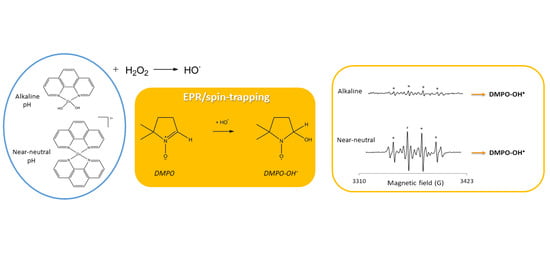
Hydroxyl Radical Generation by the H2O2/CuII/Phenanthroline System under Both Neutral and Alkaline Conditions: An EPR/Spin-Trapping Investigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Analysis Conditions
2.1.1. Chemical Preparation
2.1.2. EPR Experiments
2.2. Result Analysis: Integration and Simulations
3. Results and Discussion
3.1. Results at Near-Neutral pH
3.2. Results at Alkaline pH
4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sigman, D.S.; Graham, D.R.; D’Aurora, V.; Stern, A.M. Oxygen-dependent cleavage of DNA by the 1,10-phenanthroline cuprous complex. Inhibition of Escherichia coli DNA polymerase I. J. Biol. Chem. 1979, 254, 12269–12272. [Google Scholar] [CrossRef]
- Sigman, D.S. Nuclease activity of 1,10-phenanthroline-copper ion. Acc. Chem. Res. 1986, 19, 180–186. [Google Scholar] [CrossRef]
- Thederahn, T.B.; Kuwabara, M.D.; Larsen, T.A.; Sigman, D.S. Nuclease activity of 1,10-phenanthroline-copper: Kinetic mechanism. J. Am. Chem. Soc. 1989, 111, 4941–4946. [Google Scholar] [CrossRef]
- Oyoshi, T.; Sugiyama, H. Mechanism of DNA Strand Scission Induced by (1,10-Phenanthroline)copper Complex: Major Direct DNA Cleavage Is Not through 1′,2′-Dehydronucleotide Intermediate nor β-Elimination of Forming Ribonolactone. J. Am. Chem. Soc. 2000, 122, 6313–6314. [Google Scholar] [CrossRef]
- Chikira, M.; Tomizawa, Y.; Fukita, D.; Sugizaki, T.; Sugawara, N.; Yamazaki, T.; Sasano, A.; Shindo, H.; Palaniandavar, M.; Antholine, W.E. DNA-fiber EPR study of the orientation of Cu(II) complexes of 1,10-phenanthroline and its derivatives bound to DNA: Mono(phenanthroline)-copper(II) and its ternary complexes with amino acids. J. Inorg. Biochem. 2002, 89, 163–173. [Google Scholar] [CrossRef]
- Lu, L.-P.; Zhu, M.-L.; Yang, P. Crystal structure and nuclease activity of mono(1,10-phenanthroline) copper complex. J. Inorg. Biochem. 2003, 95, 31–36. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, J.; Li, Q.; Wang, L.; Liao, Z.; Xu, H. DNA damage by copper(II) complexes: Coordination-structural dependence of reactivities. J. Inorg. Biochem. 1999, 75, 233–240. [Google Scholar] [CrossRef]
- Sigman, D.S.; Mazumder, A.; Perrin, D.M. Chemical nucleases. Chem. Rev. 1993, 93, 2295–2316. [Google Scholar] [CrossRef]
- Simunkova, M.; Lauro, P.; Jomova, K.; Hudecova, L.; Danko, M.; Alwasel, S.; Alhazza, I.M.; Rajcaniova, S.; Kozovska, Z.; Kucerova, L.; et al. Redox-cycling and intercalating properties of novel mixed copper(II) complexes with non-steroidal anti-inflammatory drugs tolfenamic, mefenamic and flufenamic acids and phenanthroline functionality: Structure, SOD-mimetic activity, interaction with albumin, DNA damage study and anticancer activity. J. Inorg. Biochem. 2019, 194, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Lachenal, D.; Marlin, N. Production of pure cellulose from Kraft pulp by a totally chlorine-free process using catalyzed hydrogen peroxide. Ind. Crop. Prod. 2013, 49, 844–850. [Google Scholar] [CrossRef]
- Halma, M.; Lachenal, D.; Marlin, N.; Deronzier, A.; Brochier, M.C.; Zarubin, M. H2O2 oxidation of lignin model dimers catalyzed by copper(II)– phenanthroline. Ind. Crop. Prod. 2015, 74, 514–522. [Google Scholar] [CrossRef]
- Walger, E.; Rivollier, C.; Marlin, N.; Mortha, G. Activated hydrogen peroxide decolorization of a model azo dye-colored pulp. Holzforschung 2015, 69, 677–683. [Google Scholar] [CrossRef]
- Hanna, P.M.; Mason, R.P. Direct evidence for inhibition of free radical formation from Cu(I) and hydrogen peroxide by glutathione and other potential ligands using the EPR spin-trapping technique. Arch. Biochem. Biophys. 1992, 295, 205–213. [Google Scholar] [CrossRef]
- Buettner, G.R. Spin Trapping: ESR parameters of spin adducts 1474 1528V. Free Radic. Biol. Med. 1987, 3, 259–303. [Google Scholar] [CrossRef]
- Burkitt, M.J.; Ying Tsang, S.; Ching Tam, S.; Bremner, I. Generation of 5,5-Dimethyl-1-pyrroline N-Oxide Hydroxyl and Scavenger Radical Adducts from Copper/H2O2 Mixtures: Effects of Metal Ion Chelation and the Search for High-Valent Metal–Oxygen Intermediates. Arch. Biochem. Biophys. 1995, 323, 63–70. [Google Scholar] [CrossRef]
- Finkelstein, E.; Rosen, G.M.; Rauckman, E.J. Spin trapping of superoxide and hydroxyl radical: Practical aspects. Arch. Biochem. Biophys. 1980, 200, 1–16. [Google Scholar] [CrossRef]
- Lauricella, R.; Tuccio, B. Détection et caractérisation de radicaux libres après piégeage de spins. In La Spectroscopie de Résonance Paramagnétique Électronique: Applications; EDP Sciences: Les Ulis, France, 2014; pp. 49–78. ISBN 978-2-7598-1292-9. [Google Scholar]
- Eberson, L.; Balinov, B.; Hagelin, G.; Dugstad, H.; Thomassen, T.; Forngren, B.H.; Forngren, T.; Hartvig, P.; Markides, K.; Yngve, U.; et al. Formation of Hydroxyl Spin Adducts via Nucleophilic Addition--Oxidation to 5,5-Dimethyl-1-pyrroline N-Oxide (DMPO). Acta Chem. Scand. 1999, 53, 584–593. [Google Scholar] [CrossRef] [Green Version]
- Forrester, A.R.; Hepburn, S.P. Spin traps. A cautionary note. J. Chem. Soc. C Org. 1971, 701–703. [Google Scholar] [CrossRef]
- Hanna, P.M.; Chamulitrat, W.; Mason, R.P. When are metal ion-dependent hydroxyl and alkoxyl radical adducts of 5,5-dimethyl-1-pyrroline N-oxide artifacts? Arch. Biochem. Biophys. 1992, 296, 640–644. [Google Scholar] [CrossRef]
- Romo, A.I.B.; Dibo, V.S.; Abreu, D.S.; Carepo, M.S.P.; Neira, A.C.; Castillo, I.; Lemus, L.; Nascimento, O.R.; Bernhardt, P.V.; Sousa, E.H.S.; et al. Ascorbyl and hydroxyl radical generation mediated by a copper complex adsorbed on gold. Dalton Trans. 2019, 48, 14128–14137. [Google Scholar] [CrossRef]
- Villamena, F.A.; Hadad, C.M.; Zweier, J.L. Kinetic Study and Theoretical Analysis of Hydroxyl Radical Trapping and Spin Adduct Decay of Alkoxycarbonyl and Dialkoxyphosphoryl Nitrones in Aqueous Media. J. Phys. Chem. A 2003, 107, 4407–4414. [Google Scholar] [CrossRef]
- Meissner, G.; Henglein, A.; Beck, G. Pulsradiolytische Untersuchung von Dimethylthioäther und Dimethylsulfoxyd in wäßriger Lösung. Z. Für Nat. B 1967, 22, 13–19. [Google Scholar] [CrossRef]
- Dorfman, L.M.; Adams, G.E. Reactivity of the Hydroxyl Radical in Aqueous Solutions; National Standard Reference Data System; U.S. Department of Commerce-National Bureau of Standards: Washington, DC, USA, 1973; Volume 46. [Google Scholar]
- Cederbaum, A.I.; Dicker, E.; Rubin, E.; Cohen, G. The effect of dimethylsulfoxide and other hydroxyl radical scavengers on the oxidation of ethanol by rat liver microsomes. Biochem. Biophys. Res. Commun. 1977, 78, 1254–1262. [Google Scholar] [CrossRef]
- Anbar, M.; Neta, P. A compilation of specific bimolecular rate constants for the reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals with inorganic and organic compounds in aqueous solution. Int. J. Appl. Radiat. Isot. 1967, 18, 493–523. [Google Scholar] [CrossRef]
- Duling, D.R. Simulation of multiple isotropic spin-trap EPR spectra. J. Magn. Reson. B 1994, 104, 105–110. [Google Scholar] [CrossRef] [PubMed]
- SimEPR Manual. Available online: http://www.niehs.nih.gov/research/resources/software/tox-pharm/tools/simepr/index.cfm (accessed on 23 May 2016).
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef]
- Molton, F. Simultispin: A versatile graphical user interface for the simulation of solid-state continuous wave EPR spectra. Magn. Reson. Chem. 2020, 58, 718–726. [Google Scholar] [CrossRef]
- Zalibera, M.; Rapta, P.; Staško, A.; Brindzová, L.; Brezová, V. Thermal generation of stable spin trap adducts with super-hyperfine structure in their EPR spectra: An alternative EPR spin trapping assay for radical scavenging capacity determination in dimethylsulphoxide. Free Radic. Res. 2009, 43, 457–469. [Google Scholar] [CrossRef]
- Dvoranová, D.; Barbieriková, Z.; Brezová, V. Radical intermediates in photoinduced reactions on TiO2 (an EPR spin trapping study). Molecules 2014, 19, 17279–17304. [Google Scholar] [CrossRef] [Green Version]
- Mossoba, M.M.; Makino, K.; Riesz, P.; Perkins, R.C. Long-range proton hyperfine coupling in alicyclic nitroxide radicals by electron paramagnetic resonance. J. Phys. Chem. 1984, 88, 4717–4723. [Google Scholar] [CrossRef]
- Finkelstein, E.; Rosen, G.M.; Rauckman, E.J. Spin trapping. Kinetics of the reaction of superoxide and hydroxyl radicals with nitrones. J. Am. Chem. Soc. 1980, 102, 4994–4999. [Google Scholar] [CrossRef]
- Legge, R.L.; Thompson, J.E.; Baker, J.E. Free radical-mediated formation of ethylene from 1-aminocyclopropane-1-carboxylic acid: A spin-trap study. Plant Cell Physiol 1982, 23, 171–177. [Google Scholar]
- Gilbert, B.C.; Silvester, S.; Walton, P.H. Spectroscopic, kinetic and mechanistic studies of the influence of ligand and substrate concentration on the activation by peroxides of CuI–thiolate and other CuI complexes. J. Chem. Soc. Perkin Trans. 1999, 2, 1115–1122. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Deterding, L.J.; Chatterjee, S.; Jiang, J.; Ehrenshaft, M.; Lardinois, O.; Ramirez, D.C.; Tomer, K.B.; Mason, R.P. Site-specific radical formation in DNA induced by Cu(II)–H2O2 oxidizing system, using ESR, immuno-spin trapping, LC-MS, and MS/MS. Free Radic. Biol. Med. 2011, 50, 1536–1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nerud, F.; Baldrian, P.; Gabriel, J.; Ogbeifun, D. Decolorization of synthetic dyes by the Fenton reagent and the Cu/pyridine/H2O2 system. Chemosphere 2001, 44, 957–961. [Google Scholar] [CrossRef]
- Sippola, V.O.; Krause, A.O.I. Bis(o-phenanthroline)copper-catalysed oxidation of lignin model compounds for oxygen bleaching of pulp. Catal. Today 2005, 100, 237–242. [Google Scholar] [CrossRef]
- Korpi, H.; Figiel, P.J.; Lankinen, E.; Ryan, P.; Leskelä, M.; Repo, T. On in situ prepared Cu–Phenanthroline complexes in aqueous alkaline solutions and their use in the catalytic oxidation of veratryl alcohol. Eur. J. Inorg. Chem. 2007, 2007, 2465–2471. [Google Scholar] [CrossRef]
- Gueneau, B.; Marlin, N.; Deronzier, A.; Lachenal, D. Pulp delignification with oxygen and copper(II)-polyimine complexes. Holzforschung 2014, 68. [Google Scholar] [CrossRef]
- Walger, E.; Khairumuzdanial, M.; Marlin, N.; Mortha, G.; Molton, F.; Duboc, C. Use of copper(II)-phenanthroline as a hydrogen peroxide activator for dyed pulp color-stripping—Investigation of the chemical mechanism. In Proceedings of the ISWFPC 2015 Proceedings, Vienna, Austria, 9–11 September 2015; Volume 2, pp. 250–253. [Google Scholar]










| Entry | HCOO− | DMSO | H2O2 | Additive | Details | Total Peak Area (a.u.) | DMPO–OH (%) | DMPO–CH3 (%) | DMPO–COO− (%) | DMPO–R (%) | Triplet (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (a) | |||||||||||
| 1 | CuSO4 | 1.12 | 5 | 74 | 8 | ||||||
| 2 | Cu-Phen | 1.46 | 6 | 77 | 17 | ||||||
| 3 | x | Cu-Phen | 1.29 | 38 | 54 | 8 | |||||
| 4 | x | x | Cu-Phen | 1.2 | 18 | 73 | 4.5 | 4.5 | |||
| 5 | x | x | Cu-Phen | 0.65 | 18 | 49 | 28 | 5 | |||
| 6 | x | x | Cu-Phen | +10′ b | 0.54 | 14 | 70 | 12 | 4 | ||
| 7 | x | x | Cu-Phen | H a | 0.79 | 10 | 79 | 8 | 3 | ||
| 8 | x | CuSO4 | 0.89 | 7 | 57 | 16 | |||||
| 9 | x | x | CuSO4 | 0.83 | 16 | 76 | 8 | ||||
| 10 | x | x | CuSO4 | 0.21 | 15 | 23 | 34 | 10 | |||
| 11 | x | FeSO4 | pH 3 | 0.43 | 86 | 7 | 7 | ||||
| 12 | x | CuSO4 | 0.82 | 14 | 78 | 8 | |||||
| 13 | x | Cu-Phen | 1.0 | 22 | 72 | 6 | |||||
| 14 | x | Cu-Phen | 0.79 | 19 | 11 | 58 | 12 | ||||
| 15 | x | Cu-Phen | H | 0.84 | 11 | 72 | 13 | 4 | |||
| 16 | x | / | H | 0.35 | 38 | 38 | 24 | ||||
| (b) | |||||||||||
| 17 | CuSO4 | 0.62 | 2 | 72 | 6 | ||||||
| 18 | Cu-Phen | 0.55 | 17 | 58 | 10 | ||||||
| 19 | x | Cu-Phen | +8′ c | 0.54 | 92 | 8 | |||||
| 20 | x | Cu-Phen | 0.72 | 21 | 69 | 11 | |||||
| 21 | x | x | Cu-Phen | 0.12 | 16 | 80 | 5 | ||||
| 22 | x | x | Cu-Phen | 0.72 | 17 | 47 | 28 | 8 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walger, E.; Marlin, N.; Mortha, G.; Molton, F.; Duboc, C. Hydroxyl Radical Generation by the H2O2/CuII/Phenanthroline System under Both Neutral and Alkaline Conditions: An EPR/Spin-Trapping Investigation. Appl. Sci. 2021, 11, 687. https://doi.org/10.3390/app11020687
Walger E, Marlin N, Mortha G, Molton F, Duboc C. Hydroxyl Radical Generation by the H2O2/CuII/Phenanthroline System under Both Neutral and Alkaline Conditions: An EPR/Spin-Trapping Investigation. Applied Sciences. 2021; 11(2):687. https://doi.org/10.3390/app11020687
Chicago/Turabian StyleWalger, Elsa, Nathalie Marlin, Gérard Mortha, Florian Molton, and Carole Duboc. 2021. "Hydroxyl Radical Generation by the H2O2/CuII/Phenanthroline System under Both Neutral and Alkaline Conditions: An EPR/Spin-Trapping Investigation" Applied Sciences 11, no. 2: 687. https://doi.org/10.3390/app11020687
APA StyleWalger, E., Marlin, N., Mortha, G., Molton, F., & Duboc, C. (2021). Hydroxyl Radical Generation by the H2O2/CuII/Phenanthroline System under Both Neutral and Alkaline Conditions: An EPR/Spin-Trapping Investigation. Applied Sciences, 11(2), 687. https://doi.org/10.3390/app11020687