman, M.; Stevanovic, Z.D.; Franchi, G.G.; Benítez, G. Myrtle, Basil, Rosemary, and Three-Lobed Sage as Ritual Plants in the Monotheistic Religions: An Historical–Ethnobotanical Comparison. Econ. Bot. 2020, 74, 330–355. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Chemical components and pharmacological benefits of Basil (Ocimum basilicum): A review. Int. J. Food Prop. 2020, 23, 1961–1970. [Google Scholar] [CrossRef]
- Ademiluyi, A.O.; Oyeleye, S.I.; Oboh, G. Biological activities, antioxidant properties and phytoconstituents of essential oil from sweet basil (Ocimum basilicum L.) leaves. Comp. Clin. Path. 2016, 25, 169–176. [Google Scholar] [CrossRef]
- Majdi, C.; Pereira, C.; Dias, M.I.; Calhelha, R.C.; Alves, M.J.; Rhourri-Frih, B.; Charrouf, Z.; Barros, L.; Amaral, J.S.; Ferreira, I.C.F.R. Phytochemical Characterization and Bioactive Properties of Cinnamon Basil (Ocimum basilicum cv. ‘Cinnamon’) and Lemon Basil (Ocimum × citriodorum). Antioxidants 2020, 9, 369. [Google Scholar] [CrossRef]
- Jiang, Y.; Ye, J.; Li, S.; Niinemets, Ü. Regulation of floral terpenoid emission and biosynthesis in sweet basil (Ocimum basilicum). J. Plant Growth Regul. 2016, 35, 921–935. [Google Scholar] [CrossRef]
- Avetisyan, A.; Markosian, A.; Petrosyan, M.; Sahakyan, N.; Babayan, A.; Aloyan, S.; Trchounian, A. Chemical composition and some biological activities of the essential oils from basil Ocimum different cultivars. BMC Complement. Altern. Med. 2017, 17, 60. [Google Scholar] [CrossRef] [Green Version]
- Da Costa, A.S.; Arrigoni-Blank, M.d.F.; Carvalho Filho, J.L.S.d.; de Santana, A.D.D.; Santos, D.d.A.; Alves, P.B.; Blank, A.F. Chemical Diversity in Basil (Ocimum sp.) Germplasm. Sci. World J. 2015, 2015, 352638. [Google Scholar] [CrossRef] [Green Version]
- Zheljazkov, V.D.; Callahan, A.; Cantrell, C.L. Yield and Oil Composition of 38 Basil ( Ocimum basilicum L.) Accessions Grown in Mississippi. J. Agric. Food Chem. 2008, 56, 241–245. [Google Scholar] [CrossRef]
- Liber, Z.; Carović-Stanko, K.; Politeo, O.; Strikić, F.; Kolak, I.; Milos, M.; Satovic, Z. Chemical characterization and genetic relationships among Ocimum basilicum L. cultivars. Chem. Biodivers. 2011, 8, 1978–1989. [Google Scholar] [CrossRef]
- Soran, M.-L.; Stan, M.; Niinemets, Ü.; Copolovici, L. Influence of microwave frequency electromagnetic radiation on terpene emission and content in aromatic plants. J. Plant Physiol. 2014, 171, 1436–1443. [Google Scholar] [CrossRef] [Green Version]
- Copolovici, L.; Pag, A.; Kännaste, A.; Bodescu, A.; Tomescu, D.; Copolovici, D.; Soran, M.-L.; Niinemets, Ü. Disproportionate photosynthetic decline and inverse relationship between constitutive and induced volatile emissions upon feeding of Quercus robur leaves by large larvae of gypsy moth (Lymantria dispar). Environ. Exp. Bot. 2017, 138, 184–192. [Google Scholar] [CrossRef] [Green Version]
- Barbieri, G.; Vallone, S.; Orsini, F.; Paradiso, R.; De Pascale, S.; Negre-Zakharov, F.; Maggio, A. Stomatal density and metabolic determinants mediate salt stress adaptation and water use efficiency in basil (Ocimum basilicum L.). J. Plant Physiol. 2012, 169, 1737–1746. [Google Scholar] [CrossRef]
- Prinsi, B.; Morgutti, S.; Negrini, N.; Faoro, F.; Espen, L. Insight into Composition of Bioactive Phenolic Compounds in Leaves and Flowers of Green and Purple Basil. Plants 2020, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Złotek, U.; Mikulska, S.; Nagajek, M.; Świeca, M. The effect of different solvents and number of extraction steps on the polyphenol content and antioxidant capacity of basil leaves (Ocimum basilicum L.) extracts. Saudi J. Biol. Sci. 2016, 23, 628–633. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Ashkani, S.; Baghdadi, A.; Pazoki, A.; Jaafar, H.Z.; Rahmat, A. Improvement in flavonoids and phenolic acids production and pharmaceutical quality of sweet basil (Ocimum basilicum L.) by ultraviolet-B irradiation. Molecules 2016, 21, 1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiermeier, Q. Droughts, heatwaves and floods: How to tell when climate change is to blame. Nature 2018, 560, 20–23. [Google Scholar] [CrossRef] [Green Version]
- Kalantari, Z.; Ferreira, C.S.S.; Keesstra, S.; Destouni, G. Nature-based solutions for flood-drought risk mitigation in vulnerable urbanizing parts of East-Africa. Curr. Opin. Environ. Sci. 2018, 5, 73–78. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The Role of Polyphenols in Abiotic Stress Response: The Influence of Molecular Structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G.; Shimizu, H. Plant responses to drought and rewatering. Plant Signal. Behav. 2010, 5, 649–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geber, M.A.; Dawson, T.E. Genetic variation in and covariation between leaf gas exchange, morphology, and development in Polygonum arenastrum, an annual plant. Oecologia 1990, 85, 153–158. [Google Scholar] [CrossRef]
- Lee, Y.; Rubio, M.C.; Alassimone, J.; Geldner, N. A mechanism for localized lignin deposition in the endodermis. Cell 2013, 153, 402–412. [Google Scholar] [CrossRef] [Green Version]
- Balducci, L.; Deslauriers, A.; Giovannelli, A.; Beaulieu, M.; Delzon, S.; Rossi, S.; Rathgeber, C.B.K. How do drought and warming influence survival and wood traits of Picea mariana saplings? J. Exp. Bot. 2014, 66, 377–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beikircher, B.; De Cesare, C.; Mayr, S. Hydraulics of high-yield orchard trees: A case study of three Malus domestica cultivars. Tree Physiol. 2013, 33, 1296–1307. [Google Scholar] [CrossRef]
- Duan, B.; Lu, Y.; Yin, C.; Junttila, O.; Li, C. Physiological responses to drought and shade in two contrasting Picea asperata populations. Physiol. Plant. 2005, 124, 476–484. [Google Scholar] [CrossRef]
- Jeandroz, S.; Lamotte, O. Editorial: Plant Responses to Biotic and Abiotic Stresses: Lessons from Cell Signaling. Front. Plant Sci. 2017, 8, 1772. [Google Scholar] [CrossRef] [Green Version]
- Niinemets, Ü. Uncovering the hidden facets of drought stress: Secondary metabolites make the difference. Tree Physiol. 2015, 36, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, C.; Gotoh, N.; Aoki, T.; Wada, S. Phenolics composition and antioxidant activity of sweet basil (Ocimum basilicum L.). J. Agric. Food Chem. 2003, 51, 4442–4449. [Google Scholar] [CrossRef]
- Al-Huqail, A.; El-Dakak, R.M.; Sanad, M.N.; Badr, R.H.; Ibrahim, M.M.; Soliman, D.; Khan, F. Effects of Climate Temperature and Water Stress on Plant Growth and Accumulation of Antioxidant Compounds in Sweet Basil (Ocimum basilicum L.) Leafy Vegetable. Scientifica 2020, 2020, 3808909. [Google Scholar] [CrossRef] [Green Version]
- Kalisz, A.; Jezdinský, A.; Pokluda, R.; Sękara, A.; Grabowska, A.; Gil, J. Impacts of chilling on photosynthesis and chlorophyll pigment content in juvenile basil cultivars. Hortic. Environ. Biotechnol. 2016, 57, 330–339. [Google Scholar] [CrossRef]
- Kordi, S.; Saidi, M.; Ghanbari, F. Induction of drought tolerance in sweet basil (Ocimum basilicum L.) by salicylic acid. Int. J. Agric. Food Res. 2013, 2, 18–26. [Google Scholar] [CrossRef]
- Lazarević, B.; Šatović, Z.; Nimac, A.; Vidak, M.; Gunjača, J.; Politeo, O.; Carović-Stanko, K. Application of Phenotyping Methods in Detection of Drought and Salinity Stress in Basil (Ocimum basilicum L.). Front. Plant Sci. 2021, 12, 174. [Google Scholar] [CrossRef] [PubMed]
- Barickman, T.C.; Olorunwa, O.J.; Sehgal, A.; Walne, C.H.; Reddy, K.R.; Gao, W. Yield, Physiological Performance, and Phytochemistry of Basil (Ocimum basilicum L.) under Temperature Stress and Elevated CO2 Concentrations. Plants 2021, 10, 1072. [Google Scholar] [CrossRef]
- Copolovici, L.; Kännaste, A.; Pazouki, L.; Niinemets, Ü. Emissions of green leaf volatiles and terpenoids from Solanum lycopersicum are quantitatively related to the severity of cold and heat shock treatments. J. Plant Physiol. 2012, 169, 664–672. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Copolovici, L.; Hüve, K. High within-canopy variation in isoprene emission potentials in temperate trees: Implications for predicting canopy-scale isoprene fluxes. J. Geophys. Res. Biogeosci. 2010, 115, G04029. [Google Scholar] [CrossRef] [Green Version]
- Kännaste, A.; Copolovici, L.; Niinemets, Ü. Gas chromatography–mass spectrometry method for determination of biogenic volatile organic compounds emitted by plants. In Plant Isoprenoids; Humana Press: New York, NY, USA, 2014; pp. 161–169. [Google Scholar]
- Toome, M.; Randjärv, P.; Copolovici, L.; Niinemets, Ü.; Heinsoo, K.; Luik, A.; Noe, S.M. Leaf rust induced volatile organic compounds signalling in willow during the infection. Planta 2010, 232, 235–243. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Kuhn, U.; Harley, P.C.; Staudt, M.; Arneth, A.; Cescatti, A.; Ciccioli, P.; Copolovici, L.; Geron, C.; Guenther, A. Estimations of isoprenoid emission capacity from enclosure studies: Measurements, data processing, quality and standardized measurement protocols. Biogeosciences 2011, 8, 2209–2246. [Google Scholar] [CrossRef] [Green Version]
- Opriş, O.; Copaciu, F.; Soran, M.L.; Ristoiu, D.; Niinemets, Ü.; Copolovici, L. Influence of nine antibiotics on key secondary metabolites and physiological characteristics in Triticum aestivum: Leaf volatiles as a promising new tool to assess toxicity. Ecotoxicol. Environ. 2013, 87, 70–79. [Google Scholar] [CrossRef]
- Pag, A.I.; Radu, D.G.; Popa, M.I.; Sirghie, C. Flaxseed cake-a sustainable source of antioxidant and antibacterial extracts. Cell. Chem. Technol. 2014, 48, 265–273. [Google Scholar]
- Moisa, C.; Copolovici, L.; Pop, G.; Imbrea, I.; Lupitu, A.; Nemeth, S.; Copolovici, D. Wastes resulting from aromatic plants distillation-bio-sources of antioxidants and phenolic compounds with biological active principles. Farmacia 2018, 66, 289–295. [Google Scholar]
- Damalas, C.A. Improving drought tolerance in sweet basil (Ocimum basilicum) with salicylic acid. Sci. Hortic. 2019, 246, 360–365. [Google Scholar] [CrossRef]
- Cornic, G. Drought stress inhibits photosynthesis by decreasing stomatal aperture – not by affecting ATP synthesis. Trends Plant Sci. 2000, 5, 187–188. [Google Scholar] [CrossRef]
- Agurla, S.; Gahir, S.; Munemasa, S.; Murata, Y.; Raghavendra, A.S. Mechanism of Stomatal Closure in Plants Exposed to Drought and Cold Stress. Adv. Exp. Med. Biol. 2018, 1081, 215–232. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought-inhibition of Photosynthesis in C3 Plants: Stomatal and Non-stomatal Limitations Revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, M.B.; Hall, K.C. Early stomatal closure in waterlogged pea plants is mediated by abscisic acid in the absence of foliar water deficits. Plant Cell Environ. 1987, 10, 121–130. [Google Scholar] [CrossRef]
- Blanke, M.M.; Cooke, D.T. Effects of flooding and drought on stomatal activity, transpiration, photosynthesis, water potential and water channel activity in strawberry stolons and leaves. Plant Growth Regul. 2004, 42, 153–160. [Google Scholar] [CrossRef]
- Salazar, C.; Hernández, C.; Pino, M.T. Plant water stress: Associations between ethylene and abscisic acid response. Chil. J. Agric. Res. 2015, 75, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Ameye, M.; Allmann, S.; Verwaeren, J.; Smagghe, G.; Haesaert, G.; Schuurink, R.C.; Audenaert, K. Green leaf volatile production by plants: A meta-analysis. New Phytol. 2018, 220, 666–683. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Sugimoto, K.; Mano, J.; Ozawa, R.; Takabayashi, J. Differential metabolisms of green leaf volatiles in injured and intact parts of a wounded leaf meet distinct ecophysiological requirements. PloS ONE 2012, 7, e36433. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xu, G.-P.; Zheng, J.; Gao, R.-F.; Zhang, R.-M.; Wu, X.-B.; Gao, Y.; Zuo, Z.-J. Physiological responses to drought stress and the emission of induced volatile organic compounds in Rosmarinus officinalis. Chin. J. Plant Ecol. 2013, 37, 454–463. [Google Scholar] [CrossRef]
- Peron, A.; Kaser, L.; Fitzky, A.C.; Graus, M.; Halbwirth, H.; Greiner, J.; Wohlfahrt, G.; Rewald, B.; Sandén, H.; Karl, T. Combined effects of ozone and drought stress on the emission of biogenic volatile organic compounds from Quercus robur L. Biogeosciences 2021, 18, 535–556. [Google Scholar] [CrossRef]
- Wenda-Piesik, A. Volatile Organic Compound Emissions by Winter Wheat Plants (Triticum aestivum L.) under Fusarium spp. Infestation and Various Abiotic Conditions. Pol. J. Environ. Stud. 2011, 20, 1335–1342. [Google Scholar]
- Carlo, N.; Silvia, S.; Stefano, B.; Paolo, S. Influence of cut number on qualitative traits in different cultivars of sweet basil. Ind. Crops Prod. 2013, 44, 465–472. [Google Scholar] [CrossRef]
- Shiga, T.; Shoji, K.; Shimada, H.; Hashida, S.-n.; Goto, F.; Yoshihara, T. Effect of light quality on rosmarinic acid content and antioxidant activity of sweet basil, Ocimum basilicum L. Plant Biotechnol. 2009, 26, 255–259. [Google Scholar] [CrossRef] [Green Version]
- Comite, E.; El-Nakhel, C.; Rouphael, Y.; Ventorino, V.; Pepe, O.; Borzacchiello, A.; Vinale, F.; Rigano, D.; Staropoli, A.; Lorito, M. Bioformulations with Beneficial Microbial Consortia, a Bioactive Compound and Plant Biopolymers Modulate Sweet Basil Productivity, Photosynthetic Activity and Metabolites. Pathogens 2021, 10, 870. [Google Scholar] [CrossRef]
- Blanch, J.-S.; Peñuelas, J.; Sardans, J.; Llusià, J. Drought, warming and soil fertilization effects on leaf volatile terpene concentrations in Pinus halepensis and Quercus ilex. Acta Physiol. Plant. 2008, 31, 207. [Google Scholar] [CrossRef]
- Ormeño, E.; Mévy, J.P.; Vila, B.; Bousquet-Mélou, A.; Greff, S.; Bonin, G.; Fernandez, C. Water deficit stress induces different monoterpene and sesquiterpene emission changes in Mediterranean species. Relationship between terpene emissions and plant water potential. Chemosphere 2007, 67, 276–284. [Google Scholar] [CrossRef] [Green Version]
- Radwan, A.; Kleinwächter, M.; Selmar, D. Impact of drought stress on specialised metabolism: Biosynthesis and the expression of monoterpene synthases in sage (Salvia officinalis). Phytochemistry 2017, 141, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Copolovici, L.; Kännaste, A.; Remmel, T.; Niinemets, Ü. Volatile organic compound emissions from Alnus glutinosa under interacting drought and herbivory stresses. Environ. Exp. Bot. 2014, 100, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Loreto, F.; Schnitzler, J.-P. Abiotic stresses and induced BVOCs. Trends Plant Sci. 2010, 15, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Liu, C.; Zheng, R.; Cai, X.; Luo, J.; Zou, J.; Wang, C. Emission and Accumulation of Monoterpene and the Key Terpene Synthase (TPS) Associated with Monoterpene Biosynthesis in Osmanthus fragrans Lour. Front. Plant Sci. 2016, 6, 1232. [Google Scholar] [CrossRef] [Green Version]
- Niinemets, Ü.; Kännaste, A.; Copolovici, L. Quantitative patterns between plant volatile emissions induced by biotic stresses and the degree of damage. Front. Plant Sci. 2013, 4, 262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghorbanpour, M. Major essential oil constituents, total phenolics and flavonoids content and antioxidant activity of Salvia officinalis plant in response to nano-titanium dioxide. Indian J. Plant Physiol. 2015, 20, 249–256. [Google Scholar] [CrossRef]
- Aranjuelo, Í.; Molero, G.; Erice, G.; Avice, J.; Nogués, S. Plant physiology and proteomics reveals the leaf response to drought in alfalfa (Medicago sativa L.). J. Exp. Bot. 2011, 62, 111–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Wang, S.; Cao, B.; Cao, D.; Leng, G.; Li, H.; Yin, L.; Shan, L.; Deng, X. Genotypic Variation in Growth and Physiological Response to Drought Stress and Re-Watering Reveals the Critical Role of Recovery in Drought Adaptation in Maize Seedlings. Front. Plant Sci. 2016, 6, 1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arzani, A. Manipulating programmed cell death pathways for enhancing salinity tolerance in crops. In Salinity Responses and Tolerance in Plants, Volume 2; Springer: Cham, Switzerland, 2018; pp. 93–118. [Google Scholar]
- Mohammadi, H.; Ghorbanpour, M.; Brestic, M. Exogenous putrescine changes redox regulations and essential oil constituents in field-grown Thymus vulgaris L. under well-watered and drought stress conditions. Ind. Crops Prod. 2018, 122, 119–132. [Google Scholar] [CrossRef]
- Raei, M.; Angaji, S.A.; Omidi, M.; Khodayari, M. Effect of abiotic elicitors on tissue culture of Aloe vera. Int. J. Biosci. 2014, 5, 74–81. [Google Scholar]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [Green Version]
- Gharibi, S.; Sayed Tabatabaei, B.E.; Saeidi, G.; Talebi, M.; Matkowski, A. The effect of drought stress on polyphenolic compounds and expression of flavonoid biosynthesis related genes in Achillea pachycephala Rech.f. Phytochemistry 2019, 162, 90–98. [Google Scholar] [CrossRef]
- Sytar, O.; Mbarki, S.; Zivcak, M.; Brestic, M. The involvement of different secondary metabolites in salinity tolerance of crops. In Salinity Responses and Tolerance in Plants, Volume 2; Springer: Cham, Switzerland, 2018; pp. 21–48. [Google Scholar]
- Yan, K.; Cui, M.; Zhao, S.; Chen, X.; Tang, X. Salinity Stress Is Beneficial to the Accumulation of Chlorogenic Acids in Honeysuckle (Lonicera japonica Thunb.). Front. Plant Sci. 2016, 7, 1563. [Google Scholar] [CrossRef] [Green Version]
- Selmar, D.; Kleinwächter, M. Influencing the product quality by deliberately applying drought stress during the cultivation of medicinal plants. Ind. Crops Prod. 2013, 42, 558–566. [Google Scholar] [CrossRef]
- Kleinwächter, M.; Selmar, D. New insights explain that drought stress enhances the quality of spice and medicinal plants: Potential applications. Agron. Sustain. Dev. 2015, 35, 121–131. [Google Scholar] [CrossRef] [Green Version]
- Pellegrini, E.; Hoshika, Y.; Dusart, N.; Cotrozzi, L.; Gérard, J.; Nali, C.; Vaultier, M.-N.; Jolivet, Y.; Lorenzini, G.; Paoletti, E. Antioxidative responses of three oak species under ozone and water stress conditions. Sci. Total Environ. 2019, 647, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Fini, A.; Guidi, L.; Ferrini, F.; Brunetti, C.; Di Ferdinando, M.; Biricolti, S.; Pollastri, S.; Calamai, L.; Tattini, M. Drought stress has contrasting effects on antioxidant enzymes activity and phenylpropanoid biosynthesis in Fraxinus ornus leaves: An excess light stress affair? J. Plant Physiol. 2012, 169, 929–939. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Copolovici, L.; Lupitu, A.; Moisa, C.; Taschina, M.; Copolovici, D.M. The Effect of Antagonist Abiotic Stress on Bioactive Compounds from Basil (Ocimum basilicum). Appl. Sci. 2021, 11, 9282. https://doi.org/10.3390/app11199282
Copolovici L, Lupitu A, Moisa C, Taschina M, Copolovici DM. The Effect of Antagonist Abiotic Stress on Bioactive Compounds from Basil (Ocimum basilicum). Applied Sciences. 2021; 11(19):9282. https://doi.org/10.3390/app11199282
Chicago/Turabian StyleCopolovici, Lucian, Andreea Lupitu, Cristian Moisa, Monica Taschina, and Dana M. Copolovici. 2021. "The Effect of Antagonist Abiotic Stress on Bioactive Compounds from Basil (Ocimum basilicum)" Applied Sciences 11, no. 19: 9282. https://doi.org/10.3390/app11199282
APA StyleCopolovici, L., Lupitu, A., Moisa, C., Taschina, M., & Copolovici, D. M. (2021). The Effect of Antagonist Abiotic Stress on Bioactive Compounds from Basil (Ocimum basilicum). Applied Sciences, 11(19), 9282. https://doi.org/10.3390/app11199282







