New Insights into the Antioxidant Compounds of Achenes and Sprouted Buckwheat Cultivated in the Republic of Moldova
Abstract
:1. Introduction
2. Materials and Methods
2.1. Buckwheat Materials
2.2. Chemicals, Reagent and Materials
2.3. The Sprouting
2.4. Preparation of Vegetative Material
2.5. Total Polyphenol Content (TPC) Analysis
2.6. Total Flavonoid Content (TFC) Analysis
2.7. Determination of Antioxidant Activity by the DPPH Method
2.8. HPLC–MS Analysis of Phenolic Compounds
2.9. FTIR Analysis
2.10. Statistics
3. Results and Discussions
3.1. Evolution of Total Polyphenol Content Assay (TPC) and Total Flavonoid Content Assay (TFC)
3.2. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Activity Assay
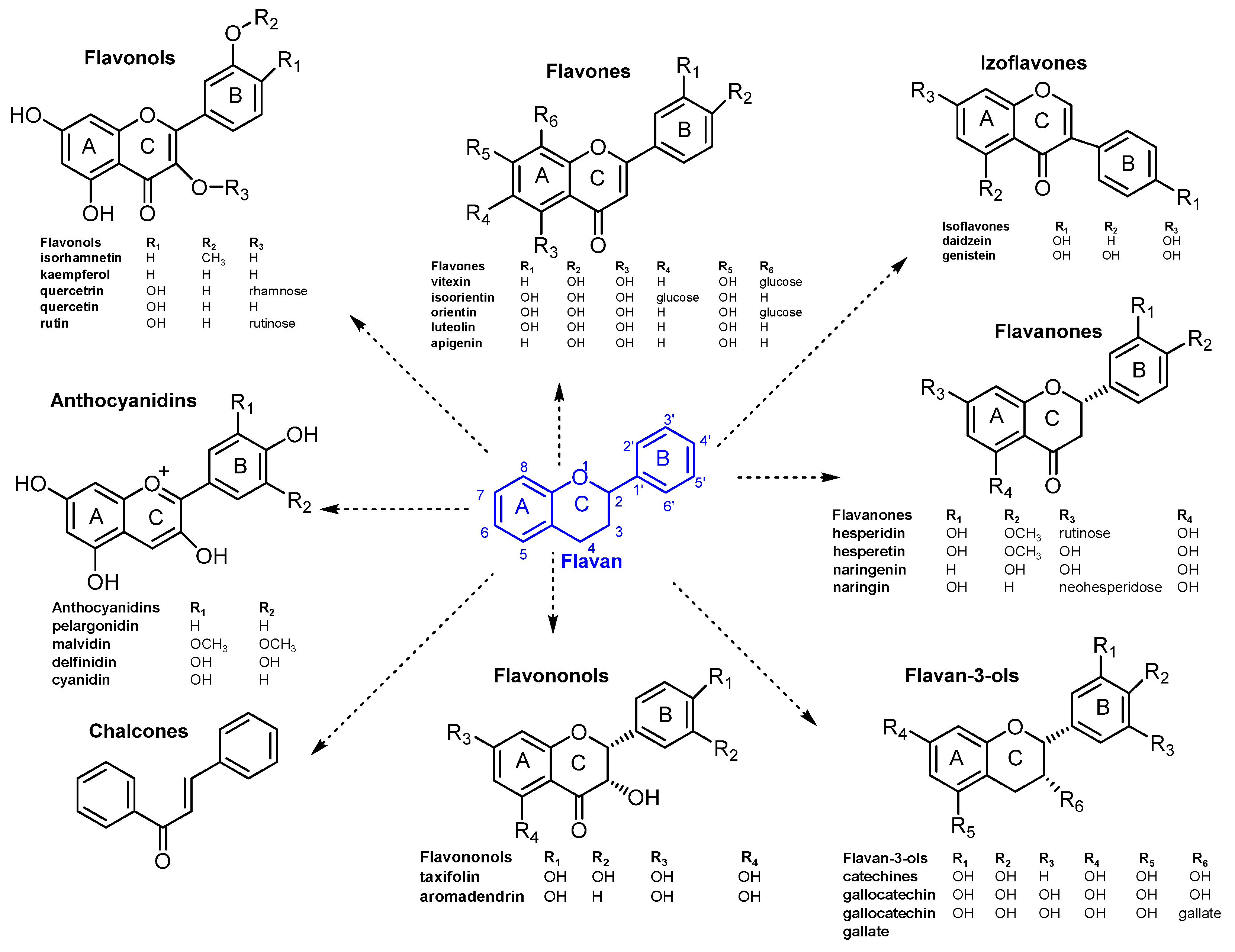
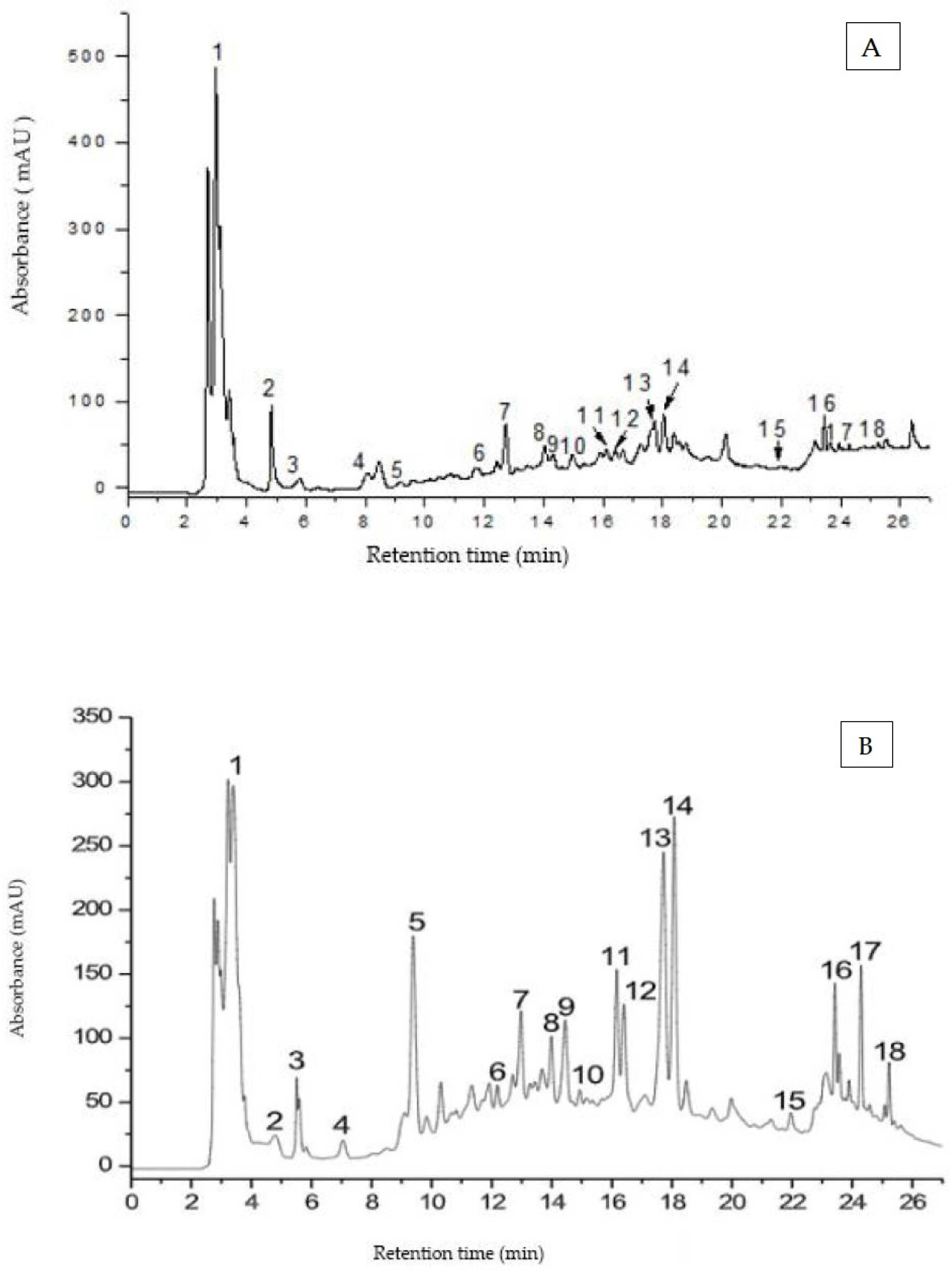
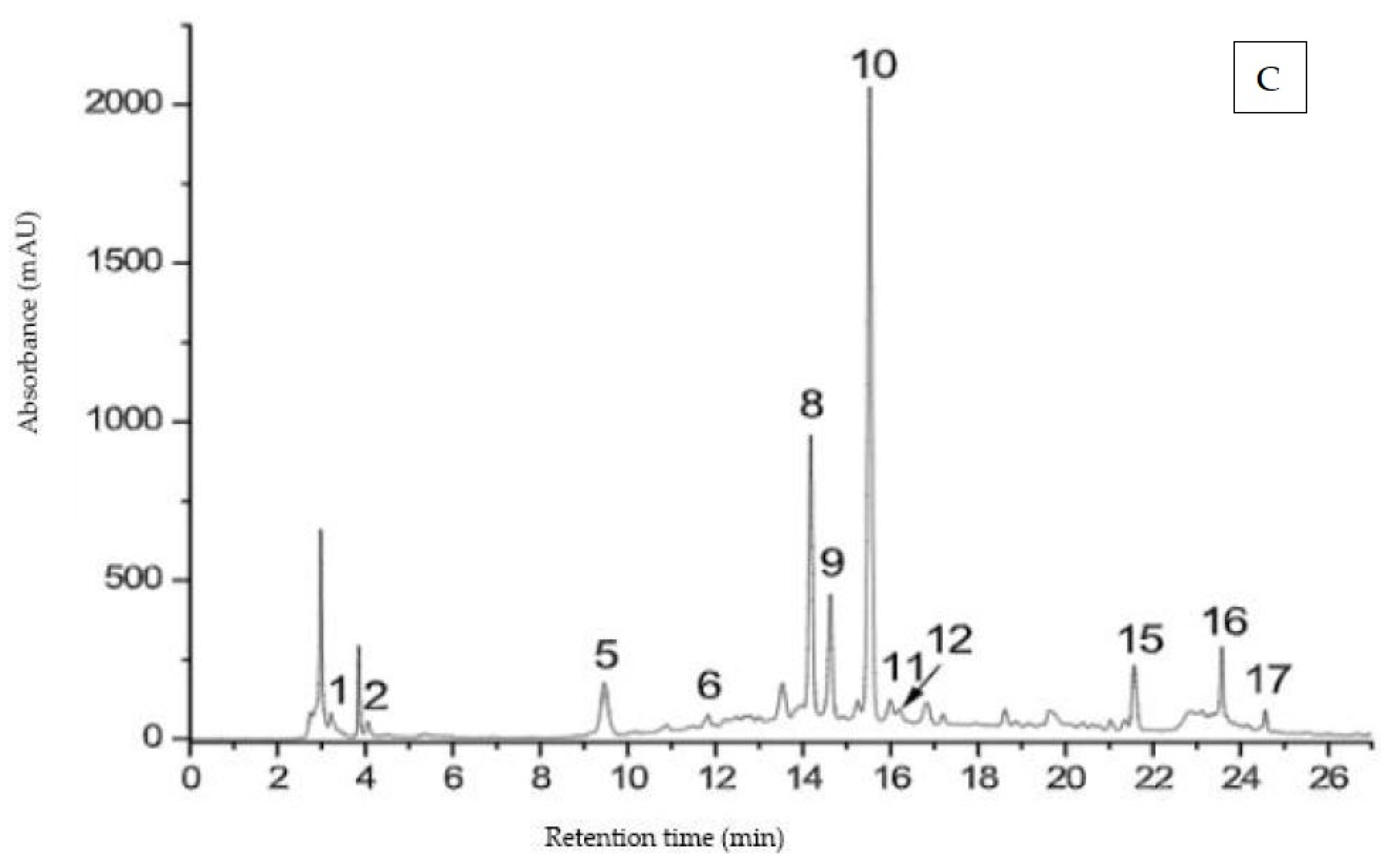
3.3. HPLC–MS Analysis of Phenolic Compounds
3.4. FTIR Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beckman, C.H. Phenolic-storing cells: Keys to programmed cell death and periderm formation in wilt disease resistance and in general defence responses in plants? Physiol. Mol. Plant Pathol. 2000, 57, 101–110. [Google Scholar] [CrossRef]
- Sanchez, A.; Schuster, T.; Burke, J.M.; Kron, K. Taxonomy of Polygonoideae (Polygonaceae): A new tribal classification. Taxon 2011, 60, 151–160. [Google Scholar] [CrossRef]
- Campbell, C.G. Buckwheat. Fagopyrum Esculentum Moench. Promoting the Conservation and Use of Underutilized and-Neglected Crops; Institute of Plant Genetics and Crop Plant Research, Gatersleben/International Plant Genetic Resources In-stitute: Rome, Italy, 1997. [Google Scholar]
- Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 19 June 2021).
- Holasova, M.; Fiedlerova, V.; Smrcinova, H.; Orsak, M.; Lachman, J.; Vavreinova, S. Buckwheat—the source of antioxidant activity in functional foods. Food Res. Int. 2002, 35, 207–211. [Google Scholar] [CrossRef]
- Sedej, I.; Sakac, M.; Misan, A.; Mandic, A. Antioxidant activity of wheat and buckwheat flours. Zb. Matice Srp. Za Prir. Nauk. 2010, 118, 59–68. [Google Scholar] [CrossRef]
- Oomah, B.D.; Mazza, G. Flavonoids and Antioxidative Activities in Buckwheat. J. Agric. Food Chem. 1996, 44, 1746–1750. [Google Scholar] [CrossRef]
- Gorinstein, S.; Lojek, A.; Milán, Č.; Pawelzik, E.; Delgado-Licon, E.; Medina, O.J.; Moreno, M.; Salas, I.A.; Goshev, I. Comparison of composition and antioxidant capacity of some cereals and pseudocereals. Int. J. Food Sci. Technol. 2008, 43, 629–637. [Google Scholar] [CrossRef]
- Gimenez-Bastida, J.A.; Zielin’ Ski, H. Buckwheat as a Functional Food and Its Effects on Health. J. Agric. Food Chem. 2015, 16, 7896–7913. [Google Scholar] [CrossRef] [PubMed]
- Draganescu, M.; Dumitru, C.; Baroiu, L.; Iancu, A.; Vizireanu, C.; Arbune, M.; Beznea, A. Antioxidant Profile of Buckwheat Honey from the Republic of Moldova. Rev. Chim. 2020, 71, 325–336. [Google Scholar] [CrossRef]
- Blaga, G.-V.; Chițescu, C.L.; Lisă, E.L.; Dumitru, C.; Vizireanu, C.; Borda, D. Antifungal residues analysis in various Romanian honey samples analysis by high resolution mass spectrometry. J. Environ. Sci. Health Part B 2020, 55, 484–494. [Google Scholar] [CrossRef]
- Segal, B.; Segal, R. Protective Food Technology; Ceres Publishing House: Bucharest, Romania, 1991. [Google Scholar]
- Kayashita, J.; Shimaoka, I.; Nakajoh, M.; Yamazaki, M.; Kato, N. Consumption of buckwheat protein lowers plasma cholesterol and raises fecal neutral sterols in cholesterol-Fed rats because of its low digestibility. J. Nutr. 1997, 127, 1395–1400. [Google Scholar] [CrossRef] [Green Version]
- Ma, M.S.; Bae, I.Y.; Lee, H.G.; Yang, C.B. Purification and identification of angiotensin I-converting enzyme inhibitory peptide from buckwheat (Fagopyrum esculentum Moench). Food Chem. 2006, 96, 36–42. [Google Scholar] [CrossRef]
- Chan, P.-K. Inhibition of tumor growth in vitro by the extract of fagopyrum cymosum (fago-c). Life Sci. 2003, 72, 1851–1858. [Google Scholar] [CrossRef]
- Kawa, J.M.; Taylor, C.G.; Przybylski, R. Buckwheat concentrate reduces serum glucose in streptozotocindiabetic rats. J. Agric. Food Chem. 2003, 51, 7287–7291. [Google Scholar] [CrossRef]
- Lorenz, K.; D’Appolonia, B. Cereal sprouts: Composition, nutritive value, food applications. Crit. Rev. Food Sci. Nutr. 1980, 13, 353–385. [Google Scholar] [CrossRef]
- Niculet, E.; Radaschin, D.S.; Nastase, F.; Draganescu, M.; Baroiu, L.; Miulescu, M.; Arbune, M.; Tatu, A.L. Influence of phytochemicals in induced psoriasis (Review). Exp. Ther. Med. 2020, 20, 3421–3424. [Google Scholar] [CrossRef] [PubMed]
- Peñas, E.; Martínez-Villaluenga, C. Advances in Production, Properties and Applications of Sprouted Seeds. Foods 2020, 9, 790. [Google Scholar] [CrossRef]
- Nam, T.G.; Lee, S.M.; Park, J.-H.; Kim, D.-O.; Baek, N.-I.; Eom, S.H. Flavonoid analysis of buckwheat sprouts. Food Chem. 2015, 170, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Koyama, M.; Nakamura, C.; Nakamura, K. Changes in phenols contents from buckwheat sprouts during growth stage. J. Food Sci. Technol. 2011, 50, 86–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinica, R.M.; Sandu, C.; Botezatu, A.V.D.; Busuioc, A.C.; Balanescu, F.; Mihaila, M.D.I.; Dumitru, C.N.; Furdui, B.; Iancu, A.V. Allantoin from Valuable Romanian Animal and Plant Sources with Promising Anti-Inflammatory Activity as a Nutricosmetic Ingredient. Sustainability 2021, 13, 10170. [Google Scholar] [CrossRef]
- Available online: https://www.globenewswire.com/news-release/2020/05/22/2037737/0/en/Nutraceuticals-market-worldwide-is-projected-to-grow-by-US-135-4-Billion.html (accessed on 1 April 2021).
- Brunori, A.; Sándor, G.; Xie, H.; Baviello, G.; Nehiba, B.; Rabnecz, G.; Végvári, G. Rutin content of the grain of 22 buckwheat (Fagopyrum esculentum Moench and Fagopyrum tataricum Gaertn.) varieties grown in Hungary. Eur. J. Plant Sci. Biotechnol. 2009, 3, 62–65. [Google Scholar]
- Klepacka, J.; Najda, A. A review of nutriyional and nutraceutical components of buckwheat. Eur. J. Plant Sci. Biotechnol. 2009, 3, 62–65. [Google Scholar]
- Ministry of Agriculture, Regional Development and the Environment, State Commission for Testing Plant Varieties. Catalog of Plant Varieties for Year 2021; Ministry of Agriculture, Regional Development and the Environment, State Commission for Testing Plant Varieties: Chişinău, Moldova, 2021. [Google Scholar]
- Anesini, C.; Ferraro, G.E.; Filip, R. Total Polyphenol Content and Antioxidant Capacity of Commercially Available Tea (Camellia sinensis) in Argentina. J. Agric. Food Chem. 2008, 56, 9225–9229. [Google Scholar] [CrossRef]
- Bozin, B.; Mimica-Dukicb, N.; Samojlikc, I.; Gorand, A.; Igic, R. Phenolics as antioxidants in garlic (Allium sativum L., Alliaceae). Food Chem. 2008, 111, 925–929. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Wijngaard, H.; Arendt, E.; Gallagher, E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking. Food Chem. 2010, 119, 770–778. [Google Scholar] [CrossRef]
- Zhang, G.; Zhicun, X.; Yuanyuan, G.; Xianxiao, H.; Yanping, Z.; Tiankui, Y. Effects of Germination on the Nutritional Properties, Phenolic Profiles, and Antioxidant Activities of Buckwheat. J. Food Sci. 2015, 80, H1111–H1119. [Google Scholar] [CrossRef]
- Busuioc, A.C.; Botezatu, A.-V.D.; Furdui, B.; Vinatoru, C.; Maggi, F.; Caprioli, G.; Dinica, R.-M. Comparative Study of the Chemical Compositions and Antioxidant Activities of Fresh Juices from Romanian Cucurbitaceae Varieties. Molecules 2020, 25, 5468. [Google Scholar] [CrossRef] [PubMed]
- Baroiu, L.; Dumitru, C.; Iancu, A.; Leșe, A.; Baroiu, N.; Drăgănescu, M. Factors That Impact the Risk of Death in Clostridium Difficile Infection. Acta Medica Mediterr. 2021, 37, 403. [Google Scholar]
- Kim, S.L.; Son, Y.K.; Hwang, J.J.; Kim, S.K.; Hur, H.S.; Park, C.H. Development and Utilization of Buckwheat Sprouts as Functional Vegetables. Fagopyrum 2001, 18, 49–54. [Google Scholar]
- Caparica, R.; Júlio, A.; Machado Araújo, M.E.; Rolim Baby, A.; Fonte, P.; Guilherme Costa, J.; Santos de Almeida, T. Anticancer Activity of Rutin and Its Combination with Ionic Liquids on Renal Cells. Biomolecules 2020, 10, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araruna, M.K.; Brito, S.A.; Morais-Braga, M.F.; Santos, K.K.; Souza, T.M.; Leite, T.R.; Costa, J.G.; Coutinho, H. Evaluation of antibiotic & antibiotic modifying activity of pilocarpine & rutin. Indian J. Med. Res. 2012, 135, 252–254. [Google Scholar]
- Pimentel, R.B.; da Costa, C.A.; Albuquerque, P.M. Antimicrobial activity and rutin identification of honey produced by the stingless bee Melipona compressipes manaosensis and commercial honey, BMC Complement. Altern. Med. Stud. 2013, 13, 151. [Google Scholar]
- Ganeshpurkar, A.; Bansal, D.; Dubey, S.; Dubey, N. Experimental studies on bioactive potential of rutin. Chron. Young-Sci. 2013, 4, 153. [Google Scholar] [CrossRef]
- Johann, S.; Mendes, B.G.; Missau, F.C.; Rezende, M.A.; Pizzollati, M.G. Antifungal activity of five species of Polygala. Braz. J. Microbiol. 2011, 42, 1065–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y. Rutin has therapeutic effect on septic arthritis caused by Candida albicans. Int. Immunopharmacol. 2009, 9, 207–211. [Google Scholar] [CrossRef]
- Tongjaroenbuangam, W.; Ruksee, N.; Chantiratikul, P.; Pakdeenarong, N.; Kongbuntad, W.; Govitrapong, P. Neuroprotective effects of quercetin, rutin and okra (Abelmoschus esculentus Linn.) in dexamethasone-treated mice. Neurochem. Int. 2011, 59, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Ugusman, A.; Zakaria, Z.; Chua, K.H.; Nordin, N.A.M.M.; Mahdy, Z.A. Role of Rutin on Nitric Oxide Synthesis in Human Umbilical Vein Endothelial Cells. Sci. World J. 2014, 2014, 169370. [Google Scholar] [CrossRef]
- Nieoczym, D.; Socała, K.; Raszewski, G.; Wlaź, P. Effect of quercetin and rutin in some acute seizure models in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 54, 50–58. [Google Scholar] [CrossRef]
- Selvaraj, G.; Kaliamurthi, S.; Sambandam, R.T.; Vivekanandan, L.; Balasubramanian, T. Anti-nociceptive effect in mice of thillai flavonoid rutin. Biomed. Environ. Sci. 2014, 27, 295–299. [Google Scholar] [PubMed]
- Guardia, T.; Rotelli, A.E.; Juarez, A.O.; Pelzer, L.E. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Il Farm. 2001, 56, 683–687. [Google Scholar] [CrossRef]
- Horcajada, M.-N.; Sanchez, C.; Scalfo, F.M.; Drion, P.; Comblain, F.; Taralla, S.; Donneau, A.-F.; Offord, E.; Henrotin, Y. Oleuropein or rutin consumption decreases the spontaneous development of osteoarthritis in the Hartley guinea pig. Osteoarthr. Cartil. 2015, 23, 94–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanashiro, A.; Andrade, D.C.; Kabeya, L.M.; Turato, W.M.; Faccioli, L.H.; Uyemura, S.A.; Lucisano-Valim, Y.M. Modulatory effects of rutin on biochemical and hematological parameters in hypercholesterolemic Golden Syrian hamsters. An. Acad. Bras. Ciências 2009, 81, 67–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.W.; Hwang, I.K.; Lim, S.S.; Yoo, K.-Y.; Li, H.; Kim, Y.S.; Kwon, D.Y.; Moon, W.K.; Kim, D.-W.; Won, M.-H. Germinated Buckwheat extract decreases blood pressure and nitrotyrosine immunoreactivity in aortic endothelial cells in spontaneously hypertensive rats. Phytother. Res. 2009, 23, 993–998. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, J.S.; Lee, T.R. Germination of Buckwheat Grain: Effects on Minerals, Rutin, Tannins and Colour. In Proceedings of the 9th International Symposium on Buckwheat, Prague, Czech Republic, 18–22 August 2004. [Google Scholar]
- Kim, S.-J.; Zaidul, I.; Suzuki, T.; Mukasa, Y.; Hashimoto, N.; Takigawa, S.; Noda, T.; Matsuura-Endo, C.; Yamauchi, H. Comparison of phenolic compositions between common and tartary buckwheat (Fagopyrum) sprouts. Food Chem. 2008, 110, 814–820. [Google Scholar] [CrossRef]
- Parasuraman, S.; David, A.V.A.; Arulmoli, R. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, P.K.; Agrawal, C.; Blunden, G. Quercetin: Antiviral Significance and Possible COVID-19 Integrative Considerations. Nat. Prod. Commun. 2020, 15, 1–10. [Google Scholar] [CrossRef]
- Ahmed, A.K.; Albalawi, Y.; SShora, H.A.; Abdelseed, H.K.; Al-Kattan, A.N. Effects of Quadruple Therapy: Zinc, Quercetin, Bromelain and Vitamin C on the Clinical Outcomes of Patients Infected with COVID-19. Int. J. Endocrinol. 2020, 1, 18–21. [Google Scholar]
- Baroiu, L.; Dumitru, C.; Iancu, A.; Leșe, A.-C.; Drăgănescu, M.; Baroiu, N.; Anghel, L. COVID-19 impact on the liver. World J. Clin. Cases 2021, 9, 3814–3825. [Google Scholar] [CrossRef]
- Gonga, G.; Guanc, Y.Y.; Zhangd, Z.L.; Rahmane, K.; Wangf, S.J.; Zhoug, S.; Luana, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Adachi, S.-I.; Kondo, S.; Sato, Y.; Yoshizawa, F.; Yagasaki, K. Anti-hyperuricemic effect of isorhamnetin in cultured hepatocytes and model mice: Structure–activity relationships of methylquercetins as inhibitors of uric acid production. Cytotechnology 2019, 71, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Sun, G.; Luo, Y. Protective effect of isorhamnetin on oxidative stress induced by H2O2 in H9C2 cells. Chin. Pharmacol. Bull. 2015, 3, 853–860. [Google Scholar]
- Yun, L.; Guibo, S.; Xi, D.; Min, W.; Meng, Q.; Yingli, Y.; Xiaobo, S. Isorhamnetin attenuates atherosclerosis by inhibiting macrophage apoptosis via PI3K/AKT activation and HO-1 induction. PLoS ONE 2015, 3, e0120259. [Google Scholar]
- Habtamu, A.; Melaku, Y. Antibacterial and Antioxidant Compounds from the Flower Extracts of Vernonia amygdalina. Adv. Pharmacol. Sci. 2018, 2018, 4083736. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Tang, C.; Tan, S.; Duan, J.; Tian, H.; Yang, Y. Cardioprotective effect of isorhamnetin against myocardial ischemia reperfusion (I/R) injury in isolated rat heart through attenuation of apoptosis. J. Cell. Mol. Med. 2020, 24, 6253–6262. [Google Scholar] [CrossRef] [Green Version]
- Coulibaly, A.; Chen, J. Evolution of Energetic Compounds, Antioxidant Capacity, Some Vitamins and Minerals, Phytase and Amylase Activity during the Germination of Foxtail Millet. Am. J. Food Technol. 2010, 6, 40–51. [Google Scholar] [CrossRef]
- Hanefeld, M.; Herrmann, K. On the occurrence of proanthocyanidins, leucoanthocyanidins and catechins in vegetables. Z. Fur Lebensm.-Unters. Und-Forsch. 1976, 161, 243–248. [Google Scholar] [CrossRef]
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted Grains. Compr. Rev. Food Sci. F 2019, 11, 421. [Google Scholar]
- Watanabe, M. Catechins as Antioxidants from Buckwheat (Fagopyrum esculentum Moench) Groats. J. Agric. Food Chem. 1998, 46, 839–845. [Google Scholar] [CrossRef]
- Durazzo, A.; Zaccaria, M.; Polito, A.; Maiani, G.; Carcea, M. Lignan Content in Cereals, Buckwheat and Derived Foods. Foods 2013, 2, 53–63. [Google Scholar] [CrossRef]
- Blomquist, C.H.; Lima, P.H.; Hotchkiss, J.R. Inhibition of 3α-hydroxysteroid dehydrogenase (3α-HSD) activity of human lung microsomes by genistein, daidzein, coumestrol and C18-, C19- and C21-hydroxysteroids and ketosteroids. Steroids 2005, 70, 507–514. [Google Scholar] [CrossRef]
- Stuart, B. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons: West Sussex, UK, 2004; ISBN 0470854278. [Google Scholar]
- Brajdes, C.; Bahrim, G.; Dinica, R.; Vizireanu, C. Phenolics composition and their biochemical stability confirmation by IN VITRO gastrointestinal conditions simulation, for a new functional fermented beverage based on sprouted buckwheat. Rom. Biotechnol. Lett. 2013, 18, 8832–8842. [Google Scholar]
- Chiş, A.; Fetea, F.; Abdelmoumen, T.; Socaciu, C. Application of FTIR Spectroscopy for a Rapid Determination of Some Hydrolytic Enzymes Activity on Sea Buckthorn Substrate. Rom. Biotechnol. Lett. 2010, 15, 5378–5744. [Google Scholar]


| Buckwheat Seeds | After 3 Days of Sprouting | After 7 Days of Sprouting | Authors | |
|---|---|---|---|---|
| TPC, mg GAE/g dwb | 4.91 ± 0.15 3.03 ± 0.09 3.23 ± 0.14 | 6.14 ± 0.22 8.42 ± 0.44 6.70 ± 0.12 | 22.57 ± 0.71 | Our study [31] [30] |
| TFC, mg GAE/g dwb | 4.05 ± 0.09 4.17 ± 0.11 | 5.57 ± 0.32 11.69 ± 0.87 | 21.72 ± 0.76 | Our study [31] |
| Nr. Peak | Retention Time tR (min) | [M-H]+ (m/z) | UV λmax (nm) | Compound Name |
|---|---|---|---|---|
| 1 | 3.2 | 379, 363 | 280 | Hidroxysecoisolariciresinol (lignan) |
| 2 | 4.7 | 307, 202 | 280 | Epigallocatechin (EGC) |
| 3 | 5.5 | 459, 202 | 280 | Epigallocatechingallat (EGCG) |
| 4 | 7.0 | 459, 202 | 280 | Gallocatechingallat (GCG) |
| 5 | 9.4 | 269, 183 | 240, 300 | Coumestrol (isoflavonoid) |
| 6 | 12.1 | 291, 202 | 280 | Catechin |
| 7 | 12.9 | 363, 247, 163 | 280 | Secoisolariciresinol (lignan) |
| 8 | 14.0 | 449, 287 | 270, 350 | Orientin |
| 9 | 14.5 | 449, 287 | 270, 350 | Isoorientin |
| 10 | 15.4 | 433, 271 | 270, 340 | Vitexin |
| 11 | 16.1 | 611, 475, 303 | 250, 360 | Rutin |
| 12 | 16.4 | 493, 475, 317 | 250, 320 | Isorhamnetin glucuronid |
| 13 | 17.7 | 435, 303 | 270, 330 | Quercetin arabinosid |
| 14 | 18.1 | 449, 433, 303 | 270, 330 | Quercetin ramnosid (Quercitrin) |
| 15 | 21.9 | 303 | 260, 370 | Quercetin |
| 16 | 23.4 | 317 | 260, 370 | Isorhamnetin |
| 17 | 24.3 | 287 | 240, 330 | Luteolin |
| 18 | 25.2 | 271 | 240, 320 | Apigenin |
| Validation Parameters | Calibration Equation | R2 | LOD µg/g | LOQµg/g | Precision (RSD, %) | Repetability (RSD, %) | |
|---|---|---|---|---|---|---|---|
| Intra-Day | Inter-Day | ||||||
| Hidroxi-secolariciresinol | Y = 11307X + 4493 | 0.992 | 10.40 | 31.52 | 3.15 | 2.85 | 4.31 |
| Epigallocatechin | Y = 11198X + 2849 | 0.993 | 9.65 | 29.24 | 2.49 | 2.62 | 3.56 |
| Epigallocatechingallat | Y = 3240X − 3511 | 0.990 | 16.84 | 51.03 | 2.36 | 4.1 | 2.82 |
| Gallocatechingallat | Y = 8671X + 2133 | 0.994 | 18.21 | 55.18 | 1.89 | 2.36 | 4.27 |
| Coumestrol | Y = 4735X − 2771 | 0.995 | 0.82 | 2.48 | 2.23 | 3.04 | 3.95 |
| Catechin | Y = 1435X + 305 | 0.993 | 0.35 | 1.06 | 2.81 | 5.01 | 3.97 |
| Secolariciresinol | Y = 1001X − 261 | 0.998 | 1.65 | 5.00 | 2.65 | 3.25 | 4.04 |
| Orientin | Y = 5100X − 4382 | 0.998 | 2.31 | 7.00 | 3.45 | 3.89 | 5.01 |
| Isoorientin | Y = 1294X + 525 | 0.991 | 1.15 | 3.48 | 4.25 | 3.87 | 3.26 |
| Vitexin | Y = 7555X + 2105 | 0.998 | 2.47 | 7.48 | 3.29 | 4.32 | 3.57 |
| Rutin | Y = 3022X + 156 | 0.996 | 11.02 | 33.39 | 2.47 | 2.65 | 2.12 |
| Isorhamnetin glucuronid | Y = 43279X − 1900 | 0.996 | 3.45 | 10.45 | 2.43 | 3.16 | 5.04 |
| Quercetin arabinosid | Y = 74639X + 2708 | 0.994 | 8.31 | 25.18 | 1.26 | 2.08 | 3.44 |
| Quercetin ramnosid | Y = 54104X − 5319 | 0.991 | 9.03 | 27.36 | 3.11 | 3.15 | 2.27 |
| Quercetin | Y = 53615X − 1292 | 0.992 | 0.32 | 0.97 | 4.48 | 1.58 | 32.23 |
| Isorhamnetin | Y = 69535X − 2208 | 0.993 | 10.5 | 31.82 | 3.04 | 4.28 | 4.98 |
| Luteolin | Y = 14473X − 4389 | 0.995 | 4.10 | 12.42 | 2.97 | 3.18 | 3.61 |
| Apigenin | Y = 24574X + 5685 | 0.995 | 6.02 | 18.24 | 3.65 | 3.78 | 3.18 |
| No. | Compound | Buckwheat’s Achenes, μg/g dwb | Buckwheat’s Achenes after 3 Days of Sprouting, μg/dwb | Buckwheat’s Achenes after 7 Days of Sprouting, μg/g dwb |
|---|---|---|---|---|
| 1 | Hydroxysecoisolariciresinol | 886.70 ± 19.02 | 604.52 ± 25.01 | 145.52 ± 4.88 |
| 2 | EGC | 131.58 ± 15.22 | 28.22 ± 6.22 | 85.55 ± 5.33 |
| 3 | EGCG | 44.20 ± 5.24 | 88.40 ± 10.39 | n.d. |
| 4 | GCG | 56.13 ± 6.66 | 32.47 ± 8.77 | n.d. |
| 5 | Coumestrol | 34.47 ± 4.87 | 395.23 ± 25.1 | 917.71 ± 15.74 |
| 6 | Catechin | 108.97 ± 5.21 | 139.49 ± 7.46 | 170 ± 8.54 |
| 7 | Secoisolariciresinol | 323.51 ± 6.98 | 229.67 ± 10.85 | n.d. |
| 8 | Orientin | 178.93 ± 7.09 | 230.28 ± 9.56 | 2361.50 ± 24.99 |
| 9 | Isoorientin | 114.28 ± 8.04 | 342.86 ± 9.34 | 1475.85 ± 35.88 |
| 10 | Vitexin | 155.74 ± 6.79 | 173.22 ± 12.40 | 5115.31 ± 37.65 |
| 11 | Rutin | 37.52 ± 3.77 | 420.47 ± 13.56 | 558.47 ± 8.22 |
| 12 | Isorhamnetin glucuronid | 39.23 ± 6.85 | 326.15 ± 9.91 | 385.17 ± 7.35 |
| 13 | Quercetin arabinosid | 299.19 ± 24.02 | 713.36 ± 27.44 | 885.17 ± 16.43 |
| 14 | Quercetin ramnosid (Quercitrin) | 227.04 ± 16.54 | 547.38 ± 12.74 | n.d. |
| 15 | Quercetin | 73.37 ± 3.63 | 173.31 ± 8.87 | 279.75 ± 9.58 |
| 16 | Isorhamnetin | 130.83 ± 5.44 | 183.03 ± 11.57 | 618.17 ± 8.33 |
| 17 | Luteolin | 52.02 ± 3.44 | 260.13 ± 8.89 | 184.91 ± 4.75 |
| 18 | Apigenin | 54.58 ± 3.98 | 111.21 ± 12.47 | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumitru, C.; Dinica, R.M.; Bahrim, G.-E.; Vizireanu, C.; Baroiu, L.; Iancu, A.V.; Draganescu, M. New Insights into the Antioxidant Compounds of Achenes and Sprouted Buckwheat Cultivated in the Republic of Moldova. Appl. Sci. 2021, 11, 10230. https://doi.org/10.3390/app112110230
Dumitru C, Dinica RM, Bahrim G-E, Vizireanu C, Baroiu L, Iancu AV, Draganescu M. New Insights into the Antioxidant Compounds of Achenes and Sprouted Buckwheat Cultivated in the Republic of Moldova. Applied Sciences. 2021; 11(21):10230. https://doi.org/10.3390/app112110230
Chicago/Turabian StyleDumitru, Caterina, Rodica Mihaela Dinica, Gabriela-Elena Bahrim, Camelia Vizireanu, Liliana Baroiu, Alina Viorica Iancu, and Miruna Draganescu. 2021. "New Insights into the Antioxidant Compounds of Achenes and Sprouted Buckwheat Cultivated in the Republic of Moldova" Applied Sciences 11, no. 21: 10230. https://doi.org/10.3390/app112110230
APA StyleDumitru, C., Dinica, R. M., Bahrim, G.-E., Vizireanu, C., Baroiu, L., Iancu, A. V., & Draganescu, M. (2021). New Insights into the Antioxidant Compounds of Achenes and Sprouted Buckwheat Cultivated in the Republic of Moldova. Applied Sciences, 11(21), 10230. https://doi.org/10.3390/app112110230









