The Double Face of Metals: The Intriguing Case of Chromium
Abstract
1. Introduction
2. Chemical Form and Properties of Chromium
3. Bioavailability, Absorption and Excretion of Cr(III) and Cr(VI)
4. The Role of Chromium and Its Mechanism of Action in the Body
5. Chromium(III) in Diabetes and Supplements
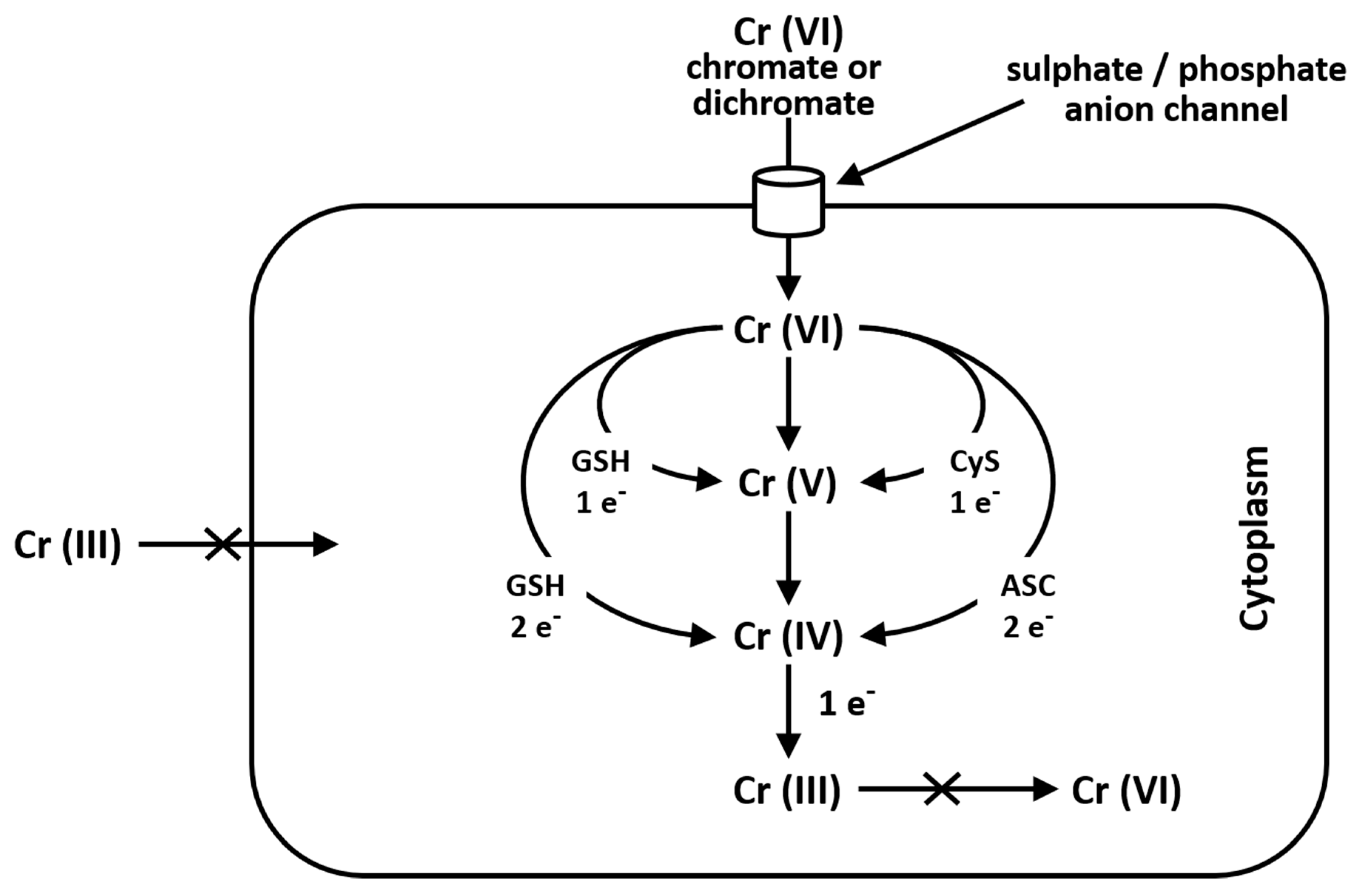
6. Toxicity of Chromium(VI)
7. Chromium Epigenetic Effects on DNA, Histones and MicroRNAs
8. Chromium Remediation
9. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ac | acetyl (Figure 3) |
| 6MeA | 6-methyladenosine |
| C | cytosine (Figure 3) |
| 5MeC | 5-methylcytosine |
| K | lysine (Figure 3) |
| AMPK | AMP protein kinase |
| CAFs | cancer-associated fibroblasts |
| COD | chemical oxygen demand |
| CRDC | chromium-dinicocysteinate |
| CrHis | chromium-histidinate |
| CrPic | chromium-picolinate |
| Cys | cysteine |
| E2 | 17β-estradiol |
| EMT | epithelial-mesenchymal transition |
| EPA | Environmental Protection Agency |
| GLUT4 | glucose transporter 4 |
| GTF | glucose tolerance factor |
| GSH | reduced glutathione |
| H3K9 | histone H3 lysine 9 |
| H3K4 | histone H3 lysine 4 |
| H3K27 | histone H3 lysine 27 |
| H3R2 | histone H3 histidine 2 |
| HATs | histone acetyltransferase |
| 16HBE | human bronchial epithelial |
| HDACs | histone deacetylases |
| HDL | high density lipoprotein |
| HMG-CoA | 3 hydroxy-3-methylglutaryl coenzyme A |
| HS | heat stress |
| ICAM-1 | intercellular adhesion molecule-1 |
| IL-6 | interleukin-6 |
| IL-8 | interleukin-8 |
| IRI | ischemia-reperfusion injury |
| JHDMs | Jumonji-C domain-containing histone demethylases |
| LDL | low density lipoprotein |
| LDL-c | low-density lipoprotein cholesterol |
| LMWCr | low-molecular-weight chromium-binding substance |
| LPO | lipid peroxidation |
| MAPK | mitogen-activated protein kinase |
| MMTV | mouse mammary tumor virus |
| MOF | males absent on the first |
| mRNA | RNA messenger |
| miRNA | microRNA |
| NBC | niacin-bound chromium |
| NO | nitric oxide |
| Nupr1 | nuclear protein 1 |
| PVF-CNT | Poly(vinyl)ferrocene-carbon nanotube |
| ROS | reactive oxygen species |
| T2DM | type 2 diabetes mellitus |
| TET | ten-eleven translocation |
| TNF- | tumor necrosis factor- |
| USEPA | US Environmental Protection Agency |
| WHO | World Health Organization |
References
- Vincent, J.B. New evidence against chromium as an essential trace element. J. Nutr. 2017, 147, 2212–2219. [Google Scholar] [PubMed]
- Lin, C.C.; Huang, Y.L. Chromium, zinc and magnesium status in type 1 diabetes. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.B. Effects of chromium supplementation on body composition, human and animal health, and insulin and glucose metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Baumgard, L.H.; Rhoads, R.P., Jr. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef]
- Bin-Jumah, M.; El-Hack, A.M.E.; Abdelnour, S.A.; Hendy, Y.A.; Ghanem, H.A.; Alsafy, S.A.; Khafaga, A.F.; Noreldin, A.E.; Shaheen, H.; Samak, D.; et al. Potential use of chromium to combat thermal stress in animals: A review. Sci. Total Environ. 2020, 707, 135996. [Google Scholar] [CrossRef]
- Filippini, T.; Cilloni, S.; Malavolti, M.; Violi, F.; Malagoli, C.; Tesauro, M.; Bottecchi, I.; Ferrari, A.; Vescovi, L.; Vinceti, M. Dietary intake of cadmium, chromium, copper, manganese, selenium and zinc in a Northern Italy community. J. Trace Elem. Med. Biol. 2018, 50, 508–517. [Google Scholar]
- Anderson, R.A. Chromium as an essential nutrient for humans. Regul. Toxicol. Pharmacol. 1997, 26, S35–S41. [Google Scholar] [CrossRef]
- Cefalu, W.T.; Hu, F.B. Role of chromium in human health and in diabetes. Diabetes Care 2004, 27, 2741–2751, Erratum in 2013, 36, 2872. [Google Scholar] [CrossRef]
- DesMarias, T.L.; Costa, M. Mechanisms of chromium-induced toxicity. Curr. Opin. Toxicol. 2019, 14, 1–7. [Google Scholar]
- Lau, F.C.; Bagchi, M.; Sen, C.K.; Bagchi, D. Nutrigenomic basis of beneficial effects of chromium (III) on obesity and diabetes. Mol. Cell. Biochem. 2008, 317, 1–10. [Google Scholar] [PubMed]
- Suksomboon, N.; Poolsup, N.; Yuwanakorn, A. Systematic review and meta-analysis of the efficacy and safety of chromium supplementation in diabetes. J. Clin. Pharm. Ther. 2014, 39, 292–306. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Chromium supplementation in human health, metabolic syndrome, and diabetes. In Essential Metals in Medicine: Therapeutic Use and Toxicity of Metal Ions in the Clinic; De Gruyter: Berlin, Germany, 2019; pp. 231–252. [Google Scholar]
- Staniek, H. The combined effects of Cr(III) propionate complex supplementation and iron excess on copper and zinc status in rats. J. Trace Elem. Med. Biol. 2019, 53, 49–54. [Google Scholar] [CrossRef] [PubMed]
- White, P.E.; Vincent, J.B. Systematic review of the effects of chromium (III) on chickens. Biol. Trace Elem. Res. 2019, 188, 99–126. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, Y.S. Chromium and leather: A review on the chemistry of relevance for allergic contact dermatitis to chromium. J. Leather Sci. Eng. 2020, 2, 1–15. [Google Scholar] [CrossRef]
- Levina, A.; Lay, P.A. Chemical properties and toxicity of chromium (III) nutritional supplements. Chem. Res. Toxicol. 2008, 21, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Milačič, R.; Ščančar, J. Cr speciation in foodstuffs, biological and environmental samples: Methodological approaches and analytical challenges–a critical review. TrAC Trends Anal. Chem. 2020, 127, 115888. [Google Scholar] [CrossRef]
- Eastmond, D.A.; MacGregor, J.T.; Slesinski, R.S. Trivalent chromium: Assessing the genotoxic risk of an essential trace element and widely used human and animal nutritional supplement. Crit. Rev. Toxicol. 2008, 38, 173–190. [Google Scholar] [CrossRef]
- Ferreira, L.M.; Cunha-Oliveira, T.; Sobral, M.C.; Abreu, P.L.; Alpoim, M.C.; Urbano, A.M. Impact of carcinogenic chromium on the cellular response to proteotoxic stress. Int. J. Mol. Sci. 2019, 20, 4901. [Google Scholar] [CrossRef]
- Jobby, R.; Jha, P.; Yadav, A.K.; Desai, N. Biosorption and biotransformation of hexavalent chromium [Cr(VI)]: A comprehensive review. Chemosphere 2018, 207, 255–266. [Google Scholar] [CrossRef]
- Pellerin, C.; Booker, S.M. Reflections on hexavalent chromium: Health hazards of an industrial heavyweight. Environ. Health Perspect. 2000, 108, A40–A407. [Google Scholar] [CrossRef]
- Coetzee, J.J.; Bansal, N.; Chirwa, E.M.N. Chromium in environment, its toxic effect from chromite-mining and ferrochrome industries, and its possible bioremediation. Expo. Health 2020, 12, 51–62. [Google Scholar] [CrossRef]
- Tumolo, M.; Ancona, V.; De Paola, D.; Losacco, D.; Campanale, C.; Massarelli, C.; Uricchio, V.F. Chromium Pollution in European Water, Sources, Health Risk, and Remediation Strategies: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 5438. [Google Scholar] [CrossRef] [PubMed]
- IARC (International Agency for Research on Cancer). Chromium (VI) Compounds. Monograph 100C; WHO: Geneva, Switzerland, 1990. [Google Scholar]
- Chowdhury, S.R.; Yanful, E.K. Arsenic and chromium removal by mixed magnetite–maghemite nanoparticles and the effect of phosphate on removal. J. Environ. Manag. 2010, 91, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human health and environmental toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef]
- Sepehr, M.N.; Nasseri, S.; Zarrabi, M.; Samarghandi, M.R.; Amrane, A. Removal of Cr(III) from tanning effluent by Aspergillus niger in airlift bioreactor. Sep. Purif. Technol. 2012, 96, 256–262. [Google Scholar] [CrossRef]
- Korkmaz, D. Precipitation titration: “determination of chloride by the Mohr method”. Methods 2001, 2, 1–6. [Google Scholar]
- Li, J.; Luo, G.; He, L.; Xu, J.; Lyu, J. Analytical approaches for determining chemical oxygen demand in water bodies: A review. Crit. Rev. Anal. Chem. 2018, 48, 47–65. [Google Scholar] [CrossRef]
- Guo, W.L.; Chen, M.; Pan, W.L.; Zhang, Q.; Xu, J.X.; Lin, Y.C.; Li, L.; Liu, B.; Bai, W.D.; Zhang, Y.Y.; et al. Hypoglycemic and hypolipidemic mechanism of organic chromium derived from chelation of Grifola frondosa polysaccharide-chromium (III) and its modulation of intestinal microflora in high fat-diet and STZ-induced diabetic mice. Int. J. Biol. Macromol. 2020, 145, 1208–1218. [Google Scholar] [CrossRef]
- Pantelić, M.; Jovanović, L.J.; Prodanović, R.; Vujanac, I.; Durić, M.; Culafić, T.; Vranješ-Đurić, S.; Korićanac, G.; Kirovski, D. The impact of the chromium supplementation on insulin signalling pathway in different tissues and milk yield in dairy cows. J. Anim. Physiol. Anim. Nutr. 2018, 102, 41–55. [Google Scholar]
- Lewicki, S.; Zdanowski, R.; Krzyzowska, M.; Lewicka, A.; Debski, B.; Niemcewicz, M.; Goniewicz, M. The role of chromium III in the organism and its possible use in diabetes and obesity treatment. Ann. Agric. Environ. Med. 2014, 21, 331–335. [Google Scholar] [CrossRef]
- Lapenna, D.; Ciofani, G. Chromium and human low-density lipoprotein oxidation. J. Trace Elem. Med. Biol. 2020, 59, 126411. [Google Scholar] [CrossRef] [PubMed]
- Hausladen, D.M.; Fendorf, S. Hexavalent chromium generation within naturally structured soils and sediments. Environ. Sci. Technol. 2017, 51, 2058–2067. [Google Scholar] [CrossRef] [PubMed]
- Clodfelder, B.J.; Chang, C.; Vincent, J.B. Absorption of the biomimetic chromium cation triaqua-mu3-oxo-mu-hexapropionatotrichromium(III) in rats. Biol. Trace Elem. Res. 2004, 98, 159–169. [Google Scholar] [CrossRef]
- Hininger, I.; Benaraba, R.; Osman, M.; Faure, H.; Roussel, A.M.; Anderson, R.A. Safety of trivalent chromium complexes: No evidence for DNA damage in human HaCaT keratinocytes. Free Rad. Biol. Med. 2007, 42, 1759–1765. [Google Scholar] [CrossRef]
- Salnikow, K.; Zhitkovich, A. Genetic and epigenetic mechanisms in metal carcinogenesis and cocarcinogenesis: Nickel, arsenic, and chromium. Chem. Res. Toxicol. 2008, 21, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Zhitkovich, A. Importance of chromium-DNA adducts in mutagenicity and toxicity of chromium(VI). Chem. Res. Toxicol. 2005, 18, 3–11. [Google Scholar] [CrossRef]
- Qu, Q.; Li, X.; An, F.; Jia, G.; Liu, L.; Watanabe-Meserve, H.; Koenig, K.; Coehn, B.; Costa, M.; Roy, N.; et al. CrVI exposure and biomarkers: Cr in erythrocytes in relation to exposure and polymorphisms of genes encoding anion transport proteins. Biomarkers 2008, 13, 467–477. [Google Scholar] [CrossRef] [PubMed]
- De Flora, S. Threshold mechanisms and site specificity in chromium(VI) carcinogenesis. Carcinogenesis 2000, 21, 533–541. [Google Scholar] [CrossRef]
- Zhitkovich, A. Chromium in drinking water: Sources, metabolism, and cancer risks. Chem. Res. Toxicol. 2011, 24, 1617–1629. [Google Scholar] [CrossRef]
- Sun, H.; Brocato, J.; Costa, M. Oral chromium exposure and toxicity. Curr. Environ. Health Rep. 2015, 2, 295–303. [Google Scholar] [CrossRef]
- Pavesi, T.; Moreira, J.C. Mechanisms and individuality in chromium toxicity in humans. J. Appl. Toxicol. 2020, 40, 1183–1197. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhou, X.; Chen, H.; Li, Q.; Costa, M. Modulation of histone methylation and MLH1 gene silencing by hexavalent chromium. Toxicol. Appl. Pharmacol. 2009, 237, 258–266. [Google Scholar] [PubMed]
- Arita, A.; Shamy, M.Y.; Chervona, Y.; Clancy, H.A.; Sun, H.; Hall, M.N.; Qu, Q.; Gamble, M.-V.; Costa, M. The effect of exposure to carcinogenic metals on histone tail modifications and gene expression in human subjects. J. Trace Elem. Med. Biol. 2012, 26, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Chervona, Y.; Costa, M. The control of histone methylation and gene expression by oxidative stress, hypoxia, and metals. Free Radic. Biol. Med. 2012, 53, 1041–1047. [Google Scholar] [CrossRef]
- Chen, D.; Kluz, T.; Fang, L.; Zhang, X.; Sun, H.; Jin, C.; Costa, M. Hexavalent chromium (Cr(VI)) down-regulates acetylation of histone H4 at lysine 16 through induction of stressor protein Nupr1. PLoS ONE 2016, 11, e0157317. [Google Scholar] [CrossRef]
- Kumpulainen, J.T. Chromium content of foods and diets. Biol. Trace Elem. Res. 1992, 32, 9–18. [Google Scholar] [CrossRef]
- Roussel, A.-M.; Andriollo-Sanchez, M.; Ferry, M.; Bryden, N.A.; Anderson, R.A. Food chromium content, dietary chromium intake and related biological variables in French free-living elderly. Br. J. Nutr. 2007, 98, 326–331. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc: A Report of the Panel on Micronutrients; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the safety of trivalent chromium as a nutrient added for nutritional purposes to foodstuffs for particular nutritional uses and foods intended for the general population (including food supplements). EFSA J. 2010, 8, 1882. [Google Scholar]
- Moffat, I.; Martinova, N.; Seidel, C.; Thompson, C.M. Hexavalent chromium in drinking water. J. Am. Water Work. Assoc. 2018, 110, E22–E35. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- EC (European Commission). Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Level of Trivalent Chromium, expressed in 4 April 2003. Available online: http://ec.europa.eu/food/fs/sc/scf/out197_en.pdf (accessed on 29 April 2010).
- Laschinsky, N.; Kottwitz, K.; Freund, B.; Dresow, B.; Fischer, R.; Nielsen, P. Bioavailability of chromium (III)-supplements in rats and humans. Biometals 2012, 25, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Zha, L.Y.; Xu, Z.R.; Wang, M.Q.; Gu, L.Y. Effects of chromium nanoparticle dosage on growth, body composition, serum hormones and tissue chromium in Sprague-Dawley rats. J. Zhejiang Univ. Sci. B 2007, 8, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Haldar, S.; Ghosh, T.K. Production and carcase traits in broiler chickens given diets supplemented with inorganic trivalent chromium and an organic acid blend. Br. Poult. Sci. 2008, 49, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Lukaski, H.C. Effects of chromium (III) as a nutritional supplement. In The Nutritional Biochemistry of Chromium (III); Elsevier: Amsterdam, The Netherlands, 2019; pp. 31–77. [Google Scholar]
- Vincent, J.B.; Lukaski, H.C. Chromium. Adv. Nutr. 2018, 9, 505–506. [Google Scholar] [CrossRef]
- Clodfelder, B.J.; Emamaullee, J.; Hepburn, D.D.; Chakov, N.E.; Nettles, H.S.; Vincent, J.B. The trail of chromium(III) in vivo from the blood to the urine: The roles of transferrin and chromodulin. J. Biol. Inorg. Chem. 2001, 6, 608–617. [Google Scholar] [CrossRef]
- Carocci, A.; Catalano, A.; Sinicropi, M.S.; Genchi, G. Oxidative stress and neurodegeneration: The involvement of iron. Biometals 2018, 31, 715–735. [Google Scholar] [CrossRef]
- Edwards, K.C.; Kim, H.; Vincent, J.B. Release of trivalent chromium from serum transferrin is sufficiently rapid to be physiologically relevant. J. Inorg. Biochem. 2020, 202, 110901. [Google Scholar] [CrossRef]
- Bjørklund, G.; Aaseth, J.; Skalny, A.V.; Suliburska, J.; Skalnaya, M.G.; Nikonorov, A.A.; Tinkov, A.A. Interactions of iron with manganese, zinc, chromium, and selenium as related to prophylaxis and treatment of iron deficiency. J. Trace Elem. Med. Biol. 2017, 41, 41–53. [Google Scholar] [CrossRef]
- Clodfelder, B.J.; Vincent, J.B. The time-dependent transport of chromium in adult rats from the bloodstream to the urine. J. Biol. Inorg. Chem. 2005, 10, 383–393. [Google Scholar] [CrossRef]
- Kirman, C.R.; Suh, M.; Proctor, D.M.; Hays, S.M. Improved physiologically based pharmacokinetic model for oral exposures to chromium in mice, rats, and humans to address temporal variation and sensitive populations. Toxicol. Appl. Pharmacol. 2017, 325, 9–17. [Google Scholar] [CrossRef]
- Baj, J.; Flieger, W.; Teresiński, G.; Buszewicz, G.; Sitarz, E.; Forma, A.; Karakuła, K.; Maciejewski, R. Magnesium, calcium, potassium, sodium, phosphorus, selenium, zinc, and chromium levels in alcohol use disorder: A review. J. Clin. Med. 2020, 9, 1901. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, K.; Mertz, W. Chromium(III) and the glucose tolerance factor. Arch. Biochem. Biophys. 1959, 85, 292–295. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Trace Elements in Human Nutrition and Health; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Lai, M.H.; Chen, Y.Y.; Cheng, H.H. Chromium yeast supplementation improves fasting plasma glucose and LDL-cholesterol in streptozotocin-induced diabetic rats. Int. J. Vitam. Nutr. Res. 2006, 76, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Schrauzer, G.N. Interactive effects of selenium and chromium on mammary tumor development and growth in MMTV-infected female mice and their relevance to human cancer. Biol. Trace Elem. Res. 2006, 109, 281–292. [Google Scholar] [CrossRef]
- Vincent, J.B. Is the pharmacological mode of action of chromium(III) as a second messenger? Biol. Trace Elem. Res. 2015, 166, 7–12. [Google Scholar] [CrossRef]
- Chen, G.; Liu, P.; Pattar, G.R.; Tackett, L.; Bhonagiri, P.; Strawbridge, A.B.; Elmendorf, J.S. Chromium activates glucose transporter 4 trafficking and enhances insulin-stimulated glucose transport in 3T3-L1 adipocytes via a cholesterol-dependent mechanism. Mol. Endocrinol. 2006, 20, 857–870. [Google Scholar] [CrossRef]
- Pattar, G.R.; Tackett, L.; Liu, P.; Elmendorf, J.S. Chromium picolinate positively influences the glucose transporter system via affecting cholesterol homeostasis in adipocytes cultured under hyperglycemic diabetic conditions. Mutat. Res. 2006, 610, 93–100. [Google Scholar] [CrossRef][Green Version]
- Raja, N.S.; Sankaranarayanan, K.; Dhathathreyan, A.; Nair, B.U. Interaction of chromium(III) complexes with model lipid bilayers: Implications on cellular uptake. Biochim. Biophys. Acta 2011, 1808, 332–340. [Google Scholar] [CrossRef]
- Adam, C.; Wohlfarth, J.; Haussmann, M.; Sennefelder, H.; Rodin, A.; Maler, M.; Martin, S.F.; Goebeler, M.; Schmidt, M. Allergy-inducing chromium compounds trigger potent innate immune stimulation via ROS-dependent inflammasome activation. J. Investig. Dermatol. 2017, 137, 367–376. [Google Scholar] [CrossRef]
- Bregnbak, D.; Johansen, J.D.; Jellesen, M.S.; Zachariae, C.; Menné, T.; Thyssen, J.P. Chromium allergy and dermatitis: Prevalence and main findings. Contact Dermat. 2015, 73, 261–280. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Wang, J.; Jia, H. Metagenome-wide association studies: Fine-mining the microbiome. Nat. Rev. Microbiol. 2016, 14, 508–522. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, S.R.; Defronzo, R.A. The insulin resistance syndrome: Physiological considerations. Diab. Vasc. Dis. Res. 2007, 4, 13–19. [Google Scholar] [CrossRef] [PubMed]
- San Mauro, I.; Ruiz-Leon, A.M.; Camina Martin, M.A.; Garicano-Vilar, E.; Collado-Yurrita, L.; de Mateo-Silleras, B.; del Río, P.R. Chromium supplementation in patients with type 2 diabetes and high risk of type 2 diabetes: A meta-analysis of randomized controlled trials. Nutr. Hosp. 2016, 33, 156–161. [Google Scholar] [CrossRef]
- Penumathsa, S.V.; Thirunavukkarasu, M.; Samuel, M.; Zhan, L.; Maulik, G.; Bagchi, M.; Bagchi, D.; Maulik, N. Niacin bound chromium treatment induces myocardial Glut-4 translocation and caveolar interaction via Akt, AMPK and eNOS phosphorylation in streptozotocin induced diabetic rats after ischemia-reperfusion injury. Biochim. Biophys. Acta 2009, 1792, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Kahlon, G.; Moorehead, L.; Dhawan, R.; Lieblong, B.; Stapleton, T.; Caldito, G.; Hoeldtke, R.; Levine, S.N.; Farrington Bass, P. Effect of chromium dinicocysteinate supplementation on circulating levels of insulin, TNF-α, oxidative stress and insulin resistance in type 2 diabetic patients: Randomized, double-blind, placebo-controlled study. Mol. Nutr. Food Res. 2012, 56, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Saiyed, Z.M.; Lugo, J.P. Impact of chromium dinicocysteinate supplementation on inflammation, oxidative stress, and insulin resistance in type 2 diabetic subjects: An exploratory analysis of a randomized, double–blind, placebo–controlled study. Food Nutr. Res. 2016, 60, 31762. [Google Scholar] [CrossRef] [PubMed]
- Abebe, W.; Liu, J.Y.; Wimborne, H.; Mozaffari, M.S. Effects of chromium picolinate on vascular reactivity and cardiac ischemia-reperfusion injury in spontaneously hypertensive rats. Pharmacol. Rep. 2010, 62, 674–682. [Google Scholar] [CrossRef]
- Grande, F.; Parisi, O.I.; Mordocco, R.A.; Rocca, C.; Puoci, F.; Scrivano, L.; Quintieri, A.M.; Cantafio, P.; Ferla, S.; Brancale, A.; et al. Quercetin derivatives as novel antihypertensive agents: Synthesis and physiological characterization. Eur. J. Pharm. Sci. 2016, 82, 161–170. [Google Scholar] [CrossRef]
- Hu, G.; Zheng, P.; Feng, H.; Jia, G. Imbalance of oxidative and reductive species involved in chromium(VI)-induced toxic effects. React. Oxyg. Species 2017, 3, 1–11. [Google Scholar]
- Rizza, P.; Pellegrino, M.; Caruso, A.; Iacopetta, D.; Sinicropi, M.S.; Rault, S.; Lancelot, J.C.; El-Kashef, H.; Lesnard, A.; Rochais, C.; et al. 3-(Dipropylamino)-5-hydroxybenzofuro[2,3-f]quinazolin-1(2H)-one (DPA-HBFQ-1) plays an inhibitory role on breast cancer cell growth and progression. Eur. J. Med. Chem. 2016, 107, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Wilbur, S.; Abadin, H.; Fay, M.; Yu, D.; Tencza, B.; Ingerman, L.; Klotzbach, J.; James, S. Toxicological Profile for Chromium; Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profiles: Atlanta, GA, USA, 2012.
- Rowbotham, A.L.; Levy, L.S.; Shuker, L.K. Chromium in the Environment: An Evaluation of Exposure of the UK General Population and Possible Adverse Health Effects. J. Toxicol. Environ. Health 2000, 3, 145–178. [Google Scholar]
- Meaza, I.; Speer, R.M.; Toyoda, J.H.; Lu, H.; Wise, S.S.; Croom-Perez, T.J.; Aboueissa, A.E.; Wise Sr, J.P. Prolonged exposure to particulate Cr (VI) is cytotoxic and genotoxic to fin whale cells. J. Trace Elem. Med. Biol. 2020, 126562. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, M.; Pei, L.; Zhang, R.; Liu, X.; Wei, L.; Yang, M.; Xu, Q. Oxidative stress and DNA damage in a long-term hexavalent chromium exposed population in North China: A cross-sectional study. BMJ Open 2018, 8, 021470. [Google Scholar] [CrossRef] [PubMed]
- Paustenbach, D.; Finley, B.; Mowat, F.; Kerger, B. Human health risk and exposure assessment of chromium (VI) in tap water. J. Toxicol. Environ. Health Part A 2003, 66, 1295–1339. [Google Scholar] [CrossRef] [PubMed]
- Vaiopoulou, E.; Gikas, P. Regulations for chromium emissions to the aquatic environment in Europe and elsewhere. Chemosphere 2020, 254, 126876. [Google Scholar] [CrossRef]
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 419. [Google Scholar] [CrossRef]
- Ertani, A.; Mietto, A.; Borin, M.; Nardi, S. Chromium in agricultural soils and crops: A review. Water Air Soil Pollut. 2017, 228, 190. [Google Scholar] [CrossRef]
- GracePavithra, K.; Jaikumar, V.; Kumar, P.S.; SundarRajan, P. A review on cleaner strategies for chromium industrial wastewater: Present research and future perspective. J. Clean. Prod. 2019, 228, 580–593. [Google Scholar] [CrossRef]
- Aslam, S.; Yousafzai, A.M. Chromium toxicity in fish: A review article. J. Entomol. Zool. Stud. 2017, 5, 1483–1488. [Google Scholar]
- Chen, M.; Lu, G.; Wu, J.; Yang, C.; Niu, X.; Tao, X.; Shi, Z.; Yi, X.; Dang, Z. Migration and fate of metallic elements in a waste mud impoundment and affected river downstream: A case study in Dabaoshan Mine, South China. Ecotoxicol. Environ. Saf. 2018, 164, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Guo, C.; Zeng, Y.; Tu, Z.; Ji, Y.; Reinfelder, J.R.; Chen, M.; Huang, W.; Lu, G.; Yi, X.; et al. The behavior of chromium and arsenic associated with redox transformation of schwertmannite in AMD environment. Chemosphere 2019, 222, 945–953. [Google Scholar] [CrossRef]
- Wang, B.J.; Sheu, H.M.; Guo, Y.L.; Lee, Y.H.; Lai, C.S.; Pan, M.H.; Wang, Y.J. Hexavalent chromium induced ROS formation, Akt, NF-kappaB, and MAPK activation, and TNF-alpha and IL-1alpha production in keratinocytes. Toxicol. Lett. 2010, 198, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, B., Jr.; Chiu, H.W.; Lee, Y.L.; Li, C.Y.; Wang, Y.J.; Lee, Y.H. Pterostilbene attenuates hexavalent chromium-induced allergic contact dermatitis by preventing cell apoptosis and inhibiting IL-1β-related NLRP3 inflammasome activation. J. Clin. Med. 2018, 7, 489. [Google Scholar] [CrossRef]
- Hegazy, R.; Salama, A.; Mansour, D.; Hassan, A. Renoprotective effect of lactoferrin against chromium-induced acute kidney injury in rats: Involvement of IL-18 and IGF-1 inhibition. PLoS ONE 2016, 11, e0151486. [Google Scholar] [CrossRef]
- Sahu, B.D.; Koneru, M.; Bijargi, S.R.; Kota, A.; Sistla, R. Chromium-induced nephrotoxicity and ameliorative effect of carvedilol in rats: Involvement of oxidative stress, apoptosis and inflammation. Chem. Biol. Interact. 2014, 223, 69–79. [Google Scholar] [CrossRef]
- Wu, Y.H.; Lin, J.C.; Wang, T.Y.; Lin, T.J.; Yen, M.C.; Liu, Y.H.; Wu, P.L.; Chen, F.W.; Shih, Y.L.; Yeh, I.J. Hexavalent chromium intoxication induces intrinsic and extrinsic apoptosis in human renal cells. Mol. Med. Rep. 2020, 21, 851–857. [Google Scholar] [CrossRef]
- Molina-Jijón, E.; Tapia, E.; Zazueta, C.; El Hafidi, M.; Zatarain-Barrón, Z.L.; Hernández-Pando, R.; Pedraza-Chaverri, J. Curcumin prevents Cr(VI)-induced renal oxidant damage by a mitochondrial pathway. Free Radic. Biol. Med. 2011, 51, 1543–1557. [Google Scholar] [CrossRef]
- Barhoma, R.A. The role of eugenol in the prevention of chromium-induced acute kidney injury in male albino rats. Alexandria J. Med. 2018, 54, 711–715. [Google Scholar] [CrossRef]
- Santonen, T.; Alimonti, A.; Bocca, B.; Duca, R.C.; Galea, K.S.; Godderis, L.; Göen, T.; Gomes, B.C.; Hanser, O.; Iavicoli, I.; et al. Setting up a collaborative European human biological monitoring study on occupational exposure to hexavalent chromium. Environ. Res. 2019, 177, 108583. [Google Scholar] [CrossRef]
- Núñez, O.; Fernández-Navarro, P.; Martín-Méndez, I.; Bel-Lan, A.; Locutura, J.F.; López-Abente, G. Arsenic and chromium topsoil levels and cancer mortality in Spain. Environ. Sci. Pollut. Res. Int. 2016, 23, 17664–17675. [Google Scholar] [CrossRef] [PubMed]
- El-Atta, H.M.A.; El-Bakary, A.A.; Attia, A.M.; Lotfy, A.; Khater, S.S.; Elsamanoudy, A.Z.; Abdalla, H.A. DNA fragmentation, caspase 3 and prostate-specific antigen genes expression induced by arsenic, cadmium, and chromium on nontumorigenic human prostate cells. Biol. Trace Elem. Res. 2014, 162, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cai, K.; Feng, Q.; Xu, Y.; Zhang, Z. Chromium (VI) promotes cell migration through targeting epithelial-mesenchymal transition in prostate cancer. Toxicol. Lett. 2019, 300, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Li, Y.; Luo, L.; Xie, Y.; Zeng, M.; Wang, A.; Chen, H.; Zhong, C. Role of mitochondrial electron transport chain dysfunction in Cr(VI)-induced cytotoxicity in L-02 hepatocytes. Cell. Physiol. Biochem. 2014, 33, 1013–1025. [Google Scholar]
- Zhong, X.; Zhong, C. Mitochondrial biogenesis in response to chromium(VI) toxicity in human liver cells. Int. J. Mol. Sci. 2017, 18, 1877. [Google Scholar] [CrossRef]
- Yan, J.; Huang, H.; Liu, Z.; Shen, J.; Ni, J.; Han, J.; Wang, R.; Lin, D.; Hu, B.; Jin, L. Hedgehog signaling pathway regulates hexavalent chromium-induced liver fibrosis by activation of hepatic stellate cells. Toxicol. Lett. 2020, 320, 1–8. [Google Scholar] [CrossRef]
- Salama, A.; Hegazy, R.; Hassan, A. Intranasal chromium induces acute brain and lung injuries in rats: Assessment of different potential hazardous effects of environmental and occupational exposure to chromium and introduction of a novel pharmacological and toxicological animal model. PLoS ONE 2016, 11, e0168688. [Google Scholar]
- Urbano, A.M.; Ferreira, L.M.; Alpoim, M.C. Molecular and cellular mechanisms of hexavalent chromium–induced lung cancer: An updated perspective. Curr. Drug Metab. 2012, 13, 284–305. [Google Scholar] [CrossRef]
- Han, B.; Li, S.; Lv, Y.; Yang, D.; Li, J.; Yang, Q.; Wu, P.; Lv, Z.; Zhang, Z. Dietary melatonin attenuates chromium-induced lung injury via activating the Sirt1/Pgc-1α/Nrf2 pathway. Food Funct. 2019, 10, 5555–5565. [Google Scholar] [CrossRef]
- Lv, Y.; Jiang, H.; Li, S.; Han, B.; Liu, Y.; Yang, D.; Zhang, Z. Sulforaphane prevents chromium-induced lung injury in rats via activation of the Akt/GSK-3β/Fyn pathway. Environ. Pollut. 2020, 259, 113812. [Google Scholar] [CrossRef]
- Banu, S.K.; Stanley, J.A.; Sivakumar, K.K.; Arosh, J.A.; Taylor, R.J.; Burghardt, R.C. Chromium VI−Induced developmental toxicity of placenta is mediated through spatiotemporal dysregulation of cell survival and apoptotic proteins. Reprod. Toxicol. 2017, 68, 171–190. [Google Scholar] [CrossRef]
- Banu, S.K.; Stanley, J.A.; Sivakumar, K.K.; Arosh, J.A.; Burghardt, R.C. Resveratrol protects the ovary against chromium-toxicity by enhancing endogenous antioxidant enzymes and inhibiting metabolic clearance of estradiol. Toxicol. Appl. Pharmacol. 2016, 303, 65–78. [Google Scholar] [CrossRef]
- Koturbash, I.; Beland, F.A.; Pogribny, I.P. Role of epigenetic events in chemical carcinogenesis—a justification for incorporating epigenetic evaluations in cancer risk assessment. Toxicol. Mech. Methods 2011, 21, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Pillai, R.S.; Bhattacharyya, S.N.; Filipowicz, W. Repression of protein synthesis by miRNAs: How many mechanisms? Trends Cell. Biol. 2007, 17, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Mott, J.L.; Mohr, A.M. Overview of microRNA biology. Semin. Liver Dis. 2015, 35, 3–11. [Google Scholar]
- Lou, J.; Wang, Y.; Yao, C.; Jin, L.; Wang, X.; Xiao, Y.; Wu, N.; Song, P.; Song, Y.; Tan, Y.; et al. Role of DNA methylation in cell cycle arrest induced by Cr(VI) in two cell lines. PLoS ONE 2013, 8, e71031. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Li, P.; Li, Y.; Wang, T.; Gao, X.; Zhang, W.; Jia, G. Methylation levels of P16 and TP53 that are involved in DNA strand breakage of 16HBE cells treated by hexavalent chromium. Toxicol. Lett. 2016, 249, 15–21. [Google Scholar] [CrossRef]
- Hu, G.; Li, P.; Cui, X.; Li, Y.; Zhang, J.; Zhai, X.; Yu, S.; Tang, S.; Zhao, Z.; Wang, J.; et al. Cr(VI)-induced methylation and down-regulation of DNA repair genes and its association with markers of genetic damage in workers and 16HBE cells. Environ. Pollut. 2018, 238, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Yang, L.; Huang, H.; Pang, L.; Hu, G.; Liu, Q.; Yuan, J.; Liu, J.; Xia, Y.; Zhuang, Z. Chromium(VI) causes down regulation of biotinidase in human bronchial epithelial cells by modifications of histone acetylation. Toxicol. Lett. 2011, 205, 140–145. [Google Scholar] [CrossRef]
- Xia, B.; Ren, X.; Zhuang, Z.; Yang, L.; Huang, H.; Pang, L.; Wu, D.; Luo, J.; Tan, Y.; Liu, J.; et al. Effect of hexavalent chromium on histone biotinylation in human bronchial epithelial cells. Toxicol. Lett. 2014, 228, 241–247. [Google Scholar] [CrossRef]
- Chong, T.L.; Ahearn, E.L.; Cimmino, L. Reprogramming the epigenome with vitamin C. Front. Cell Dev. Biol. 2019, 7, 128. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, K.A.; Vaghjiani, R.J.; Nemec, A.A.; Klei, L.R.; Barchowsky, A. Cr(VI)-stimulated STAT3 tyrosine phosphorylation and nuclear translocation in human airway epithelial cells requires Lck. Biochem. J. 2007, 402, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Pandey, A.; Chowdhuri, D.K. MiRNA profiling provides insights on adverse effects of Cr(VI) in the midgut tissues of Drosophila melanogaster. J. Hazard. Mater. 2015, 283, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.L.; Zhang, H.Y.; Shi, J.X.; Langrish, T.A.G. Adsorption of chromium(VI) from aqueous solutions using cross-linked magnetic chitosan beads. Ind. Eng. Chem. Res. 2009, 48, 2646–2651. [Google Scholar] [CrossRef]
- Santhosh, C.; Daneshvar, E.; Kollu, P.; Peraniemi, S.; Grace, A.N.; Bhatnagar, A. Magnetic SiO2-CoFe2O4 nanoparticles decorated on graphene oxide as efficient adsorbents for the removal of anionic pollutants from water. Chem. Eng. J. 2017, 322, 472–487. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Gupta, A.; Kaur, A.; Malik, D. Alleviation of hexavalent chromium by using microorganisms: Insight into the strategies and complications. Water Sci. Technol. 2019, 79, 411–424. [Google Scholar] [CrossRef]
- Singh, V.; Chauhan, P.K.; Kanta, R.; Dhewa, T.; Kumar, V. Isolation and characterization of Pseudomonas resistant to heavy metals contaminants. Int. J. Pharm. Sci. Rev. Res. 2010, 3, 164–167. [Google Scholar]
- Gong, D.; Ye, F.; Pang, C.; Lu, Z.; Shang, C. Isolation and Characterization of Pseudomonas sp. Cr13 and Its Application in Removal of Heavy Metal Chromium. Curr. Microbiol. 2020, 77, 3661–3670. [Google Scholar] [CrossRef]
- Pang, B.; Lv, L.; Pang, C.; Ye, F.; Shang, C. Optimization of Growth Conditions of Acinetobacter sp. Cr1 for Removal of Heavy Metal Cr Using Central Composite Design. Curr. Microbiol. 2020. [Google Scholar] [CrossRef]
- Guo, S.; Xiao, C.; Zhou, N.; Chi, R. Speciation, toxicity, microbial remediation and phytoremediation of soil chromium contamination. Environ. Chem. Lett. 2020, 1–19. [Google Scholar] [CrossRef]
- Su, X.; Kushima, A.; Halliday, C.; Zhou, J.; Li, J.; Hatton, T.A. Electrochemically-mediated selective capture of heavy metal chromium and arsenic oxyanions from water. Nat. Commun. 2018, 9, 1–9. [Google Scholar]
- Petrella, A.; Spasiano, D.; Acquafredda, P.; De Vietro, N.; Ranieri, E.; Cosma, P.; Petruzzelli, D. Heavy metals retention (Pb (II), Cd (II), Ni (II)) from single and multimetal solutions by natural biosorbents from the olive oil milling operations. Process Saf. Environ. Prot. 2018, 114, 79–90. [Google Scholar] [CrossRef]
- Carocci, A.; Catalano, A.; Lauria, G.; Sinicropi, M.S.; Genchi, G. Lead toxicity, antioxidant defense and environment. In Reviews of Environmental Contamination and Toxicology; Springer: Cham, Switzerland, 2016; pp. 45–67. [Google Scholar]
- Ranieri, E.; Moustakas, K.; Barbafieri, M.; Ranieri, A.C.; Herrera-Melián, J.A.; Petrella, A.; Tommasi, F. Phytoextraction technologies for mercury-and chromium-contaminated soil: A review. J. Chem. Technol. Biotechnol. 2020, 95, 317–327. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Carocci, A.; Lauria, G.; Catalano, A. Mercury exposure and heart diseases. Int. J. Environ. Res. Public Health 2017, 14, 74. [Google Scholar] [CrossRef]
- Nguyen, A.; Le, B.V.; Richter, O. The Role of Mangroves in the Retention of Heavy Metal (Chromium): A Simulation Study in the Thi Vai River Catchment, Vietnam. Int. J. Environ. Res. Public Health 2020, 17, 5823. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, K.; Sudha, P.N.; Sukumaran, A. Novel chitosan based thin sheet nanofiltration membrane for rejection of heavy metal chromium. Int. J. Biol. Macromol. 2019, 132, 939–953. [Google Scholar]
- Younan, S.; Sakita, G.Z.; Albuquerque, T.R.; Keller, R.; Bremer-Neto, H. Chromium (VI) bioremediation by probiotics. J. Sci. Food Agric. 2016, 96, 3977–3982. [Google Scholar] [CrossRef]


| Atomic number | 24 |
| Atomic weight | 51.9961 u |
| Atomic radius | 130 pm |
| Electronic configuration | [Ar] 4s13d5 |
| Melting point | 1907 °C |
| Boiling point | 2672 °C |
| Density at 20 °C | 7.18 g/cm3 |
| Heat of fusion | 21 KJ/mol |
| Heat of vaporization | 342 KJ/mol |
| Pauling electronegativity number | 1.66 |
| First ionization energy | 652.4 KJ/mol |
| Second ionization energy | 1590.6 KJ/mol |
| Third ionization energy | 2987 KJ/mol |
| Form | Uses |
|---|---|
| Cr(O) | Stainless steel production |
| Alloy production | |
| Metal and alloy manufacturing | |
| Cr(III) | Metal and alloy manufacturing |
| Brick lining | |
| Chrome plating and welding | |
| Leather tanning | |
| Textiles | |
| Cr(VI) | Chrome plating and welding |
| Copying machine toner | |
| Chrome plating | |
| Leather tanning | |
| Textiles | |
| Wood preservatives |
| Food | Cr (µg/kg) |
|---|---|
| Mussels | 128 |
| Brewer’s yeast | 112 |
| Brazil nuts | 100 |
| Oysters | 57 |
| Wholemeal bread | 42 |
| Rye bread | 30 |
| Dried dates | 29 |
| Pears | 27 |
| Shrimps | 26 |
| Broccoli | 25 |
| Whole wheat flour | 21 |
| Tomatoes | 20 |
| Whole meal barley | 13 |
| Hazelnuts | 12 |
| Whole corn | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. The Double Face of Metals: The Intriguing Case of Chromium. Appl. Sci. 2021, 11, 638. https://doi.org/10.3390/app11020638
Genchi G, Lauria G, Catalano A, Carocci A, Sinicropi MS. The Double Face of Metals: The Intriguing Case of Chromium. Applied Sciences. 2021; 11(2):638. https://doi.org/10.3390/app11020638
Chicago/Turabian StyleGenchi, Giuseppe, Graziantonio Lauria, Alessia Catalano, Alessia Carocci, and Maria Stefania Sinicropi. 2021. "The Double Face of Metals: The Intriguing Case of Chromium" Applied Sciences 11, no. 2: 638. https://doi.org/10.3390/app11020638
APA StyleGenchi, G., Lauria, G., Catalano, A., Carocci, A., & Sinicropi, M. S. (2021). The Double Face of Metals: The Intriguing Case of Chromium. Applied Sciences, 11(2), 638. https://doi.org/10.3390/app11020638







