Impact of Dredged Material Disposal on Heavy Metal Concentrations and Benthic Communities in Huangmao Island Marine Dumping Area near Pearl River Estuary
Abstract
:1. Introduction
2. Materials and Methods
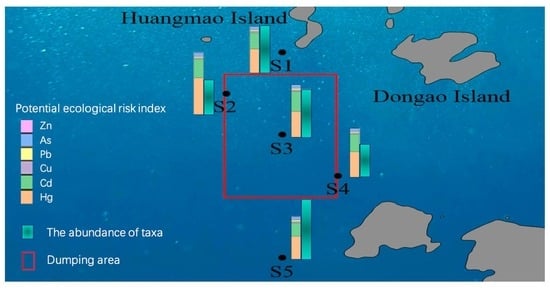
2.1. Description of the Study Area
2.2. Sampling
2.3. Laboratory Analysis
2.4. Pollution and Ecological Risk Assessment Methods
2.4.1. Nemerow Pollution Index (Pi)
2.4.2. Geochemical Accumulation Index (Igeo)
2.4.3. Integrated Potential Ecological Risk Index (RI)
2.5. Statistical Analysis
3. Results and Discussion
3.1. HMs in the Sediments
3.2. Temporal and Spatial Distribution Characteristics of HMs
3.3. Correlation Analysis between HMs and Other Environmental Factors
3.4. Pollution Assessment of HMs in Sediments
3.5. Characteristics of Benthic Community in the Dumping Area
3.6. Influence of Dumping Dredged Materials and the Pollution Status
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, K.-H.; Choi, K.-Y.; Kim, C.-J.; Kim, Y.-I.; Chung, C.-S. Assessment of the Governance System for the Management of the East Sea-Jung Dumping Site, Korea through Analysis of Heavy Metal Concentrations in Bottom Sediments. Ocean Sci. J. 2015, 50, 721–740. [Google Scholar] [CrossRef]
- Fatoki, O.S.; Mathabatha, S. An Assessment of Heavy Metal Pollution in the East London and Port Elizabeth Harbours. Water Sa 2001, 27, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Katsiaras, N.; Simboura, N.; Tsangaris, C.; Hatzianestis, I.; Pavlidou, A.; Kapsimalis, V. Impacts of Dredged-Material Disposal on the Coastal Soft-Bottom Macrofauna, Saronikos Gulf, Greece. Sci. Total Environ. 2015, 508, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Harvey, M.; Gauthier, D.; Munro, J. Temporal Changes in the Composition and Abundance of the Macro-Benthic Invertebrate Communities at Dredged Material Disposal Sites in the Anse a Beaufils, Baie Des Chaleurs, Eastern Canada. Mar. Pollut. Bull. 1998, 36, 41–55. [Google Scholar] [CrossRef]
- State Oceanic Administration of China. Bulletin of China Marine Ecological Environment Status; Ocean Press: Beijing, China, 2011–2017. [Google Scholar]
- Marmin, S.; Lesueur, P.; Dauvin, J.C.; Samson, S.; Tournier, P.; Lavanne, A.G.; Dubrulle-Brunaud, C.; Thouroude, C. An Experimental Study on Dredge Spoil of Estuarine Sediments in the Bay of Seine (France): A Morphosedimentary Assessment. Cont. Shelf Res. 2016, 116, 89–102. [Google Scholar] [CrossRef]
- Moog, O.; Stubauer, I.; Haimann, M.; Habersack, H.; Leitner, P. Effects of Harbour Excavating and Dredged Sediment Disposal on the Benthic Invertebrate Fauna of River Danube (Austria). Hydrobiologia 2018, 814, 109–120. [Google Scholar] [CrossRef]
- Guerra-Garcia, J.; Garcia-Gomez, J. Polychaete Assemblages and Sediment Pollution in a Harbour with Two Opposing Entrances. Helgol. Mar. Res. 2004, 58, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.S.; Ahmed, M.K.; Raknuzzaman, M.; Habibullah-Al-Mamun, M.; Islam, M.K. Heavy Metal Pollution in Surface Water and Sediment: A Preliminary Assessment of an Urban River in a Developing Country. Ecol. Indic. 2015, 48, 282–291. [Google Scholar] [CrossRef]
- Abdel-Ghani, N.T.; Elchaghaby, G.A. Influence of Operating Conditions on the Removal of Cu, Zn, Cd and Pb Ions from Wastewater by Adsorption. Int. J. Environ. Sci. Technol. 2007, 4, 451–456. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Song, J.; Li, X.; Li, N.; Duan, L. Distribution and Contamination of Heavy Metals in Surface Sediments of the South Yellow Sea. Mar. Pollut. Bull. 2012, 64, 2151–2159. [Google Scholar] [CrossRef]
- Gleyzes, C.; Tellier, S.; Astruc, M. Fractionation Studies of Trace Elements in Contaminated Soils and Sediments: A Review of Sequential Extraction Procedures. Trac Trends Anal. Chem. 2002, 21, 451–467. [Google Scholar] [CrossRef]
- Peng, B.; Peng, J.X.; Sun, K.F. A review on heavy metals contamination in Daya Bay and adjacent waters. Ecol. Sci. 2015, 34, 170–180. [Google Scholar]
- Bolam, S.G.; Barry, J.; Bolam, T.; Mason, C.; Rumney, H.S.; Thain, J.E.; Law, R.J. Impacts of Maintenance Dredged Material Disposal on Macrobenthic Structure and Secondary Productivity. Mar. Pollut. Bull. 2011, 62, 2230–2245. [Google Scholar] [CrossRef]
- Newell, R.C.; Seiderer, L.J.; Hitchcock, D.R. The Impact of Dredging Works in Coastal Waters: A Review of the Sensitivity to Disturbance and Subsequent Recovery of Biological Resources on the Sea Bed. Oceanogr. Mar. Biol. D 1998, 36, 127–178. [Google Scholar]
- Vandolah, R.; Calder, D.; Knott, D. Effects of Dredging and Open-Water Disposal on Benthic Macroinvertebrates in a South-Carolina Estuary. Estuaries 1984, 7, 28–37. [Google Scholar] [CrossRef]
- Roberts, R.D.; Forrest, B.M. Minimal Impact from Long-Term Dredge Spoil Disposal at a Dispersive Site in Tasman Bay, New Zealand. N. Z. J. Mar. Freshw. Res. 1999, 33, 623–633. [Google Scholar] [CrossRef] [Green Version]
- Roberts, R.D.; Gregory, M.R.; Foster, B.A. Developing an Efficient Macrofauna Monitoring Index from an Impact Study—A Dredge Spoil Example. Mar. Pollut. Bull. 1998, 36, 231–235. [Google Scholar] [CrossRef]
- State Oceanic Administration of China. Technical Guidelines for Selecting of Ocean Dumping Area (HY/T122-2009); Standard Press: Beijing, China, 2009. [Google Scholar]
- Donazar-Aramendia, I.; Sanchez-Moyano, J.E.; Garcia-Asencio, I.; Miro, J.M.; Megina, C.; Garcia-Gomez, J.C. Impact of Dredged-Material Disposal on Soft-Bottom Communities in a Recurrent Marine Dumping Area near to Guadalquivir Estuary, Spain. Mar. Environ. Res. 2018, 139, 64–78. [Google Scholar] [CrossRef]
- Wang, X.; Kong, L.; Cheng, J.; Zhao, D.; Chen, H.; Sun, R.; Yang, W.; Han, J. Distribution of Butyltins at Dredged Material Dumping Sites around the Coast of China and the Potential Ecological Risk. Mar. Pollut. Bull. 2019, 138, 491–500. [Google Scholar] [CrossRef]
- Yan, N.; Liu, W.; Xie, H.; Gao, L.; Han, Y.; Wang, M.; Li, H. Distribution and Assessment of Heavy Metals in the Surface Sediment of Yellow River, China. J. Environ. Sci. 2016, 39, 45–51. [Google Scholar] [CrossRef]
- Muller, G. Index of Geoaccumulation in Sediments of the Rhine River. Geojournal 1969, 2, 108–118. [Google Scholar]
- Hakanson, L. An Ecological Risk Index for Aquatic Pollution-Control—A Sedimentological Approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An. Approach to Statistical Analysis and Interpretation; Plymouth Marine Laboratory: Plymouth, UK, 1994. [Google Scholar]
- Zhang, Y. A Background Value Study on Heavy Metal Elements in the Sediments of Daya Bay. Trop. Ocean. 1991, 3, 76–80. [Google Scholar]
- MacDonald, D.D.; Carr, R.S.; Calder, F.D.; Long, E.R.; Ingersoll, C.G. Development and Evaluation of Sediment Quality Guidelines for Florida Coastal Waters. Ecotoxicology 1996, 5, 253–278. [Google Scholar] [CrossRef]
- Long, E.; Macdonald, D.; Smith, S.; Calder, F. Incidence of Adverse Biological Effects Within Ranges of Chemical Concentrations in Marine and Estuarine Sediments. Environ. Manag. 1995, 19, 81–97. [Google Scholar] [CrossRef]
- Rauret, G. Extraction Procedures for the Determination of Heavy Metals in Contaminated Soil and Sediment. Talanta 1998, 46, 449–455. [Google Scholar] [CrossRef]
- Bonsignore, M.; Manta, D.S.; Mirto, S.; Quinci, E.M.; Ape, F.; Montalto, V.; Gristina, M.; Traina, A.; Sprovieri, M. Bioaccumulation of Heavy Metals in Fish, Crustaceans, Molluscs and Echinoderms from the Tuscany Coast. Ecotoxicol. Environ. Saf. 2018, 162, 554–562. [Google Scholar] [CrossRef]
- Lao, Q.; Su, Q.; Liu, G.; Shen, Y.; Chen, F.; Lei, X.; Qing, S.; Wei, C.; Zhang, C.; Gao, J. Spatial Distribution of and Historical Changes in Heavy Metals in the Surface Seawater and Sediments of the Beibu Gulf, China. Mar. Pollut. Bull. 2019, 146, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Adila, S.; Mamattursun, E.; Alimujiang, K. Assessment of Pollution and Ecological Risk of Heavy Metals in Surface Dust in Karamay City. Asian J. Ecotoxicol. 2021, 16, 310–322. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Lu, S.; Zhang, D.; Tong, Z.; Yan, Y.; Hu, B. Interannual Variation, Ecological Risk and Human Health Risk of Heavy Metals in Oyster-Cultured Sediments in the Maowei Estuary, China, from 2011 to 2018. Mar. Pollut. Bull. 2020, 154, 111039. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Liu, Y.; Yuan, Y.; Zhou, C.Y.; Qu, L. Enrichment of Heavy Metals in the Surface Sediments from the Dumping Areas in Bohai Sea and Assessment of Their Potential Ecological Risk. Mar. Sci. Bull. 2014, 33, 340–346. [Google Scholar]
- Zhang, N.X.; Cao, C.H.; Ren, R.Z.; Sun, X. Heavy Metals in the Surface Sediment of the Dumping Ground Outside Jiaozhou Bay and Their Potential Ecological Risk. HuanjingKexue 2011, 32, 1315–1320. [Google Scholar]
- Liu, G.Q.; Liu, B.L.; Qing, S.M.; Xing, S.K. Assessment on Pollution and Potential Ecological Risk of Heavy Metals in the Sediments of the Temporary Marine Dumping Area of Fangchenggang. Ecol. Sci. 2013, 32, 177–182. [Google Scholar]
- Chen, C.F.; Chen, C.W.; Ju, Y.R.; Kao, C.M.; Dong, C.D. Impact of Disposal of Dredged Material on Sediment Quality in the Kaohsiung Ocean Dredged Material Disposal Site, Taiwan. Chemosphere 2018, 191, 555–565. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, R.Z.; Wu, F.C.; Wang, J.W.; Zhang, N.X. Analysis on the Distribution of Surface Sediment Heavy Metals Contamination and the Influencing Factors of Temporary Ocean Dumping Site in Lanshan Port. Trans. Oceanol. Limnol. 2012, 1, 130–136. [Google Scholar]
- Hilton, J.; Davison, W.; Ochsenbein, U. A Mathematical-Model for Analysis of Sediment Core Data—Implications for Enrichment Factor Calculations and Trace-Metal Transport Mechanisms. Chem. Geol. 1985, 48, 281–291. [Google Scholar] [CrossRef]
- Sundaray, S.K.; Nayak, B.B.; Lin, S.; Bhatta, D. Geochemical Speciation and Risk Assessment of Heavy Metals in the River Estuarine Sediments-A Case Study: Mahanadi Basin, India. J. Hazard. Mater. 2011, 186, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, J.; Xu, M.; Zhao, L.; Wang, Z. Spatial Distribution, Source Apportionment and Ecological Risk Assessment of Heavy Metals in the Sediments of Haizhou Bay National Ocean Park, China. Mar. Pollut. Bull. 2019, 149, 110651. [Google Scholar] [CrossRef]
- Tang, H.; Ke, Z.; Yan, M.; Wang, W.; Nie, H.; Li, B.; Zhang, J.; Xu, X.; Wang, J. Concentrations, Distribution, and Ecological Risk Assessment of Heavy Metals in Daya Bay, China. Water 2018, 10, 780. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Lei, K.; Zhang, X.; Xu, L.; Lin, C.; Yang, Y. Contamination and Ecological Risks of Toxic Metals in the Hai River, China. Ecotoxicol. Environ. Saf. 2018, 164, 210–218. [Google Scholar] [CrossRef]
- Lin, L.H.; Wei, H.J.; Huang, H.M. Contamination status and bioaccumulation of the heavy metals in the surface sediments and benthos in Daya Bay. Ecol. Sci. 2017, 36, 173–181. [Google Scholar]
- Sarr, A.B.; Joao Benetti, C.; Fernandez-Diaz, M.; Garrido, J. The Microhabitat Preferences of Water Beetles in Four Rivers in Ourense Province, Northwest Spain. Limnetica 2013, 32, 1–9. [Google Scholar]
- de Castro Vasconcelos, M.; Melo, A.S. An Experimental Test of the Effects of Inorganic Sediment Addition on Benthic Macroinvertebrates of a Subtropical Stream. Hydrobiologia 2008, 610, 321–329. [Google Scholar] [CrossRef]
- Pallo, P.; Widbom, B.; Olafsson, E. A Quantitative Survey of the Benthic Meiofauna in the Gulf of Riga (Eastern Baltic Sea), with Special Reference to the Structure of Nematode Assemblages. Ophelia 1998, 49, 117–139. [Google Scholar] [CrossRef]
- Fonseca, E.M.; Fernandes, J.R.; Lima, L.S.; Delgado, J.; Correa, T.R.; Costa, P.M.S.; Baptista Neto, J.A.; Aguiar, V.M.C. Effects of Dredged Sediment Dumping on Trace Metals Concentrations and Macro Benthic Assemblage at the Continental Shelf Adjacent to a Tropical Urbanized Estuary. Ocean. Coast. Manag. 2020, 196, 105299. [Google Scholar] [CrossRef]
- Quan, F. Intertidal Pollution on Benthic Macro-Arthropod Community in Mangrove Forest. Master’s Thesis, Hainan Normal University, Hainan, China, 2011. [Google Scholar]
- Jia, H.B.; Hu, H.Y.; Tang, J.L.; Wang, Y.M.; Chai, H.P. Effect of Heavy Metals on Macro-Benthos in Surface Sediments in Changjiang Estuary and Adjacent Sea. Mar. Environ. Sci. 2011, 30, 809–813. [Google Scholar]
- Li, X.Z.; Li, B.Q.; Wang, H.F. Community Structure of Macrobenthos in Coastal Water off Rushan, Southern Shandong Peninsula, and the Relationships with Environmental Factors. Acta Oceanol. Sin. 2009, 28, 81–93. [Google Scholar]
- Xu, R.; Yang, Y.; Li, Z. Diffusion of Heavy Metals from Marine Environment to Shellfish. Mar. Sci. Bull. 2007, 26, 117–120. [Google Scholar]
- Simonini, R.; Ansaloni, I.; Cavallini, F.; Graziosi, F.; Iotti, M.; N’Siala, G.M.; Mauri, M.; Montanari, G.; Preti, M.; Prevedelli, D. Effects of Long-Term Dumping of Harbor-Dredged Material on Macrozoobenthos at Four Disposal Sites along the Emilia-Romagna Coast (Northern Adriatic Sea, Italy). Mar. Pollut. Bull. 2005, 50, 1595–1605. [Google Scholar] [CrossRef]








| Hg | As | Cu | Pb | Cd | Zn | References | |
|---|---|---|---|---|---|---|---|
| 2011 | 0.050–0.090 | 14.2–17.4 | 20.2–31.5 | 29.7–32.3 | 0.15–0.21 | 108–117 | |
| 2012 | 0.056–0.14 | 16.4–19.6 | 21.1–29.6 | 23.0–54.7 | 0.16–0.27 | 97.4–122 | |
| 2013 | 0.054–0.086 | 16.1–17.8 | 19.8–36.3 | 19.9–36.0 | 0.16–0.34 | 66.1–116 | |
| 2014 | 0.052–0.12 | 14.2–17.9 | 20.3–26.7 | 21.6–33.4 | 0.16–0.28 | 62.2–122 | This study |
| 2016 | 0.053–0.26 | 14.2–19.8 | 21.8–25.4 | 25.4–32.8 | 0.22–0.32 | 88.6–119 | |
| 2017 | 0.030–0.050 | 7.90–10.9 | 13.1–21.7 | 17.4–35.6 | 0.11–0.24 | 50.0–83.0 | |
| Average a ± standard deviation | 0.080 ± 0.041 | 15.1 ± 3.20 | 24.5 ± 4.40 | 30.8 ± 7.70 | 0.21 ± 0.061 | 97.6 ± 20.5 | |
| Coefficient of variation (CV%) b | 0.51 | 0.21 | 0.18 | 0.25 | 0.29 | 0.21 | |
| Class I c | 0.2 | 20 | 35 | 60 | 0.5 | 150 | |
| Background | 0.03 | 7.7 | 15 | 20 | 0.07 | 65 | [26] |
| TEL d | 0.13 | 7.24 | 18.7 | 30.2 | 0.68 | 124 | [27] |
| PEL e | 0.7 | 41.6 | 108 | 112 | 4.21 | 271 | [27] |
| ERL f | 0.15 | 8.2 | 34 | 46.7 | 1.2 | 150 | [28] |
| ERM j | 0.71 | 70 | 270 | 218 | 9.6 | 410 | [28] |
| Locate | Hg | As | Cu | Pb | Cd | Zn | Reference |
|---|---|---|---|---|---|---|---|
| mg/kg | |||||||
| Huangmao Island Dumping Area | 0.03–0.26 | 7.9–19.8 | 13.1–36.3 | 17.4–54.7 | 0.10–0.34 | 50–122 | This study |
| Hulu Island Port Dumping Area | 0.31–0.36 | 6.07–10.5 | 15.6–20.7 | 12–18.5 | 0.17–0.63 | 63.1–126 | [34] |
| Jinzhou Port Dumping Area | 0.10–0.26 | 4.97–8.62 | 0.15–0.45 | 10.7–17.5 | 0.15–0.45 | 22.8–234 | |
| Tianjin Dumping Area | 0.016–0.023 | 16–25 | 0.112–0.149 | 20.5–23.4 | 0.112–0.149 | 53.9–66.5 | |
| Huangye Port Dumping Area | 0.016–0.019 | 50.9–73.1 | 0.167–0.23 | 17.2–23.7 | 0.156–0.201 | 6.37–7.71 | |
| Laizhou Port Dumping Area | 0.015–0.061 | 6.91–10.71 | 0.22–0.24 | 19.1–23.5 | 0.22–0.24 | 58.6–64.2 | |
| Marine Dumping Area outside Jiaozhou Bay | 0.038–0.082 | NA | 2.79–3.84 | 0.99–1.32 | 0.09–0.13 | 3.73–16.16 | [35] |
| Fangchenggang Dumping Area | 0.063–0.071 | 9.02–9.89 | 13.8–16.7 | 26.2–32.1 | 0.02–0.11 | 31.7–79.5 | [36] |
| Dumping Site at Taiwan Shelf | NA | NA | 12.5–15.6 | 15.6–18.5 | NA | 101.3–124.7 | [37] |
| Lanshan Port Temporary Marine Dumping Area | 0.038–0.082 | NA | 10.32–13.67 | 7–9.15 | 0.15–0.17 | 22.59–28.15 | [38] |
| TOC | Hg | As | Cu | Pb | Cd | Zn | Oils | Sulfides | |
|---|---|---|---|---|---|---|---|---|---|
| TOC | 1 | ||||||||
| Hg | 0.139 | 1 | |||||||
| As | 0.493 | 0.657 ** | 1 | ||||||
| Cu | 0.663 ** | 0.245 | 0.484 | 1 | |||||
| Pb | 0.438 | 0.06 | 0.103 | 0.44 | 1 | ||||
| Cd | 0.017 | 0.419 | 0.723 ** | 0.179 | 0.187 | 1 | |||
| Zn | 0.661 ** | 0.269 | 0.549 * | 0.766 ** | 0.515 * | 0.254 | 1 | ||
| Oils | 0.363 | −0.016 | 0.16 | 0.164 | 0.45 | 0.243 | 0.451 | 1 | |
| Sulfides | 0.213 | 0.017 | −0.249 | 0.052 | −0.002 | −0.556 * | −0.164 | 0.012 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, W.; Jiang, Z.; Peng, X.; Yang, Z.; Cai, W.; Yu, H.; Ye, J. Impact of Dredged Material Disposal on Heavy Metal Concentrations and Benthic Communities in Huangmao Island Marine Dumping Area near Pearl River Estuary. Appl. Sci. 2021, 11, 9412. https://doi.org/10.3390/app11209412
Tao W, Jiang Z, Peng X, Yang Z, Cai W, Yu H, Ye J. Impact of Dredged Material Disposal on Heavy Metal Concentrations and Benthic Communities in Huangmao Island Marine Dumping Area near Pearl River Estuary. Applied Sciences. 2021; 11(20):9412. https://doi.org/10.3390/app11209412
Chicago/Turabian StyleTao, Wei, Zhongchen Jiang, Xiaojuan Peng, Zhenxiong Yang, Weixu Cai, Huili Yu, and Jianjun Ye. 2021. "Impact of Dredged Material Disposal on Heavy Metal Concentrations and Benthic Communities in Huangmao Island Marine Dumping Area near Pearl River Estuary" Applied Sciences 11, no. 20: 9412. https://doi.org/10.3390/app11209412