Detoxification of Molasses and Production of Mycelial Mass and Valuable Metabolites by Morchella Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Species, Substrates and Culture Conditions
2.2. Analytical Methods
2.2.1. Characteristics and Specifications of Molasses
2.2.2. Broth and Mycelial Analyses
3. Results and Discussion
3.1. Beet Molasses
3.2. Biomass Production, Sugars and Nitrogen Consumption
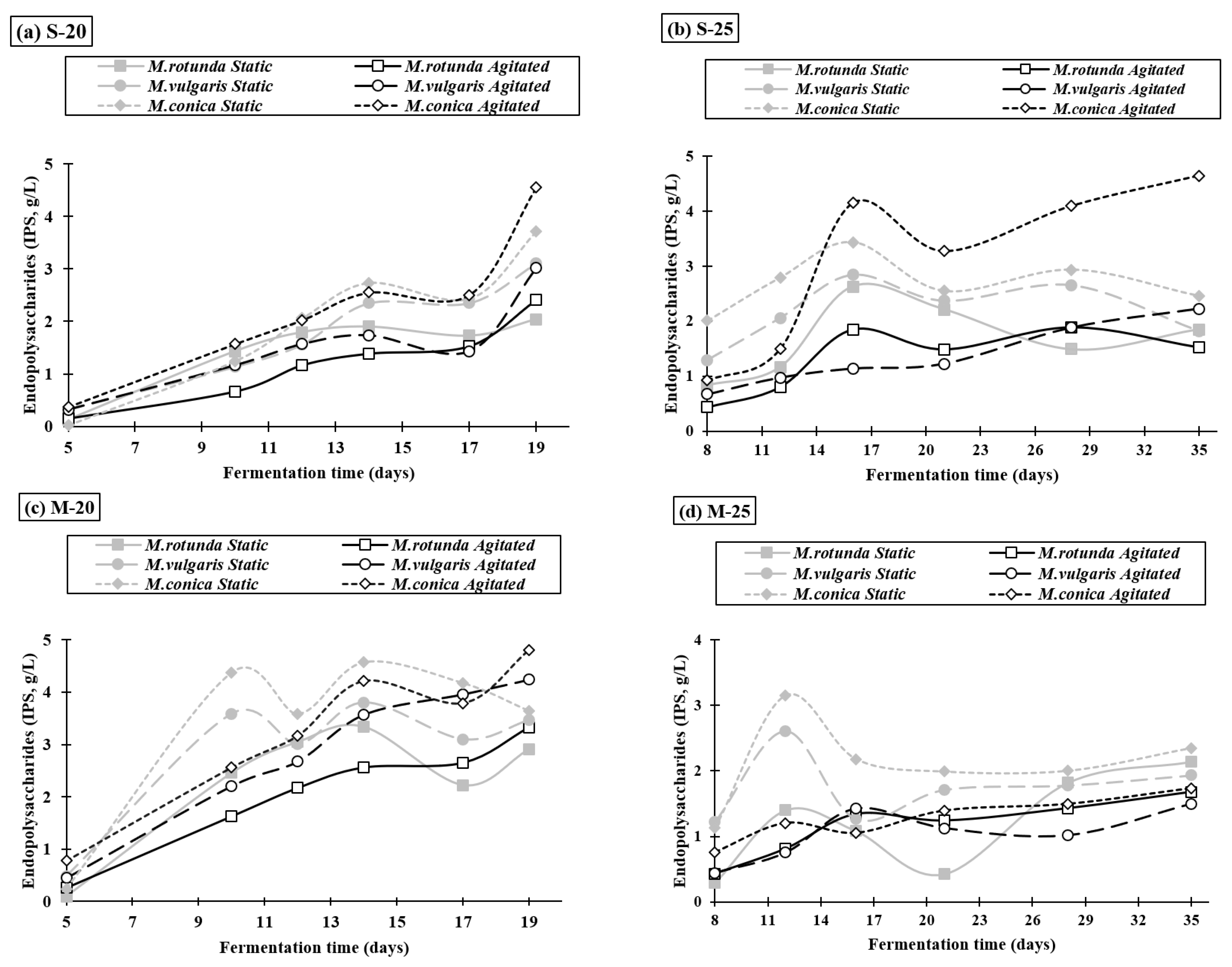
3.3. Extracellular Polysaccharides Production
3.4. Intracellular Polysaccharide Synthesis and Composition
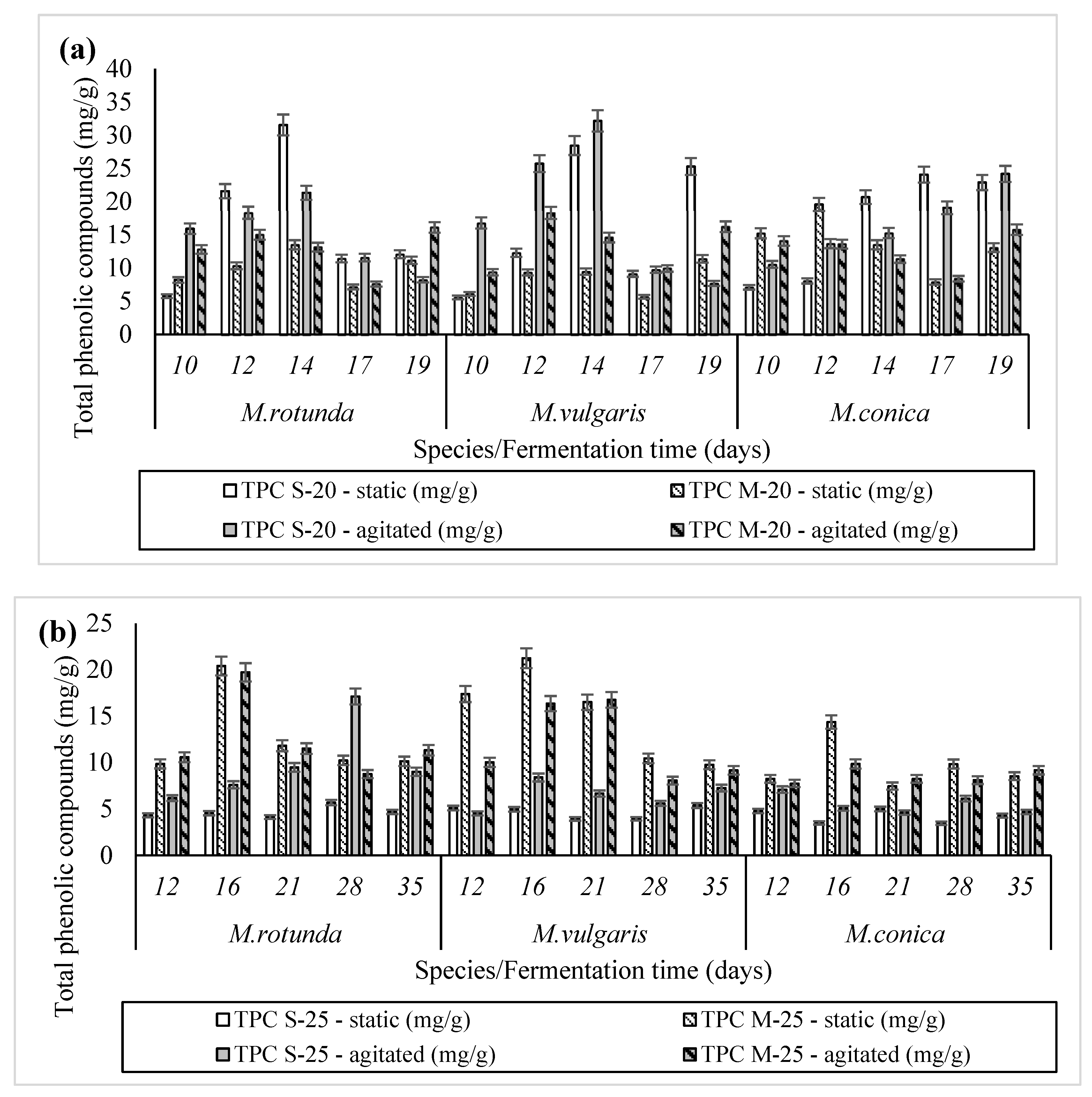
3.5. Removal of Phenolic Compounds–Decolorization
3.6. Fungal Phenolic Compounds—Antioxidant Components Synthesis
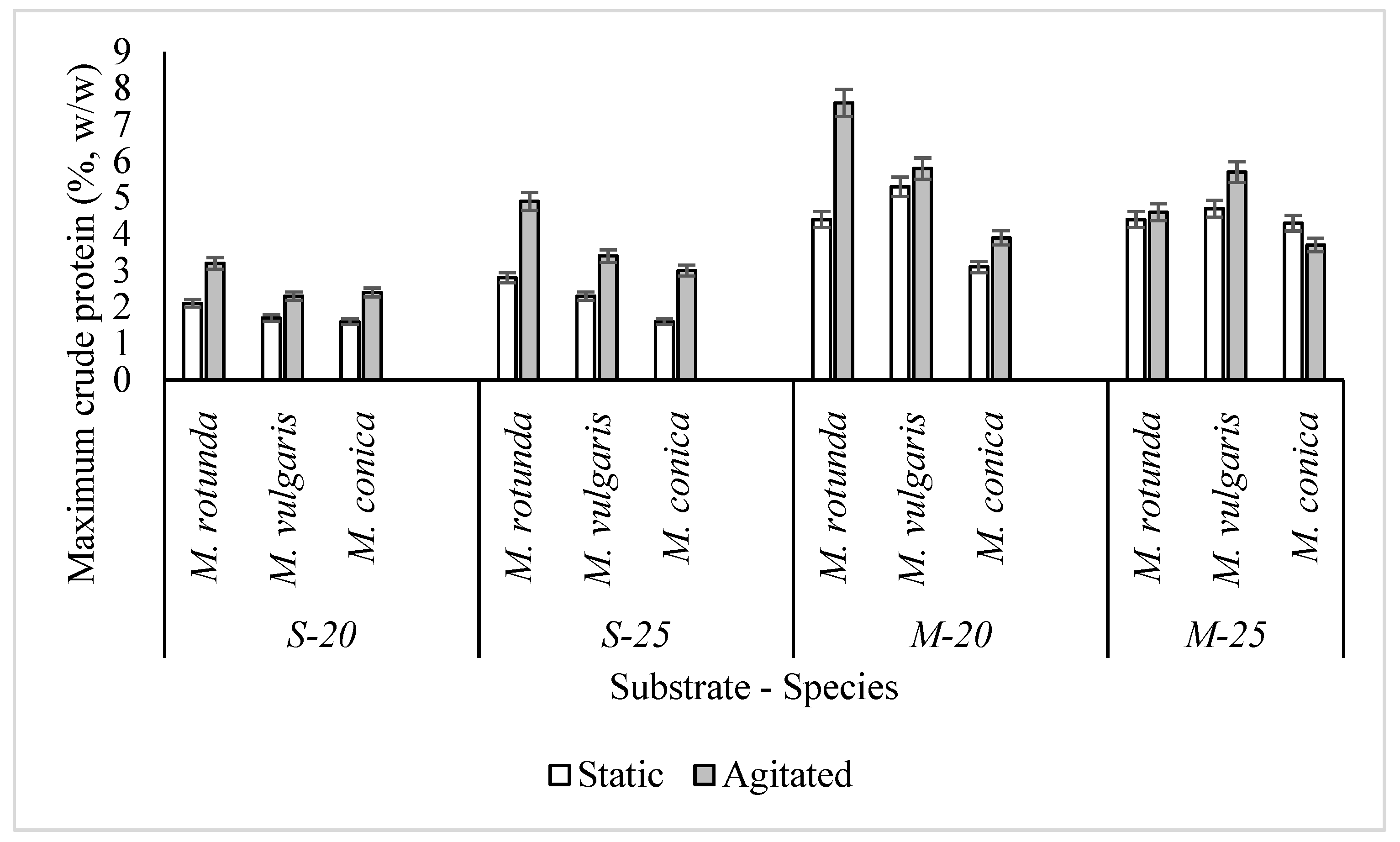
3.7. Protein
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Barros, L.; Cruz, T.; Baptista, P.; Estevinho, L.M.; Ferreira, I.C. Wild and Commercial Mushrooms as Source of Nutrients and Nutraceuticals. Food Chem. Toxicol. 2008, 46, 2742–2747. [Google Scholar] [CrossRef]
- Liu, C.; Sun, Y.; Mao, Q.; Guo, X.; Li, P.; Liu, Y.; Xu, N. Characteristics and Antitumor Activity of Morchella esculenta Polysaccharide Extracted by Pulsed Electric Field. Int. J. Mol. Sci. 2016, 17, 986. [Google Scholar] [CrossRef] [Green Version]
- Usman, M.; Murtaza, G.; Ditta, A. Nutritional, Medicinal, and Cosmetic Value of Bioactive Compounds in Button Mushroom (Agaricus bisporus): A Review. Appl. Sci. 2021, 11, 5943. [Google Scholar] [CrossRef]
- Xu, N.; Lu, Y.; Hou, J.; Liu, C.; Sun, Y. A Polysaccharide Purified from Morchella conica Pers. Prevents Oxidative Stress Induced by H2O2 in Human Embryonic Kidney (HEK) 293T Cells. Int. J. Mol. Sci. 2018, 19, 4027. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Zhou, X.; Meng, Q.; Wang, M.; Zhang, Y.; Fu, S. Secondary Metabolites and Antiradical Activity of Liquid Fermentation of Morchella sp. Isolated from Southwest China. Molecules 2019, 24, 1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasser, S.P. Medicinal Mushrooms as a Source of Antitumor and Immunomodulating Polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar] [PubMed]
- Barros, L.; Ferreira, M.-J.; Queiros, B.; Ferreira, I.C.; Baptista, P. Total Phenols, Ascorbic Acid, β-Carotene and Lycopene in Portuguese Wild Edible Mushrooms and Their Antioxidant Activities. Food Chem. 2007, 103, 413–419. [Google Scholar] [CrossRef]
- Prasad, P.; Chauhan, K.; Kandari, L.S.; Maikhuri, R.K.; Purohit, A.; Bhatt, R.P.; Rao, K.S. Morchella esculenta (Guchhi): Need for Scientific Intervention for Its Cultivation in Central Himalaya. Curr. Sci. 2002, 82, 1098–1100. [Google Scholar]
- Jacobs, M.E. Beta-Alanine and Tanning Polymorphisms. Comp. Biochem. Physiol. B 1982, 72, 173–177. [Google Scholar] [CrossRef]
- Papadaki, A.; Diamantopoulou, P.; Papanikolaou, S.; Philippoussis, A. Evaluation of Biomass and Chitin Production of Morchella Mushrooms Grown on Starch-Based Substrates. Foods 2019, 8, 239. [Google Scholar] [CrossRef] [Green Version]
- Güler, P.; Arkan, O. Cultural Characteristics of Morchella esculenta Mycelium on Some Nutrients. Turk. J. Biol. 2000, 24, 783–794. [Google Scholar]
- Winder, R.S. Cultural Studies of Morchella elata. Mycol. Res. 2006, 110, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Sarris, D.; Philippoussis, A.; Mallouchos, A.; Diamantopoulou, P. Valorization of Low-Cost, Carbon-Rich Substrates by Edible Ascomycetes and Basidiomycetes Grown on Liquid Cultures. FEMS Microbiol. Lett. 2020, 367, fnaa168. [Google Scholar] [CrossRef]
- Fazenda, M.L.; Seviour, R.; McNeil, B.; Harvey, L.M. Submerged Culture Fermentation of “Higher Fungi”: The Macrofungi. Adv. Appl. Microbiol. 2008, 63, 33–103. [Google Scholar]
- Gang, J.; Fang, Y.; Wang, Z.; Liu, Y. Fermentation Optimization and Antioxidant Activities of Mycelia Polysaccharides from Morchella esculenta Using Soybean Residues. Afr. J. Biotechnol. 2013, 12, 1239–1249. [Google Scholar]
- Mukhopadhyay, R.; Guha, A.K. A Comprehensive Analysis of the Nutritional Quality of Edible Mushroom Pleurotus Sajor-Caju Grown in Deproteinized Whey Medium. LWT-Food Sci. Technol. 2015, 61, 339–345. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Papanikolaou, S.; Aggelis, G.; Philippoussis, A. Adaptation of Volvariella volvacea Metabolism in High Carbon to Nitrogen Ratio Media. Food Chem. 2016, 196, 272–280. [Google Scholar] [CrossRef]
- Kumar, P.; Chandra, R. Decolourisation and Detoxification of Synthetic Molasses Melanoidins by Individual and Mixed Cultures of Bacillus spp. Bioresour. Technol. 2006, 97, 2096–2102. [Google Scholar] [CrossRef]
- Silván, J.M.; van de Lagemaat, J.; Olano, A.; del Castillo, M.D. Analysis and Biological Properties of Amino Acid Derivates Formed by Maillard Reaction in Foods. J. Pharm. Biomed. Anal. 2006, 41, 1543–1551. [Google Scholar] [CrossRef]
- Brock, T.D. Studies on the Nutrition of Morchella esculenta Fries. Mycologia 1951, 43, 402–422. [Google Scholar] [CrossRef]
- Kaul, T.N. Physiological Studies on Morchella Species. I. Carbon Util. Bull. Bot. Soc. Ben. 1977, 31, 35–42. [Google Scholar]
- Philippoussis, A. Study of the fungi morphogenesis of the genus Morchella. Ph.D. Thesis, Agricultural University of Athens, Athens, Greece, May 2003. [Google Scholar]
- Meng, F.; Liu, X.; Jia, L.; Song, Z.; Deng, P.; Fan, K. Optimization for the Production of Exopolysaccharides from Morchella esculenta SO-02 in Submerged Culture and Its Antioxidant Activities in Vitro. Carbohydr. Polym. 2010, 79, 700–704. [Google Scholar] [CrossRef]
- Gomes, J.; Menawat, A.S. Precise Control of Dissolved Oxygen in Bioreactors—A Model-Based Geometric Algorithm. Chem. Eng. Sci. 2000, 55, 67–78. [Google Scholar] [CrossRef]
- Tang, Y.-J.; Zhong, J.-J. Fed-Batch Fermentation of Ganoderma lucidum for Hyperproduction of Polysaccharide and Ganoderic Acid. Enzym. Microb. Technol. 2002, 31, 20–28. [Google Scholar] [CrossRef]
- Wagner, R.; Mitchell, D.A.; Lanzi Sassaki, G.; Lopes de Almeida Amazonas, M.A.; Berovič, M. Current Techniques for the Cultivation of Ganoderma lucidum for the Production of Biomass, Ganoderic Acid and Polysaccharides. Food Technol. Biotechnol. 2003, 41, 371–382. [Google Scholar]
- Papagianni, M. Fungal Morphology and Metabolite Production in Submerged Mycelial Processes. Biotechnol. Adv. 2004, 22, 189–259. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Papanikolaou, S.; Kapoti, M.; Komaitis, M.; Aggelis, G.; Philippoussis, A. Mushroom Polysaccharides and Lipids Synthesized in Liquid Agitated and Static Cultures. Part I: Screening Various Mushroom Species. Appl. Biochem. Biotechnol. 2012, 167, 536–551. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Papanikolaou, S.; Komaitis, M.; Aggelis, G.; Philippoussis, A. Patterns of Major Metabolites Biosynthesis by Different Mushroom Fungi Grown on Glucose-Based Submerged Cultures. Bioprocess Biosyst. Eng. 2014, 37, 1385–1400. [Google Scholar] [CrossRef]
- Kim, S.W.; Hwang, H.J.; Park, J.P.; Cho, Y.J.; Song, C.H.; Yun, J.W. Mycelial Growth and Exo-biopolymer Production by Submerged Culture of Various Edible Mushrooms under Different Media. Lett. Appl. Microbiol. 2002, 34, 56–61. [Google Scholar] [CrossRef] [Green Version]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater, 20th ed.; Amer Public Health Assn: Washington, DC, USA, 1998. [Google Scholar]
- Sparks, D.L.; Fendorf, S.E.; Toner IV, C.V.; Carski, T.H. Kinetic Methods and Measurements. In Methods of Soil Analysis: Part 3 Chemical Methods; Soil Science Society of America, Inc.: Madison, WI, USA; American Society of Agronomy, Inc.: Madison, WI, USA, 1996; Volume 5, pp. 1275–1307. [Google Scholar]
- Roukas, T. Ethanol Production from Non-Sterilized Beet Molasses by Free and Immobilized Saccharomyces cerevisiae Cells Using Fed-Batch Culture. J. Food Eng. 1996, 27, 87–96. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Lie, S. The EBC-ninhydrin Method for Determination of Free Alpha Amino Nitrogen. J. Inst. Brew. 1973, 79, 37–41. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Hsieh, C.; Hsu, T.-H.; Yang, F.-C. Production of Polysaccharides of Ganoderma lucidum (CCRC36021) by Reusing Thin Stillage. Process Biochem. 2005, 40, 909–916. [Google Scholar] [CrossRef]
- Liang, Y.; Sarkany, N.; Cui, Y.; Blackburn, J.W. Batch Stage Study of Lipid Production from Crude Glycerol Derived from Yellow Grease or Animal Fats through Microalgal Fermentation. Bioresour. Technol. 2010, 101, 6745–6750. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and Mechanisms of Antioxidant Activity Using the DPPH^* Free Radical Method. Food Sci. Technol. 1997, 30, 609–615. [Google Scholar]
- Molyneux, P. The Use of the Stable Free Radical Diphenylpicrylhydrazyl (DPPH) for Estimating Antioxidant Activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Musa, K.H.; Abdullah, A.; Kuswandi, B.; Hidayat, M.A. A Novel High Throughput Method Based on the DPPH Dry Reagent Array for Determination of Antioxidant Activity. Food Chem. 2013, 141, 4102–4106. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Sayadi, S.; Ellouz, R. Decolourization of Olive Mill Waste-Waters by the White-Rot Fungus Phanerochaete Chrysosporium: Involvement of the Lignin-Degrading System. Appl. Microbiol. Biotechnol. 1992, 37, 813–817. [Google Scholar] [CrossRef]
- Chang, M.-Y.; Tsai, G.-J.; Houng, J.-Y. Optimization of the Medium Composition for the Submerged Culture of Ganoderma lucidum by Taguchi Array Design and Steepest Ascent Method. Enzym. Microb. Technol. 2006, 38, 407–414. [Google Scholar] [CrossRef]
- Lee, B.C.; Bae, J.T.; Pyo, H.B.; Choe, T.B.; Kim, S.W.; Hwang, H.J.; Yun, J.W. Submerged Culture Conditions for the Production of Mycelial Biomass and Exopolysaccharides by the Edible Basidiomycete Grifola frondosa. Enzym. Microb. Technol. 2004, 35, 369–376. [Google Scholar] [CrossRef]
- Xu, C.-P.; Yun, J.W. Influence of Aeration on the Production and the Quality of the Exopolysaccharides from Paecilomyces tenuipes C240 in a Stirred-Tank Fermenter. Enzym. Microb. Technol. 2004, 35, 33–39. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Papanikolaou, S.; Katsarou, E.; Komaitis, M.; Aggelis, G.; Philippoussis, A. Mushroom Polysaccharides and Lipids Synthesized in Liquid Agitated and Static Cultures. Part II: Study of Volvariella volvacea. Appl. Biochem. Biotechnol. 2012, 167, 1890–1906. [Google Scholar] [CrossRef]
- Xing, Z.; Sun, F.; Liu, J. Studies on the Submerge-Cultured Conditions of Morchella conica. Shi Yong Jun Xue Bao Online 2004, 11, 38–43. [Google Scholar]
- Ding, Z.; Wang, W.; Wang, F.; Wang, Q.; Zhang, K. Polysaccharides Production by Submerged Fermentation of Coprinus comatus and Their Inhibitory Effects on Non-Enzymatic Glycosylation. J. Med. Plants Res. 2012, 6, 1375–1381. [Google Scholar]
- Fang, Q.-H.; Zhong, J.-J. Submerged Fermentation of Higher Fungus Ganoderma lucidum for Production of Valuable Bioactive Metabolites—Ganoderic Acid and Polysaccharide. Biochem. Eng. J. 2002, 10, 61–65. [Google Scholar] [CrossRef]
- Tang, Y.-J.; Zhu, L.-L.; Li, D.-S.; Mi, Z.-Y.; Li, H.-M. Significance of Inoculation Density and Carbon Source on the Mycelial Growth and Tuber Polysaccharides Production by Submerged Fermentation of Chinese Truffle Tuber sinense. Process Biochem. 2008, 43, 576–586. [Google Scholar] [CrossRef]
- Fakas, S.; Galiotou-Panayotou, M.; Papanikolaou, S.; Komaitis, M.; Aggelis, G. Compositional Shifts in Lipid Fractions during Lipid Turnover in Cunninghamella echinulata. Enzym. Microb. Technol. 2007, 40, 1321–1327. [Google Scholar] [CrossRef]
- Haque, M.A.; Kachrimanidou, V.; Koutinas, A.; Lin, C.S.K. Valorization of Bakery Waste for Biocolorant and Enzyme Production by Monascus purpureus. J. Biotechnol. 2016, 231, 55–64. [Google Scholar] [CrossRef]
- Taskin, M.; Ozkan, B.; Atici, O.; Aydogan, M.N. Utilization of Chicken Feather Hydrolysate as a Novel Fermentation Substrate for Production of Exopolysaccharide and Mycelial Biomass from Edible Mushroom Morchella esculenta. Int. J. Food Sci. Nutr. 2012, 63, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-Y.; Cho, E.-J.; Nam, S.-H.; Choi, J.-W.; Yun, J.-W. Production of Polysaccharide–Peptide Complexes by Submerged Mycelial Culture of an Entomopathogenic Fungus Cordyceps sphecocephala. Process Biochem. 2007, 42, 352–362. [Google Scholar] [CrossRef]
- Lee, W.Y.; Park, Y.; Ahn, J.K.; Ka, K.H.; Park, S.Y. Factors Influencing the Production of Endopolysaccharide and Exopolysaccharide from Ganoderma applanatum. Enzym. Microb. Technol. 2007, 40, 249–254. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, D.; Wan, W.; Hu, X.; Qi, Y.; Liang, Z. Enhanced Simultaneous Production of Mycelia and Intracellular Polysaccharide in Submerged Cultivation of Cordyceps jiangxiensis Using Desirability Functions. Process Biochem. 2006, 41, 1887–1893. [Google Scholar] [CrossRef]
- Ruiz-Herrera, J.; Osorio, E. Isolation and Chemical Analysis of the Cell Wall of Morchella sp. Antonie Van Leeuwenhoek 1974, 40, 57–64. [Google Scholar] [CrossRef]
- Smiderle, F.R.; Olsen, L.M.; Ruthes, A.C.; Czelusniak, P.A.; Santana-Filho, A.P.; Sassaki, G.L.; Gorin, P.A.J.; Iacomini, M. Exopolysaccharides, Proteins and Lipids in Pleurotus pulmonarius Submerged Culture Using Different Carbon Sources. Carbohydr. Polym. 2012, 87, 368–376. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, A.M.; Borja, R.; Martın, A. Aerobic–Anaerobic Biodegradation of Beet Molasses Alcoholic Fermentation Wastewater. Process Biochem. 2003, 38, 1275–1284. [Google Scholar] [CrossRef]
- Raghukumar, C.; Rivonkar, G. Decolorization of Molasses Spent Wash by the White-Rot Fungus Flavodon flavus, Isolated from a Marine Habitat. Appl. Microbiol. Biotechnol. 2001, 55, 510–514. [Google Scholar] [CrossRef]
- Sarris, D.; Matsakas, L.; Aggelis, G.; Koutinas, A.A.; Papanikolaou, S. Aerated vs Non-Aerated Conversions of Molasses and Olive Mill Wastewaters Blends into Bioethanol by Saccharomyces cerevisiae under Non-Aseptic Conditions. Ind. Crop. Prod. 2014, 56, 83–93. [Google Scholar] [CrossRef]
- Turkoglu, A.; Kivrak, I.; Mercan, N.; Duru, M.E.; Gezer, K.; Turkoglu, H. Antioxidant and Antimicrobial Activities of Morchella conica Pers. Afr. J. Biotechnol. 2006, 5, 1146–1150. [Google Scholar] [CrossRef]
- Puttaraju, N.G.; Venkateshaiah, S.U.; Dharmesh, S.M.; Urs, S.M.N.; Somasundaram, R. Antioxidant Activity of Indigenous Edible Mushrooms. J. Agric. Food Chem. 2006, 54, 9764–9772. [Google Scholar] [CrossRef]
- Gursoy, N.; Sarikurkcu, C.; Cengiz, M.; Solak, M.H. Antioxidant Activities, Metal Contents, Total Phenolics and Flavonoids of Seven Morchella Species. Food Chem. Toxicol. 2009, 47, 2381–2388. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, I.; Sahan, S.; Sahin, U.; Ekici, L.; Sagdic, O. Bioactivity and Mineral Contents of Wild-Grown Edible Morchella conica in the Mediterranean Region. J. Für Verbrauch. Und Lebensm. 2010, 5, 453–457. [Google Scholar] [CrossRef]
- Vieira, V.; Fernandes, Â.; Barros, L.; Glamočlija, J.; Ćirić, A.; Stojković, D.; Martins, A.; Soković, M.; Ferreira, I.C. Wild Morchella conica Pers. from Different Origins: A Comparative Study of Nutritional and Bioactive Properties. J. Sci. Food Agric. 2016, 96, 90–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tietel, Z.; Masaphy, S. True Morels (Morchella)—Nutritional and Phytochemical Composition, Health Benefits and Flavor: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1888–1901. [Google Scholar] [CrossRef]
- Petre, M.; Petre, V. Environmental Biotechnology for Bioconversion of Agricultural and Forestry Wastes into Nutritive Biomass. In Environmental Biotechnology: New Approaches and Prospective Applications; InTech: London, UK, 2013. [Google Scholar]
- Mshandete, A.M.; Mgonja, J.R. Submerged Liquid Fermentation of Some Tanzanian Basidiomycetes for the Production of Mycelial Biomass, Exopolysaccharides and Mycelium Protein Using Wastes Peels Media. J. Agric. Biol. 2009, 4, 1–13. [Google Scholar]
- Pandey, N.; Budhathoki, U. Protein Determination through Bradford’s Method of Nepalese Mushroom. Sci. World 2007, 5, 85–88. [Google Scholar] [CrossRef] [Green Version]


| Molasses Characteristics | |
|---|---|
| Moisture | 27.0% w/w |
| Total nitrogen | 1.8% w/w |
| Organic matter | 44.8% w/w |
| Total sugars | 510-530 g/L |
| Phenolic compounds | 33.25 g/L |
| pH | 5.50 |
| Density | 1.2438 g/mL |
| (a) M. rotunda | |||||||||
| Substrate | Time | X | TScon | Ncon | YX/S | EPS | IPS | IPS | |
| (Days) | (g/L) | (%) | (%) | (g/g) | (g/L) | (g/L) | (%, w/w) | ||
| Static | |||||||||
| S-20 | 5 | d | 0.87 ± 0.01 | 21.0 ± 0.1 | 15.3 ± 0.1 | 0.24 ± 0.01 | 0.94 ± 0.01 | 0.13 ± 0.02 | 15.0 ± 0.3 |
| 10 | c | 5.67 ± 0.73 | 49.1 ± 0.0 | 61.6 ± 2.1 | 0.68 ± 0.05 | 0.67 ± 0.06 | 1.43 ± 0.06 | 25.3 ± 0.9 | |
| 19 | a, b, e, f | 7.29 ± 0.16 | 80.5 ± 1.4 | 97.4 ± 0.2 | 0.53 ± 0.16 | 0.28 ± 0.01 | 2.04 ± 0.03 | 28.0 ± 0.4 | |
| S-25 | 8 | c, d | 5.22 ± 0.06 | 45.9 ± 0 | 82.4 ± 0.1 | 0.57 ± 0.06 | 0.87 ± 0.22 | 0.84 ± 0.06 | 16.0 ± 0.6 |
| 16 | e, f | 6.24 ± 0.05 | 65.6 ± 1.3 | 90.2 ± 2.1 | 0.48 ± 0.05 | 0.37 ± 0.01 | 2.63 ± 0.14 | 42.1 ± 2.2 | |
| 35 | a, b | 7.39 ± 0.27 | 85.2 ± 1.1 | 97.5 ± 0.1 | 0.43 ± 0.27 | 0.13 ± 0.01 | 1.85 ± 0.04 | 25.0 ± 0.5 | |
| M-20 | 5 | d | 0.62 ± 0.05 | 16.9 ± 1.3 | 11.7 ± 0.1 | 0.17 ± 0.06 | 1.66 ± 0.03 | 0.09 ± 0.11 | 15.0 ± 1.1 |
| 10 | c | 9.69 ± 0.01 | 50.2 ± 1.7 | 56.7 ± 0.2 | 0.88 ± 0.01 | 1.37 ± 0.08 | 2.46 ± 0.11 | 25.4 ± 1.1 | |
| 14 | e, f | 12.65 ± 0.38 | 77.5 ± 1.0 | 83.5 ± 0.9 | 0.74 ± 0.05 | 0.97 ± 0.06 | 3.34 ± 0.09 | 26.4 ± 0.7 | |
| 19 | a, b | 13.46 ± 0.02 | 95.2 ± 0.8 | 88.9 ± 0.3 | 0.64 ± 0.27 | 0.42 ± 0.06 | 2.92 ± 0.06 | 21.7 ± 0.4 | |
| M-25 | 8 | d | 1.80 ± 0.23 | 17.0 ± 1.7 | 23.3 ± 1.9 | 0.26 ± 0.06 | 3.94 ± 0.07 | 0.29 ± 0.10 | 16.0 ± 1.2 |
| 12 | c | 7.93 ± 0.10 | 28.9 ± 2.4 | 37.2 ± 2.1 | 0.67 ± 0.01 | 3.77 ± 0.03 | 1.40 ± 0.05 | 17.7 ± 0.6 | |
| 35 | a, b, e, f | 12.01 ± 0.04 | 59.0 ± 1.9 | 69.8 ± 0.3 | 0.50 ± 0.27 | 3.26 ± 0.11 | 2.14 ± 0.22 | 17.8 ± 1.8 | |
| Agitated | |||||||||
| S-20 | 5 | d | 0.90 ± 0.01 | 30.4 ± 0.0 | 25.4 ± 2.2 | 0.17 ± 0.01 | 1.09 ± 0.05 | 0.15 ± 0.07 | 16.7 ± 0.2 |
| 17 | c | 7.71 ± 0.13 | 85.3 ± 1.3 | 73.1 ± 0.1 | 0.53 ± 0.13 | 0.27 ± 0.01 | 1.53 ± 0.01 | 19.8 ± 0.2 | |
| 19 | a, b, e, f | 7.95 ± 0.65 | 90.0 ± 1.2 | 97.4 ± 0.4 | 0.52 ± 0.65 | 0.25 ± 0.01 | 2.40 ± 0.02 | 30.2 ± 0.3 | |
| S-25 | 8 | c,d | 3.37 ± 0.47 | 18.3 ± 0.1 | 41.4 ± 0.0 | 0.92 ± 0.47 | 0.64 ± 0.03 | 0.44 ± 0.02 | 13.0 ± 0.23 |
| 16 | f | 6.26 ± 0.17 | 70.8 ± 2.9 | 75.1 ± 0.8 | 0.44 ± 0.17 | 0.42 ± 0.04 | 1.85 ± 0.14 | 29.5 ± 2.3 | |
| 28 | e | 8.63 ± 0.75 | 80.4 ± 0.9 | 96.9 ± 1.1 | 0.54 ± 0.75 | 0.34 ± 0.01 | 1.88 ± 0.06 | 21.8 ± 0.7 | |
| 35 | a, b | 9.69 ± 0.13 | 82.1 ± 1.8 | 97.9 ± 0.1 | 0.59 ± 0.13 | 0.30 ± 0.20 | 1.52 ± 0.05 | 15.7 ± 0.5 | |
| M-20 | 12 | d | 12.23 ± 0.52 | 82.7 ± 2.5 | 78.3 ± 2.7 | 0.67 ± 0.17 | 1.15 ± 0.03 | 2.17 ± 0.06 | 17.7 ± 0.5 |
| 19 | a–c, e, f | 16.15 ± 0.58 | 95.1 ± 0.6 | 90.1 ± 0.1 | 0.77 ± 0.13 | 0.52 ± 0.01 | 3.33 ± 0.09 | 20.6 ± 0.6 | |
| M-25 | 16 | f | 7.79 ± 0.24 | 48.1 ± 2.4 | 41.3 ± 0.9 | 0.39 ± 0.17 | 2.86 ± 0.13 | 1.34 ± 0.06 | 17.2 ± 0.7 |
| 35 | a–c, d, e | 16.27 ± 0.01 | 62.5 ± 0.2 | 68.8 ± 0.3 | 0.64 ± 0.13 | 3.69 ± 0.10 | 1.68 ± 0.01 | 10.3 ± 0.1 | |
| (b) M. vulgaris | |||||||||
| Substrate | Time | X | TScon | Ncon | YX/S | EPS | IPS | IPS | |
| (Days) | (g/L) | (%) | (%) | (g/g) | (g/L) | (g/L) | (%, w/w) | ||
| Static | |||||||||
| S-20 | 5 | d | 2.34 ± 0.02 | 31.9 ± 0.0 | 37.9 ± 2.4 | 0.43 ± 0.02 | 1.20 ± 0.04 | 0.33 ± 0.02 | 14.0 ± 0.2 |
| 10 | c | 7.35 ± 0.14 | 72.5 ± 2.1 | 73.6 ± 2.1 | 0.60 ± 0.14 | 1.07 ± 0.03 | 1.14 ± 0.01 | 15.5 ± 0.1 | |
| 19 | a, b, e, f | 8.39 ± 0.26 | 88.8 ± 0.1 | 96.3 ± 0.0 | 0.56 ± 0.26 | 0.06 ± 0.01 | 3.10 ± 0.09 | 37.0 ± 1.1 | |
| S-25 | 8 | d | 6.78 ± 0.39 | 70.0 ± 0.5 | 87.4 ± 0.6 | 0.48 ± 0.39 | 0.34 ± 0.02 | 1.29 ± 0.00 | 19.0 ± 1.2 |
| 12 | c | 7.61 ± 0.06 | 72.5 ± 1.5 | 89.7 ± 0.8 | 0.53 ± 0.06 | 0.31 ± 0.03 | 2.05 ± 0.03 | 27.0 ± 0.8 | |
| 16 | e, f | 7.89 ± 0.01 | 80.1 ± 0.1 | 92.6 ± 0.8 | 0.49 ± 0.01 | 0.26 ± 0.07 | 2.84 ± 0.01 | 36.0 ± 0.3 | |
| 35 | a, b | 8.40 ± 0.24 | 89.7 ± 0.2 | 96.4 ± 0.2 | 0.47 ± 0.24 | 0.12 ± 0.00 | 1.82 ± 0.13 | 21.7 ± 1.5 | |
| M-20 | 5 | c, d | 3.47 ± 0.11 | 16.4 ± 2.4 | 22.3 ± 0.7 | 0.96 ± 0.39 | 1.82 ± 0.05 | 0.49 ± 0.01 | 14.0 ± 0.2 |
| 14 | e, f | 14.33 ± 0.03 | 91.7 ± 0.8 | 90.4 ± 1.2 | 0.71 ± 0.02 | 0.83 ± 0.01 | 3.81 ± 0.07 | 26.6 ± 0.5 | |
| 19 | a, b | 15.42 ± 0.44 | 95.9 ± 0.4 | 94.1 ± 0.2 | 0.73 ± 0.24 | 0.59 ± 0.03 | 3.47 ± 0.07 | 22.5 ± 0.4 | |
| M-25 | 8 | c | 6.79 ± 0.49 | 14.8 ± 1.3 | 45.1 ± 0.7 | 0.96 ± 0.39 | 3.39 ± 0.12 | 1.22 ± 0.13 | 18.0 ± 0.6 |
| 12 | e, f | 9.86 ± 0.25 | 37.7 ± 1.0 | 57.2 ± 0.7 | 0.64 ± 0.06 | 3.12 ± 0.23 | 2.61 ± 0.05 | 26.5 ± 0.6 | |
| 35 | a, b, d | 13.33 ± 0.42 | 71.0 ± 1.0 | 85.8 ± 0.7 | 0.46 ± 0.24 | 3.44 ± 0.07 | 1.93 ± 0.15 | 14.5 ± 1.1 | |
| Agitated | |||||||||
| S-20 | 5 | c, d | 2.34 ± 0.36 | 25.6 ± 0.6 | 25.2 ± 0.6 | 0.54 ± 0.36 | 1.18 ± 0.03 | 0.32 ± 0.01 | 13.5 ± 0.2 |
| 19 | a, b, e, f | 8.40 ± 0.53 | 98.3 ± 1.3 | 96.9 ± 0.3 | 0.50 ± 0.53 | 0.11 ± 0.01 | 3.02 ± 0.08 | 35.9 ± 1.0 | |
| S-25 | 8 | d | 4.78 ± 0.02 | 66.6 ± 2.5 | 39.0 ± 4.8 | 0.36 ± 0.02 | 0.44 ± 0.00 | 0.67 ± 0.03 | 14.0 ± 1.2 |
| 35 | a–c, e, f | 11.05 ± 0.52 | 96.6 ± 1.4 | 96.0 ± 0.0 | 0.57 ± 0.52 | 0.14 ± 0.01 | 2.22 ± 0.07 | 20.1 ± 0.6 | |
| M-20 | 19 | a–c, d, e, f | 16.81 ± 0.61 | 95.4 ± 0.3 | 90.5 ± 0.1 | 0.80 ± 0.52 | 1.40 ± 0.02 | 4.24 ± 0.08 | 25.2 ± 0.5 |
| M-25 | 16 | f | 7.35 ± 0.12 | 40.3 ± 0.1 | 59.3 ± 1.4 | 0.44 ± 0.02 | 3.11 ± 0.04 | 1.42 ± 0.23 | 19.4 ± 3.1 |
| 35 | a–c, d, e | 16.92 ± 0.74 | 76.5 ± 1.2 | 84.3 ± 0.1 | 0.54 ± 0.52 | 3.93 ± 0.17 | 1.50 ± 0.15 | 8.8 ± 0.9 | |
| (c) M. conica | |||||||||
| Substrate | Time | X | TScon | Ncon | YX/S | EPS | IPS | IPS | |
| (Days) | (g/L) | (%) | (%) | (g/g) | (g/L) | (g/L) | (%, w/w) | ||
| Static | |||||||||
| S-20 | 5 | d | 0.08 ± 0.08 | 21.5 ± 0.0 | 14.1 ± 1.9 | 0.02 ± 0.08 | 1.11 ± 0.01 | 0.02 ± 0.01 | 27.0 ± 0.3 |
| 12 | c | 6.54 ± 0.19 | 56.2 ± 2.5 | 80.2 ± 0.1 | 0.69 ± 0.14 | 0.25 ± 0.00 | 2.06 ± 0.14 | 31.5 ± 2.1 | |
| 19 | a, b, e, f | 8.50 ± 0.24 | 98.0 ± 0.5 | 96.2 ± 0.4 | 0.51 ± 0.01 | 0.07 ± 0.02 | 3.71 ± 0.10 | 43.6 ± 1.2 | |
| S-25 | 8 | d | 6.91 ± 0.44 | 86.4 ± 0.0 | 53.4 ± 0.2 | 0.40 ± 0.44 | 0.30 ± 0.03 | 2.00 ± 0.17 | 29.0 ± 0.7 |
| 16 | c, e, f | 8.40 ± 0.01 | 94.2 ± 0.4 | 78.6 ± 0.8 | 0.45 ± 0.01 | 0.12 ± 0.09 | 3.43 ± 0.01 | 40.8 ± 0.1 | |
| 35 | a, b | 8.71 ± 0.02 | 99.5 ± 0.0 | 97.0 ± 0.2 | 0.44 ± 0.02 | 0.06 ± 0.01 | 2.46 ± 0.13 | 28.2 ± 1.5 | |
| M-20 | 5 | d | 0.89 ± 0.05 | 11.3 ± 0.8 | 5.5 ± 0.3 | 0.36 ± 0.44 | 2.37 ± 0.12 | 0.24 ± 0.02 | 27.0 ± 1.0 |
| 10 | f | 12.48 ± 0.38 | 72.0 ± 1.6 | 37.2 ± 1.2 | 0.79 ± 0.09 | 1.21 ± 0.11 | 4.37 ± 0.07 | 35.1 ± 0.4 | |
| 14 | e | 14.73 ± 0.40 | 85.6 ± 0.5 | 62.3 ± 0.9 | 0.78 ± 0.01 | 0.73 ± 0.03 | 4.57 ± 0.09 | 31.0 ± 0.6 | |
| 19 | a, b | 16.02 ± 0.30 | 96.9 ± 0.6 | 85.0 ± 0.7 | 0.75 ± 0.02 | 0.55 ± 0.06 | 3.64 ± 0.10 | 22.7 ± 0.6 | |
| M-25 | 8 | c, d | 5.94 ± 0.57 | 18.1 ± 0.7 | 15.9 ± 1.2 | 0.80 ± 0.44 | 3.14 ± 0.02 | 1.13 ± 0.14 | 19.0 ± 1.3 |
| 12 | e, f | 9.48 ± 0.35 | 31.9 ± 1.2 | 34.2 ± 1.2 | 0.72 ± 0.09 | 2.97 ± 0.03 | 3.15 ± 0.08 | 33.2 ± 0.8 | |
| 35 | a, b | 14.45 ± 0.08 | 81.2 ± 0.4 | 83.6 ± 0.1 | 0.43 ± 0.02 | 1.98 ± 0.11 | 2.35 ± 0.07 | 16.3 ± 0.5 | |
| Agitated | |||||||||
| S-20 | 5 | c, d | 1.76 ± 0.94 | 13.4 ± 0.0 | 21.6 ± 0.0 | 0.77 ± 0.94 | 1.01 ± 0.06 | 0.37 ± 0.04 | 21.0 ± 0.5 |
| 19 | a, b, e, f | 10.85 ± 0.58 | 99.5 ± 0.2 | 96.7 ± 0.2 | 0.64 ± 0.58 | 0.08 ± 0.03 | 4.55 ± 0.07 | 42.0 ± 1.2 | |
| S-25 | 8 | d | 6.16 ± 0.01 | 75.9 ± 1.3 | 62.1 ± 0.1 | 0.41 ± 0.01 | 0.71 ± 0.12 | 0.92 ± 0.03 | 15.0 ± 0.4 |
| 16 | f | 8.00 ± 0.71 | 96.5 ± 1.0 | 84.9 ± 0.9 | 0.41 ± 0.71 | 0.43 ± 0.02 | 4.14 ± 0.03 | 51.8 ± 0.7 | |
| 35 | a–c, e | 12.15 ± 0.01 | 99.4 ± 0.0 | 96.5 ± 0.3 | 0.61 ± 0.01 | 0.11 ± 0.10 | 4.64 ± 0.13 | 38.2 ± 1.0 | |
| M-20 | 5 | d | 3.37 ± 1.58 | 14.6 ± 1.4 | 10.5 ± 0.2 | 0.80 ± 0.01 | 0.95 ± 0.01 | 0.78 ± 0.23 | 23.0 ± 2.4 |
| 14 | f | 13.26 ± 0.19 | 94.6 ± 1.7 | 85.1 ± 1.3 | 0.64 ± 1.15 | 0.69 ± 0.02 | 4.21 ± 0.15 | 31.8 ± 1.1 | |
| 19 | a–c, e | 18.16 ± 0.01 | 97.4 ± 0.8 | 89.6 ± 0.0 | 0.85 ± 0.01 | 0.54 ± 0.23 | 4.81 ± 0.09 | 26.5 ± 0.5 | |
| M-25 | 8 | d | 4.73 ± 0.11 | 30.9 ± 1.5 | 35.9 ± 0.8 | 0.37 ± 0.01 | 3.18 ± 0.09 | 0.76 ± 0.16 | 16.0 ± 2.6 |
| 12 | f | 5.56 ± 0.11 | 34.2 ± 0.0 | 47.3 ± 0.5 | 0.40 ± 0.21 | 2.93 ± 0.08 | 1.20 ± 0.01 | 21.7 ± 10.2 | |
| 35 | a–c, e | 18.10 ± 0.03 | 80.3 ± 1.2 | 76.3 ± 1.2 | 0.55 ± 0.01 | 1.90 ± 0.02 | 1.74 ± 0.02 | 9.6 ± 0.1 | |
| Morchella Species | Substrate | Day | Glucose | Fructose | Mannitol |
|---|---|---|---|---|---|
| Static | |||||
| M. rotunda | S-20 | 19 | 64.2 ± 3.3 | 15.1 ± 0.7 | 20.7 ± 1.3 |
| S-25 | 16 | 56.3 ± 3.9 | 24.0 ± 1.2 | 19.7 ± 0.9 | |
| M-20 | 14 | 51.6 ± 4.1 | 12.9 ± 0.7 | 35.5 ± 1.6 | |
| M-25 | 35 | 45.7 ± 5.2 | 26.1 ± 0.9 | 28.2 ± 0.8 | |
| M. vulgaris | S-20 | 19 | 63.4 ± 3.2 | 12.2 ± 0.2 | 24.4 ± 1.5 |
| S-25 | 16 | 63.4 ± 4.1 | 15.0 ± 0.9 | 21.6 ± 2.1 | |
| M-20 | 14 | 52.7 ± 2.9 | 11.8 ± 1.1 | 35.5 ± 1.6 | |
| M-25 | 12 | 57.2 ± 1.8 | 14.7 ± 0.5 | 28.1 ± 1.8 | |
| M. conica | S-20 | 19 | 63.5 ± 3.5 | 12.0 ± 0.1 | 24.5 ± 1.2 |
| S-25 | 16 | 59.0 ± 2.0 | 19.9 ± 0.9 | 21.1 ± 2.1 | |
| M-20 | 14 | 58.9 ± 1.0 | 9.7 ± 1.1 | 31.4 ± 1.7 | |
| M-25 | 12 | 54.4 ± 4.1 | 17.6 ± 0.7 | 28.0 ± 1.4 | |
| Agitated | |||||
| M. rotunda | S-20 | 19 | 58.9 ± 3.4 | 20.5 ± 0.8 | 20.6 ± 1.4 |
| S-25 | 28 | 52.3 ± 4.1 | 22.3 ± 1.2 | 25.4 ± 1.3 | |
| M-20 | 19 | 56.6 ± 4.2 | 15.9 ± 0.9 | 27.6 ± 1.7 | |
| M-25 | 35 | 36.8 ± 5.1 | 19.9 ± 0.8 | 43.3 ± 0.9 | |
| M. vulgaris | S-20 | 19 | 55.1 ± 3.3 | 22.2 ± 0.3 | 22.7 ± 1.6 |
| S-25 | 35 | 40.4 ± 4.2 | 22.6 ± 0.4 | 37.0 ± 2.5 | |
| M-20 | 19 | 61.0 ± 3.1 | 12.1 ± 1.0 | 26.8 ± 1.3 | |
| M-25 | 35 | 46.2 ± 1.9 | 12.6 ± 0.3 | 41.2 ± 1.9 | |
| M. conica | S-20 | 19 | 70.2 ± 3.6 | 11.1 ± 0.9 | 18.7 ± 1.2 |
| S-25 | 35 | 70.4 ± 2.3 | 12.3 ± 0.4 | 17.4 ± 1.5 | |
| M-20 | 19 | 61.3 ± 1.8 | 10.1 ± 0.8 | 28.6 ± 1.3 | |
| M-25 | 35 | 48.4 ± 3.7 | 11.3 ± 0.1 | 40.3 ± 0.9 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dedousi, M.; Fourtaka, K.; Melanouri, E.-M.; Argyropoulos, D.; Psallida, C.; Diamantis, I.; Papanikolaou, S.; Diamantopoulou, P. Detoxification of Molasses and Production of Mycelial Mass and Valuable Metabolites by Morchella Species. Appl. Sci. 2021, 11, 9481. https://doi.org/10.3390/app11209481
Dedousi M, Fourtaka K, Melanouri E-M, Argyropoulos D, Psallida C, Diamantis I, Papanikolaou S, Diamantopoulou P. Detoxification of Molasses and Production of Mycelial Mass and Valuable Metabolites by Morchella Species. Applied Sciences. 2021; 11(20):9481. https://doi.org/10.3390/app11209481
Chicago/Turabian StyleDedousi, Marianna, Katerina Fourtaka, Eirini-Maria Melanouri, Dimitrios Argyropoulos, Charoula Psallida, Ilias Diamantis, Seraphim Papanikolaou, and Panagiota Diamantopoulou. 2021. "Detoxification of Molasses and Production of Mycelial Mass and Valuable Metabolites by Morchella Species" Applied Sciences 11, no. 20: 9481. https://doi.org/10.3390/app11209481





