1. Introduction
In 1977, Kierstan and Bucke [
1] published alginate beads production using a simple extrusion dropwise of an alginate solution in a calcium bath. The migration of the calcium inside the alginate droplet provokes the gelification of the alginate. The method is simple, done at room temperature without any toxic compound. Therefore, it is not surprising that it has become the most usual cell encapsulation method, with more than 11,000 publications (Science Direct). Zhu [
2], for instance, had prepared calcium alginate gel beads through a system under forced high-voltage electrostatic for the encapsulation and immobilization of microorganisms, enzymes, or cells. Bahry [
3] also utilized alginate beads to immobilize
Lactobacillus rhamnosus and explore another efficient way to valorize the residual carbohydrates of carob waste instead of work directly on pods, using
Lactobacillus casei in the liquid phase. This method was demonstrated to be effective, mainly because it allows the reuse of microorganism due to easy separation of the biomass from the liquid phase.
Alginate has exceptional gelation properties. It allows the development of several alternatives to the simple alginate beads through different compositions, such as the mixture of alginate (20 g/L) with starch up to 300 g/L [
4], or shellac up to 100 g/L [
5]. Alginate also provides mechanical or gastric resistance. Besides presenting versatility in encapsulation, like core-shell capsules by inverse gelation, the extrusion of calcium suspension in alginate bath [
6], or co-extrusion of core solution inside an alginate shell solution [
5].
Initially, these methods were developed based on a simple extrusion drop by drop from a needle. It results in large capsules (2–3 mm) and a low flow rate (100 mL/h). Then, to reduce the size, different technologies were studied, such as electro-dripping, which is extrusion by applying an electrostatic potential between the needle and the gelation bath [
7]. To get a smaller size and higher flow rate, the solution must be extruded as a jet and the jet itself broken into droplets either by a vibration [
8] or a cutting tool [
9]. Equipment for the first option has been developed by several companies (Buchi, Switzerland; Nisco, Switzerland; Brace, Germany), readily available commercially.
The jet breakage by applying a vibration was proposed by Savart in 1833 [
10] and interpreted by Rayleigh in 1879 [
11]. A liquid jet breaks in droplets with a mean diameter defined by the breakage frequency, mainly jet diameter, and linear speed. If all vibrations are eliminated, and the optimum frequency is applied, they would be monodispersed droplets. In fact, a size dispersion of 5% is generally observed.
Even though the obtention of alginate beads by simple extrusion is one of the most popular encapsulation techniques used, while often used to get small, monodispersed alginate beads, the nozzle resonance technology is not fully understood and not used optimally. Especially, operators assume that the size could be controlled by simply varying the frequency.
Many articles develop the Rayleigh’s theory, taking into account the rheology of the solution, its surface tension, and the formation of satellite droplets [
12,
13,
14]. However, these models are difficult to apply for daily work. Alginate forms a shear-thinning solution but is not viscoelastic [
8]. The solution generally has relatively low viscosity (<200 mPa·s). This contribution therefore wishes to verify that a simpler model developed for Newtonian solutions could be applied to hydrogel solutions like alginate solution.
Most data relating to the size and the operating conditions are based on the alginate beads and not directly the droplets. The present contribution reports direct observations of the alginate solution droplet formation with the assistance of fast recording cameras. It evaluates the impact of several parameters, such as frequency, type of membrane, nozzle size, and vibration amplitude on droplet size. Besides, the study compares and verifies those results with mathematical theories to better understand process optimizing and the importance of selecting a well-designed vibrating system.
2. Materials and Methods
2.1. Alginate Solution
The alginate powder Algol 3001 was kindly provided by Cargill (Puteaux, France). One liter of distilled water was introduced in a beaker (1.5 L, diameter 12 cm, Fischer Scientific, Illkirch, France) and agitated using a magnetic stirrer (MINI MR standard IKA, Fischer Scientific, Illkirch, France, equipped with 5 cm magnetic bar, and turning at 300 rpm). Eighteen grams of alginate were slowly added through a fine mesh strainer cooking sieve. The resulting suspension was maintained under agitation for one hour until alginate was dissolved entirely and hydrated. Then, the solution was let stand for one hour without agitation for deaeration. Finally, it was used directly or kept in the fridge at 4 °C.
2.2. Droplet Production
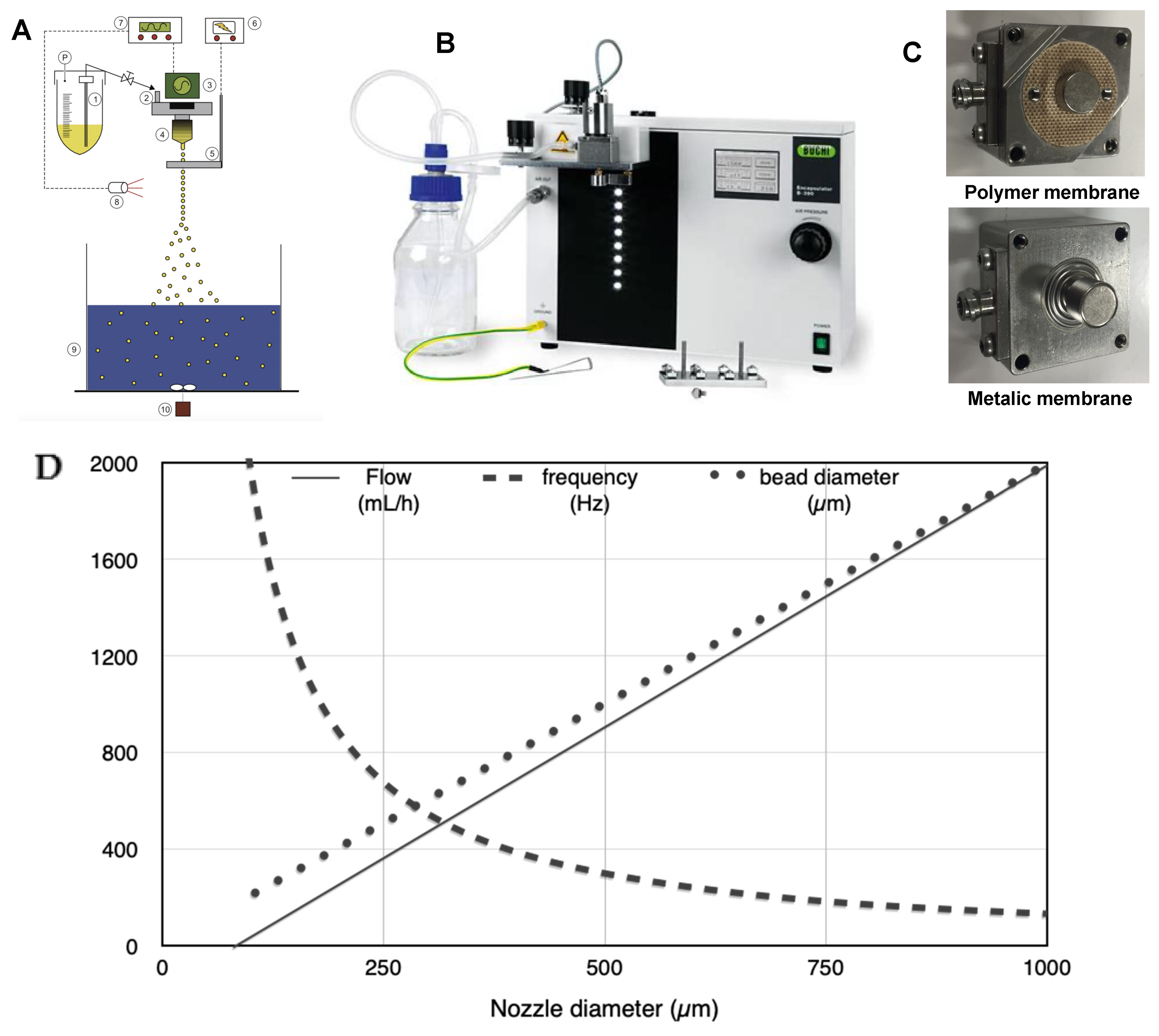
Figure 1A,B present the encapsulator B390 (Buchi, Flawil, Switzerland). The system comprises 1. Pressure bottle, 2. Dripping system, 3. Vibration system, 4. Nozzle, 5. Electrode, 6. Electric potential control, 7. Vibration controller, 8. LED/Stroboscope, 9. Gelation bath, 10. Magnetic stirrer. The dripping system is composed of a support to be connected to the nozzle and the feeding vessel (
Figure 1C). It is closed on the top by a membrane on which a magnet is attached. A vibration system with a second magnet is placed on the dripping system, allowing vibration on the membrane and subsequently on the liquid. The control block allows adjusting the frequency and the amplitude of the vibration. The liquid flow rate is controlled by applying pressure on the feeding vessel and the tube’s opening connecting the vessel to the dripping device. The control block is equipped with LED flashing at the same frequency as the applied vibration on the capillary jet.
The feeding vessel was filled with the alginate solution. The frequency and amplitude were set following supplier recommendations corresponding to the selected nozzle. The liquid flow rate was then first adjusted following the same recommendations and adapted to get optimum breakage. The experiments were carried out with different nozzles sizes (450, 750, and 1000 µm), as well as with polymer and metallic membrane (
Figure 1C).
The optimum breakage was determined by observing the droplet string in front of the LED. If optimum conditions are reached, the droplets must seem immobile, as if in each flash the droplets have the same position. However, if the optimum conditions are not reached, they seem to move up or down. As shown later, the optimum conditions constitute a range of flow rate/frequency couples. In practice, it represents an advantage but introduces a degree of incertitude while trying to fit the data with a model.
In the manual of the encapsulator B390 [
15], some ranges of operating conditions are provided.
Figure 1D presents the mean value of the frequency, the flow rate, and the resulting droplet size. These data were used as a starting point for the parameter set-up.
2.3. Droplet Formation Visualization
The droplet formation was observed using a high-speed camera (HighSpeedStar 6, Lavision, Paris, France), and images were recorded at 5000 to 7000 Hz. The images were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA). The camera was connected to PC Thinkpad X200, Lenovo running on windows XP. The video was recorded with the software Photron FASTCAM Viewer ver. 3.1.8 (Photron, Tokyo, Japan).
An example of capillary jet breakage recorded with the high-speed camera is displayed in
Figure 2. The mean size and size dispersion were obtained by measuring 50 droplets and computing the mean value and the standard deviation. Experiments were repeated a minimum of three times to provide the final value. The length before breakage was defined as the distance between the nozzle before the first droplet had been formed. Droplet velocity was obtained by following one droplet over the image and dividing the droplet traveled distance by the time.
3. Theoretical Approach
Rayleigh [
11] analyses the instability of the liquid jet and its tendency to break into droplets due to axial symmetric disturbances. He observed that the breakage occurs when the jet length is similar to the jet circumference. For Newtonian and low viscosity liquid, the optimum breakage wavelength,
, to obtain mono-dispersed droplets was given by:
where
dj is the jet diameter. The optimum frequency, ƒ
opt, is equal to:
where
is the linear velocity of the jet. According to Schneider and Hendricks [
16], uniform droplets may be produced in a range of wavelengths defined by:
This provides some freedom in selecting the conditions for droplet production. In 1931, Weber [
17] extended the Rayleigh equation considering the impacts of the liquid physicochemical properties:
where
is the fluid viscosity,
is the liquid density, and
is the surface tension. Generally, the jet diameter,
dj, is assumed equal to the internal nozzle diameter,
dn [
13]. However, at the exit of the nozzle, the jet is submitted to a pressure relaxation, leading to a jet expansion that could be expressed by [
8]
where
Wen is the Weber number related to flow inside the nozzle:
The liquid flow rate,
F, is the product of the jet section times the jet velocity:
The volume,
, of the droplets may be predicted by:
and, combining Equations (1), (2), (7) and (8), the droplet diameter,
, is given by:
6. Conclusions
Alginate beads have been used in many applications, from relatively simple food and feed applications to advanced biomedical technologies. Monodispersion is sometimes the key to success, like in the case of Langerhans Islets encapsulation but not always requested. However, reasonable control of size is important to consider an industrial transfer or sophisticated applications. Nozzle resonance method allows to get such control both size and the size dispersion, but only if well driven.
The use of a fast-video camera allows observing, more precisely, the capillary jet breakage. Previously, the analysis was done based on the alginate bead size. However, during gelation, the size of the particles contract. This process is in the function of the alginate type and concentration and calcium chloride concentration. Additionally, it was not possible to observe the breakage quality (length before breakage, formation of satellite droplets, etc.).
As pointed out by all equipment providers, the first observation is that the operator must define the optimum conditions to get mono-dispersed droplets. Equipment supplier recommendations help to define approximate parameter values. An alternative is to use the equations cited above. However, fine tune-up has to be accomplished using the LED control system.
The present work shows the importance of selecting a well-designed vibrating system (membrane material in Buchi). A nozzle resonance device may be homemade, but it requires an exact design to avoid any parasite vibration. In addition, this contribution presents data while varying the nozzle size, the frequency coupled with the flow rate to get optimum breakage, but keeping all the experiments the same alginate solution. For future experiments, we would like to modify the concentration and composition of the extruded solution. It would also be interesting to find a precise criterium to identify the optimum conditions.