In Vivo Evaluation of PVP-Gelatin-Chitosan Composite Blended with Egg-Yolk Oil for Radiodermatitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of PVP-Gelatin-Chitosan (PGC) Composite Film
2.2. Preparation of PGC/EYO Composite Film
2.3. Physicochemical Characterization of Composite
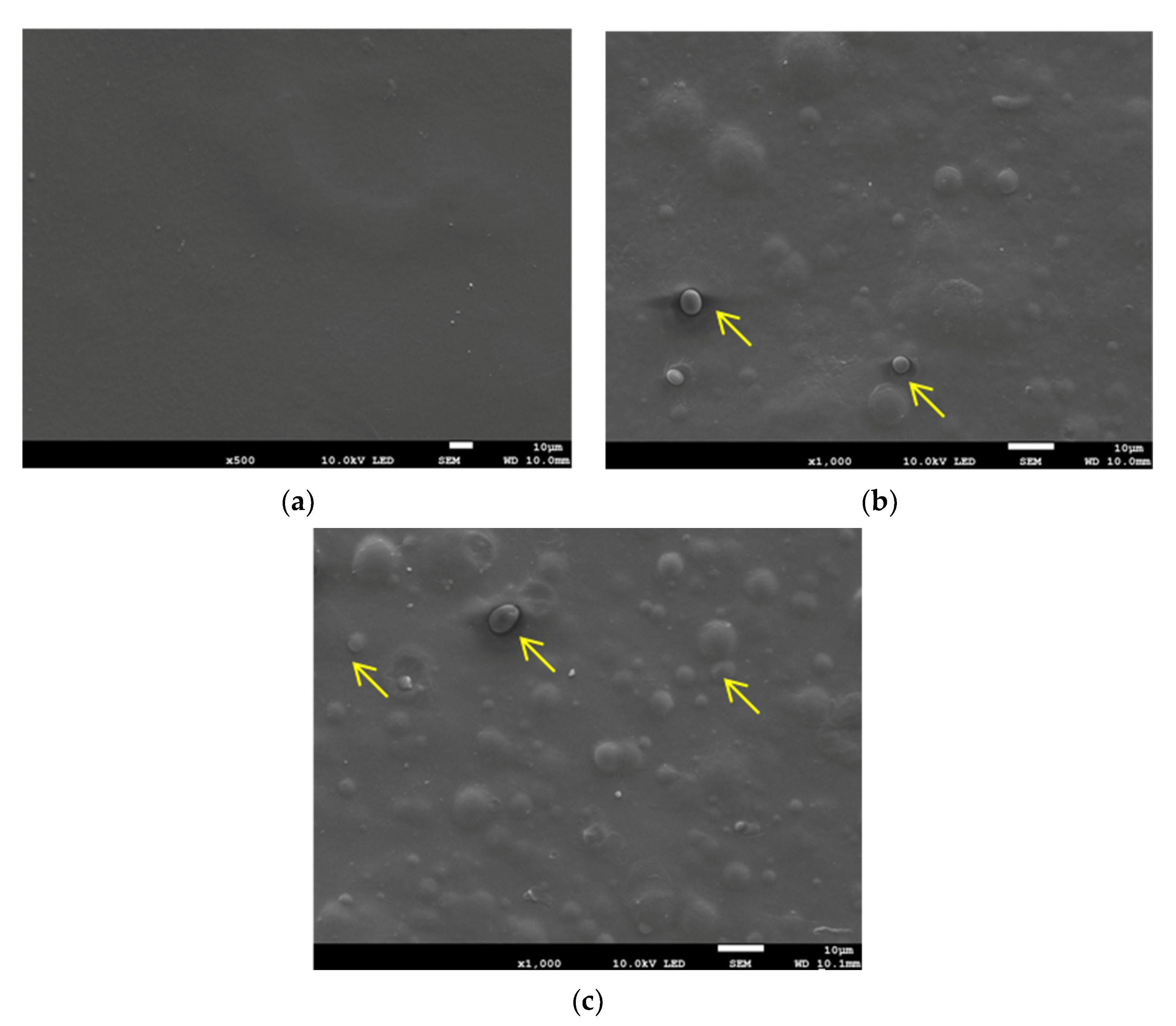
2.3.1. Surface Morphology
2.3.2. Chemical Analysis
2.3.3. Water Content Ratio
2.3.4. Mechanical Properties
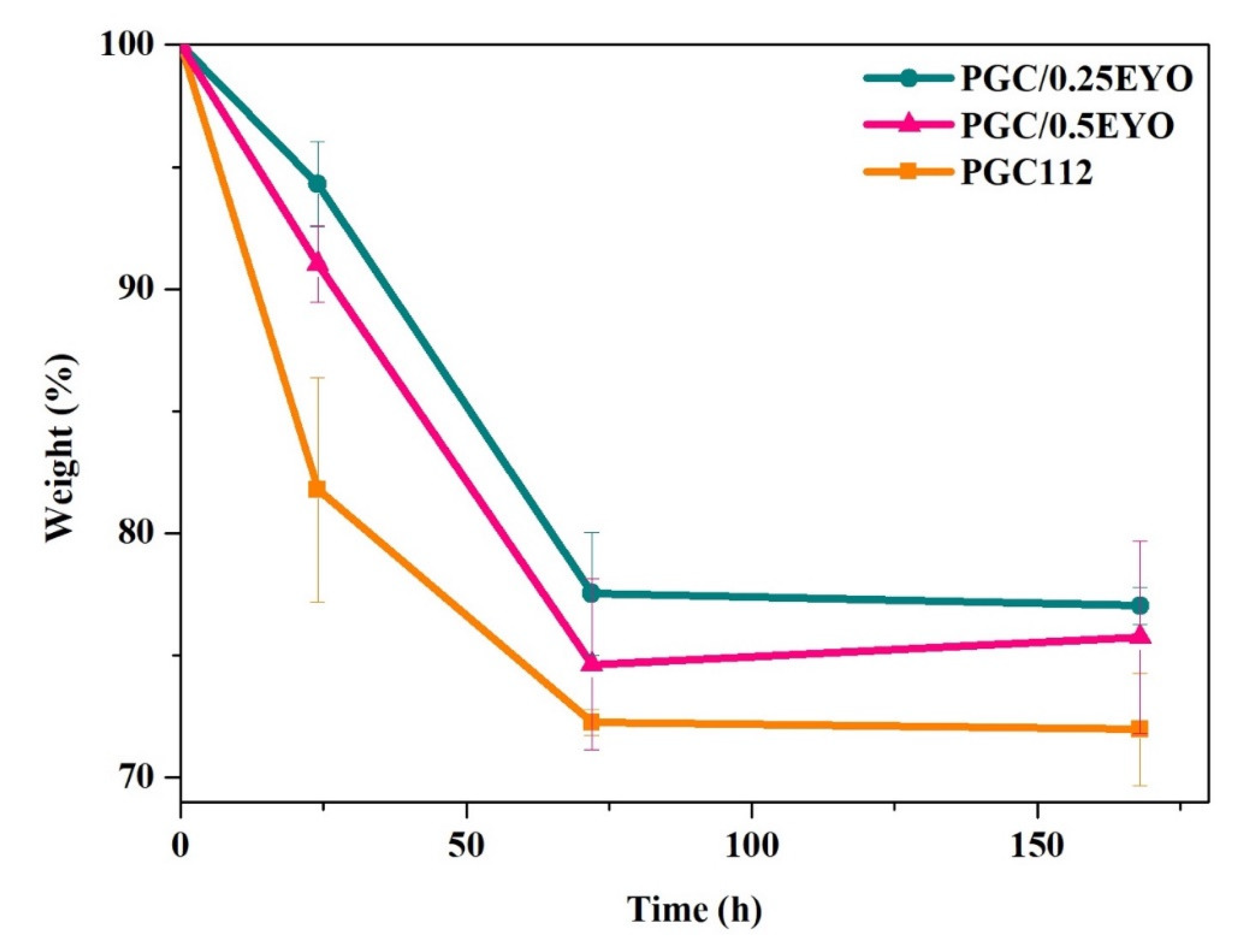
2.3.5. Degradation Rate
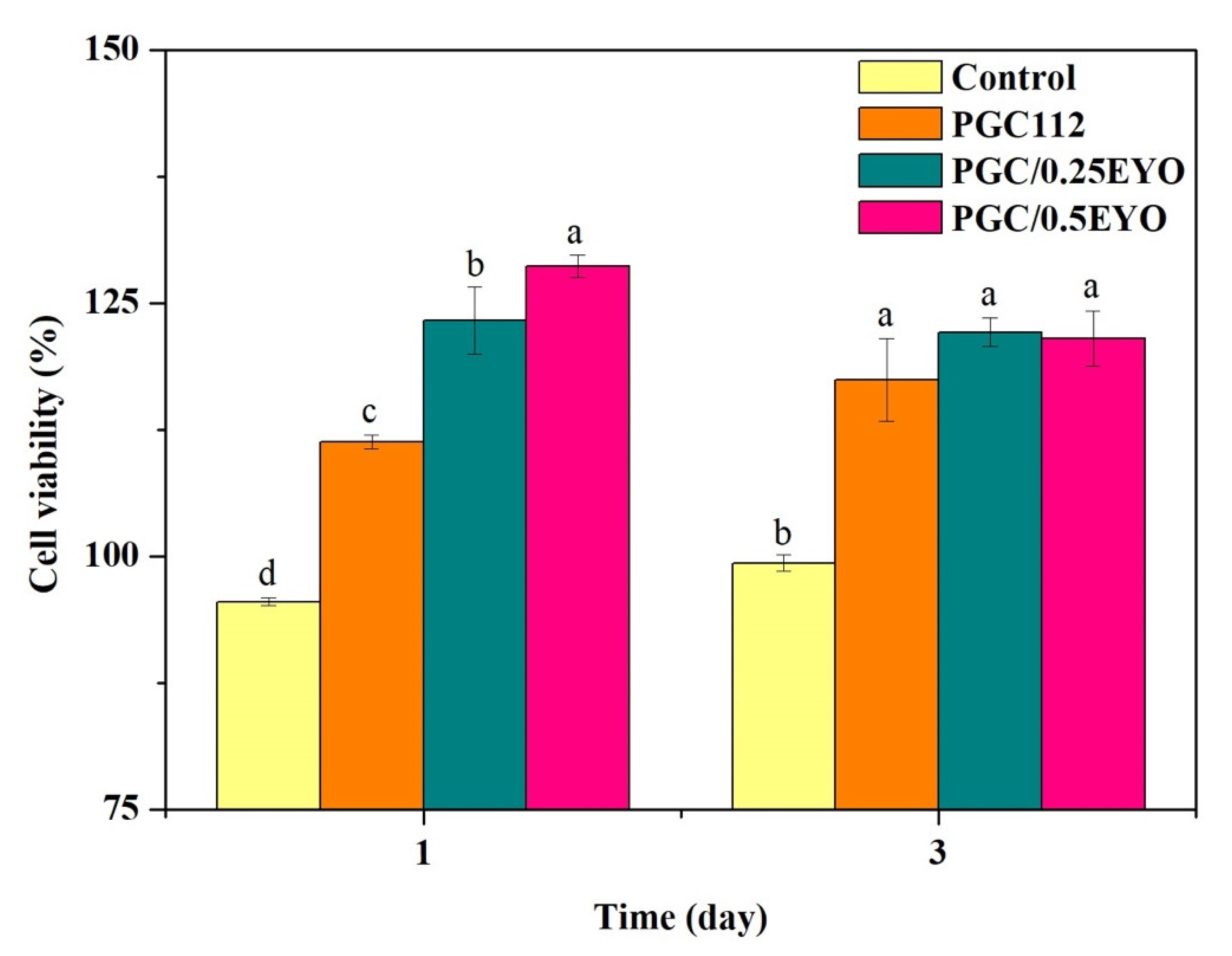
2.4. Biocompatibility
2.5. In Vivo Study
2.5.1. Animals and Experimental Design
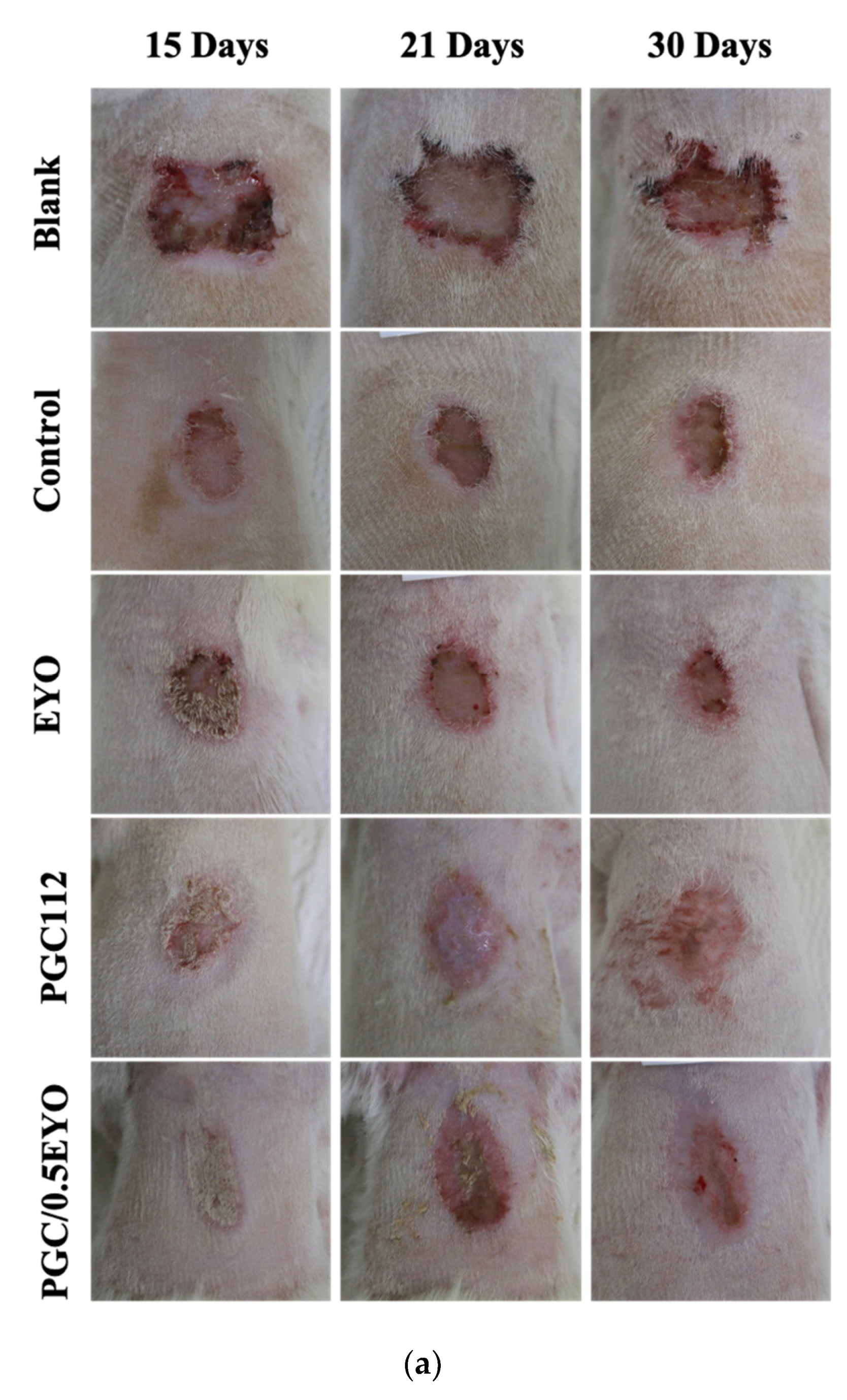
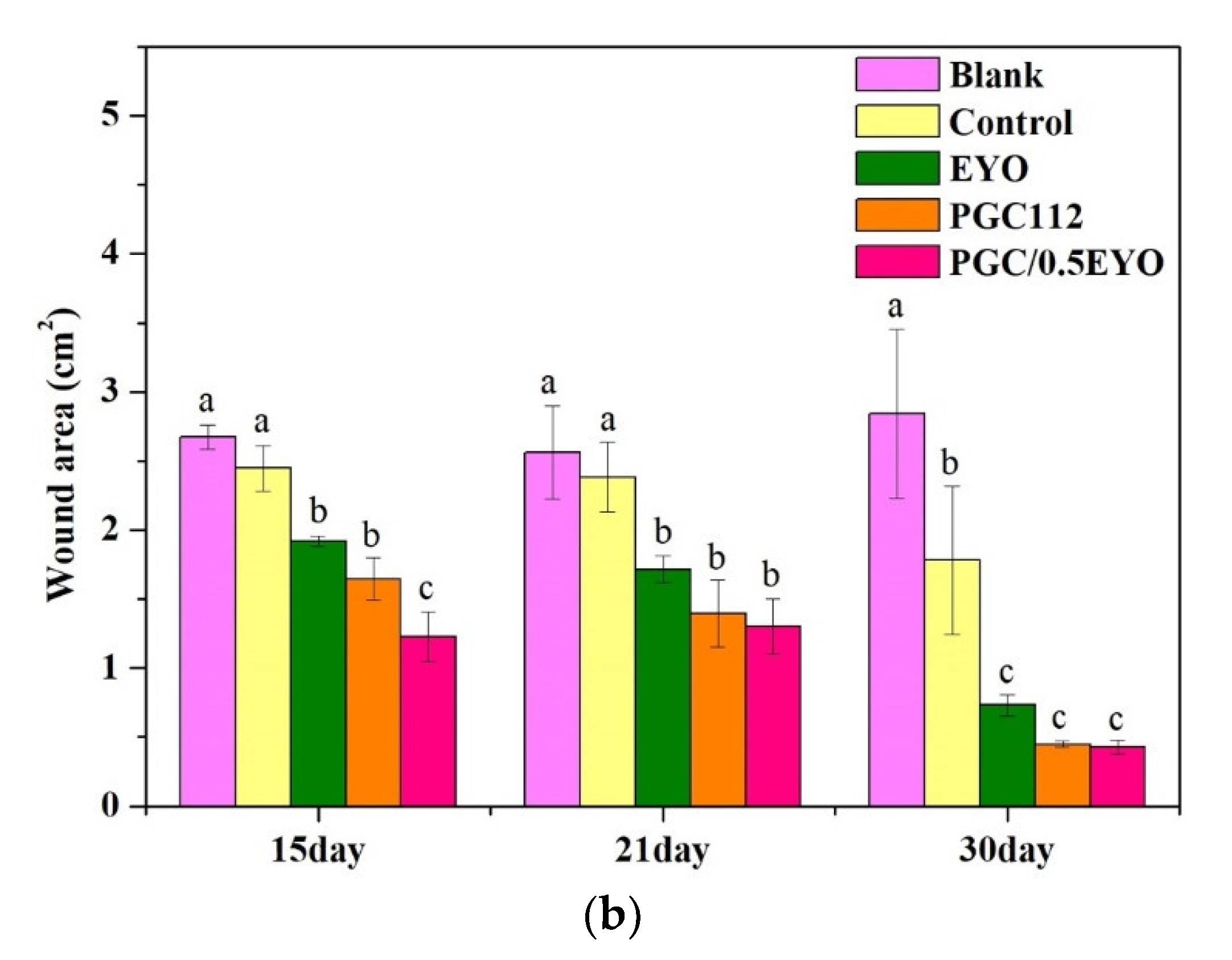
2.5.2. Analysis of the Wound Area of Radiodermatitis
2.5.3. Histological Analysis
2.6. Statistical Analysis
3. Result and Discussion
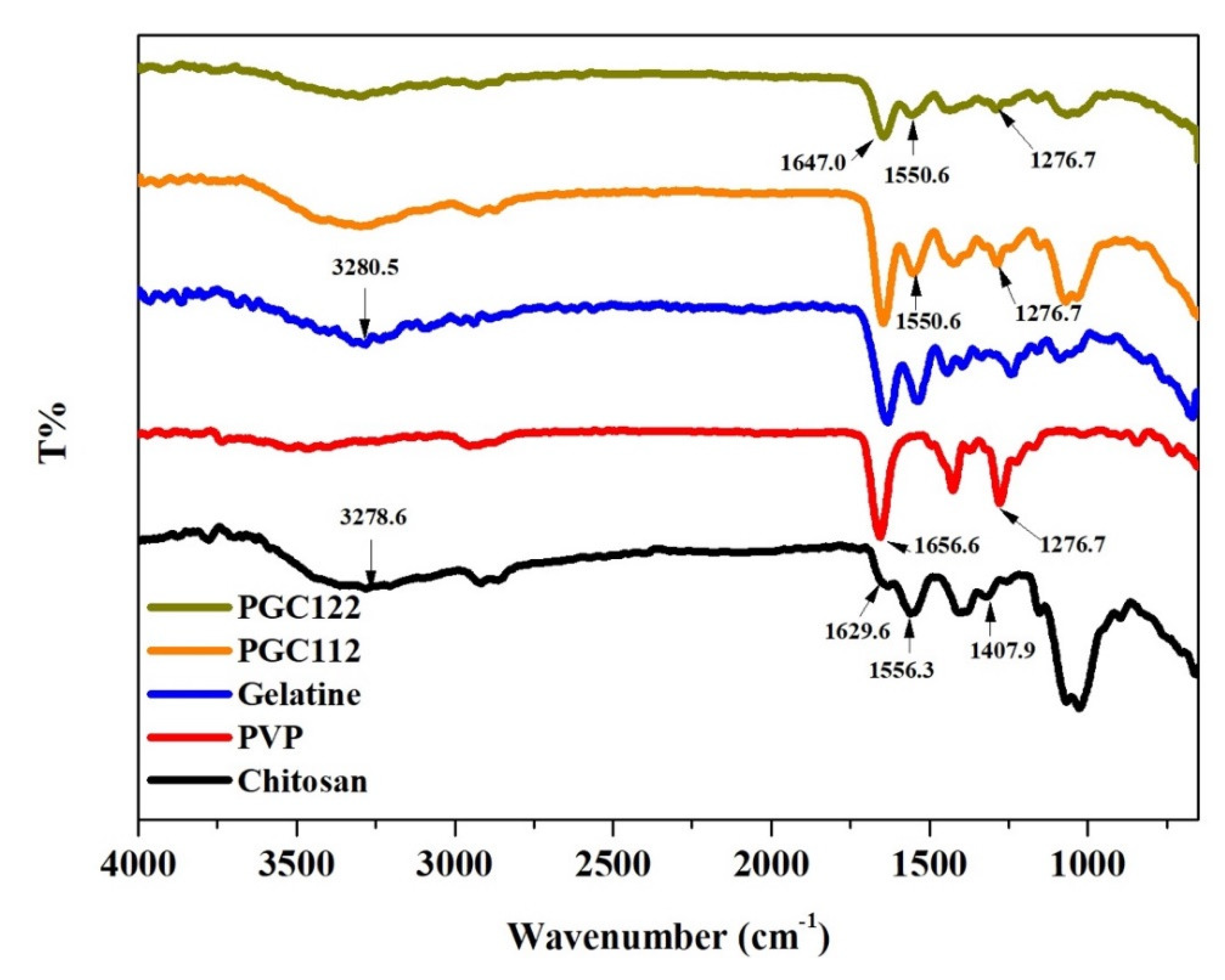
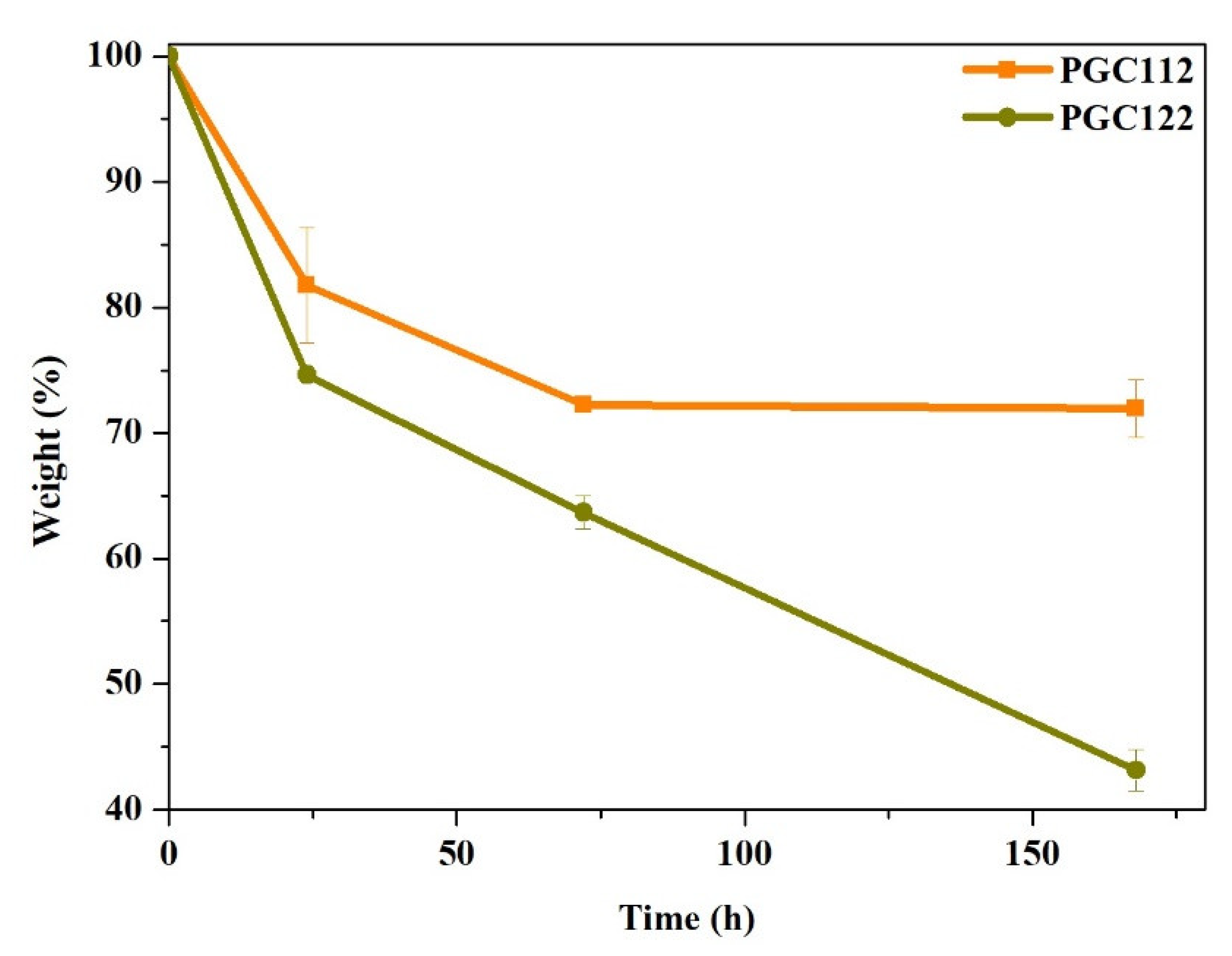
3.1. Characterization of PGC Composite Films
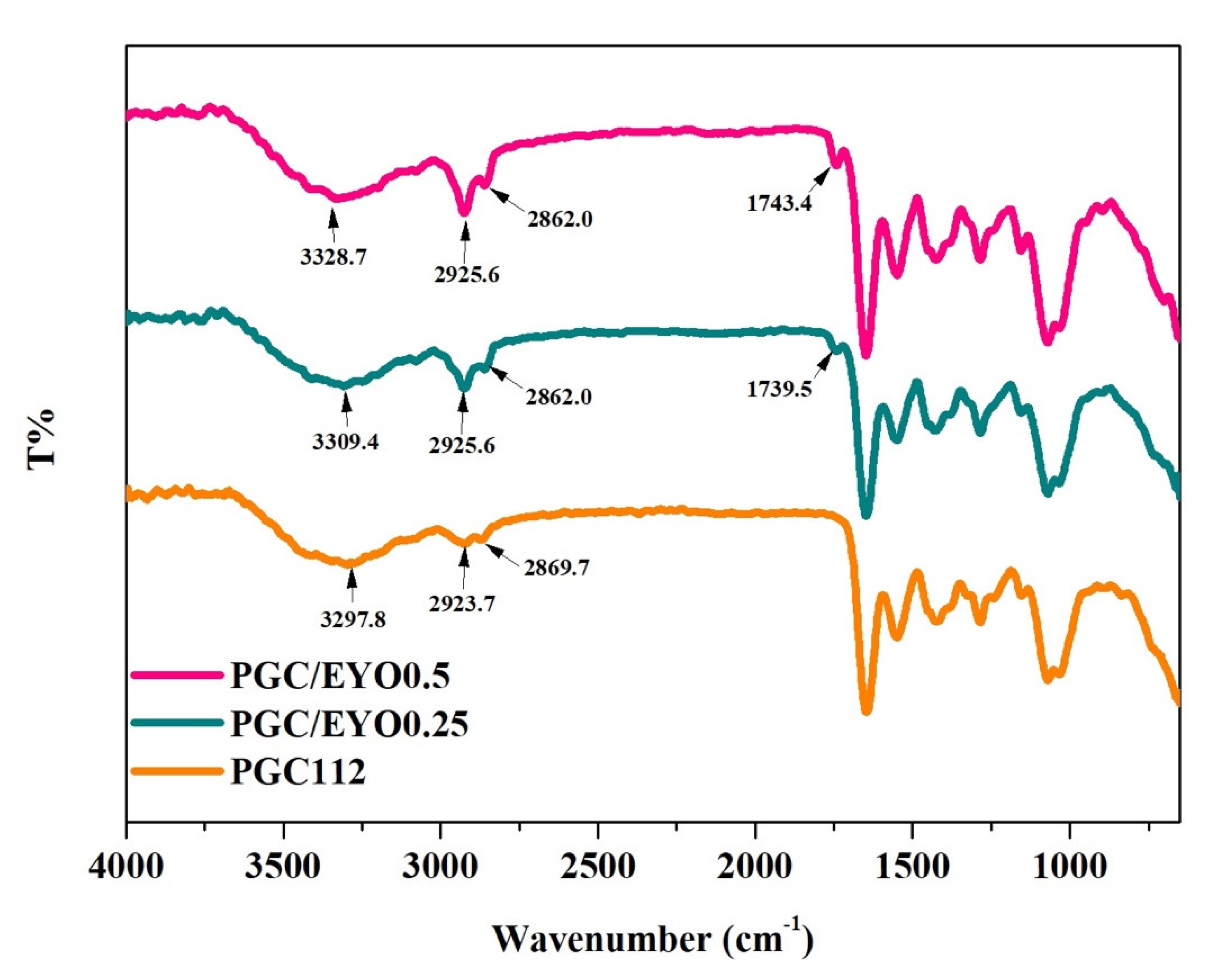
3.2. Characterization of PGC/EYO Composite Films
3.3. Biocompatibility of PGC/EYO Composite Films
3.4. In Vivo Evaluation
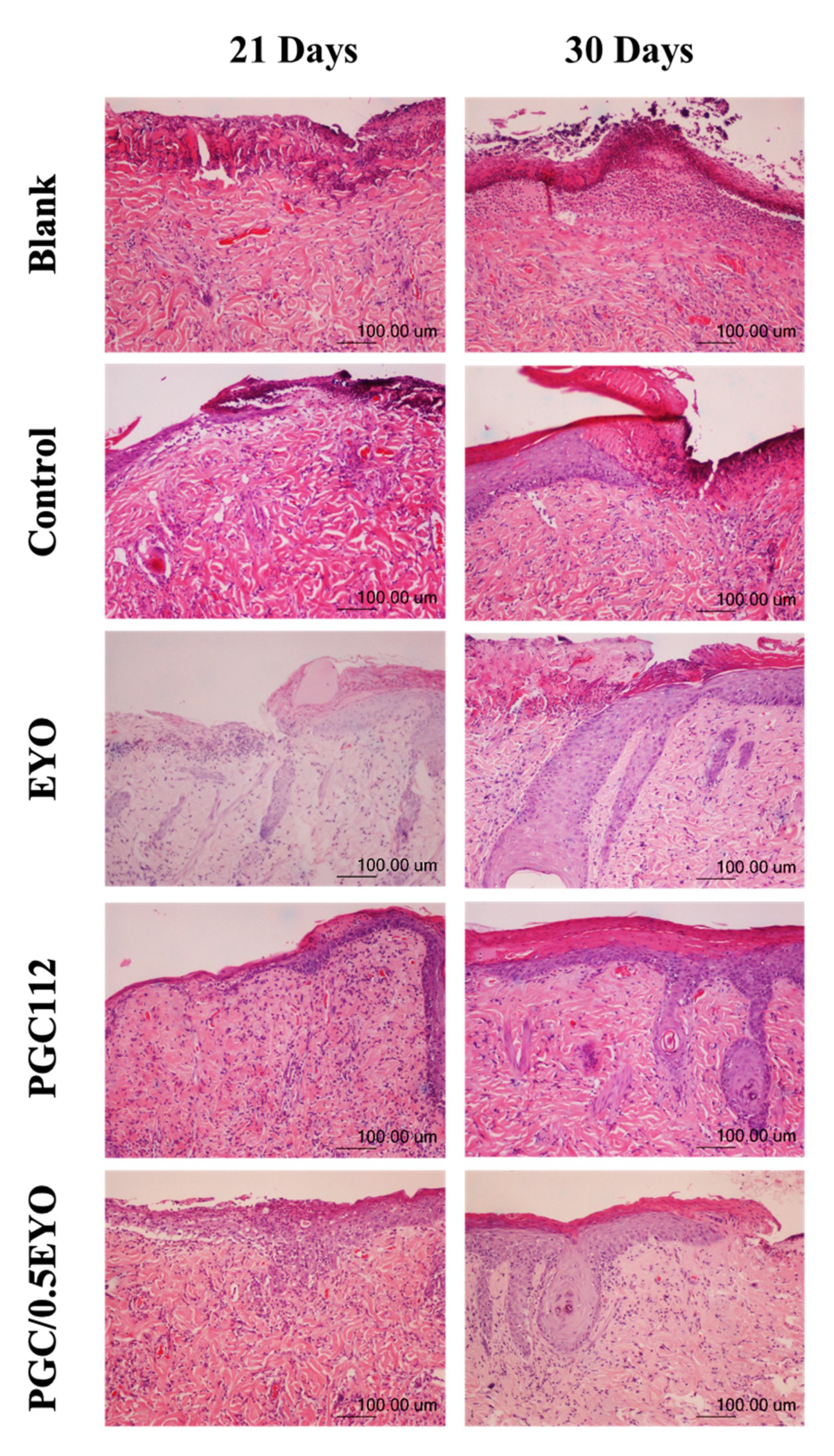
3.4.1. Histological Examination
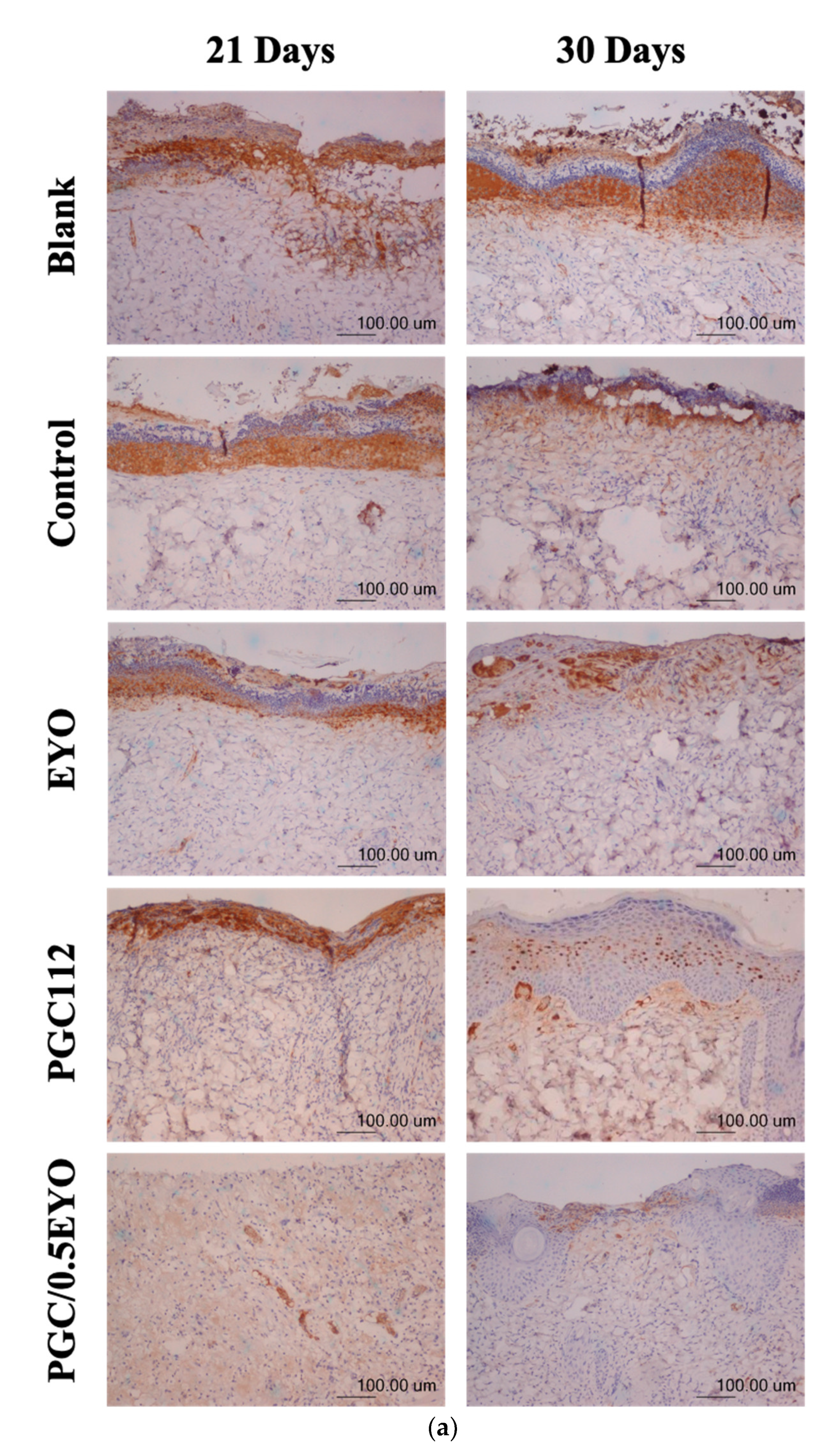
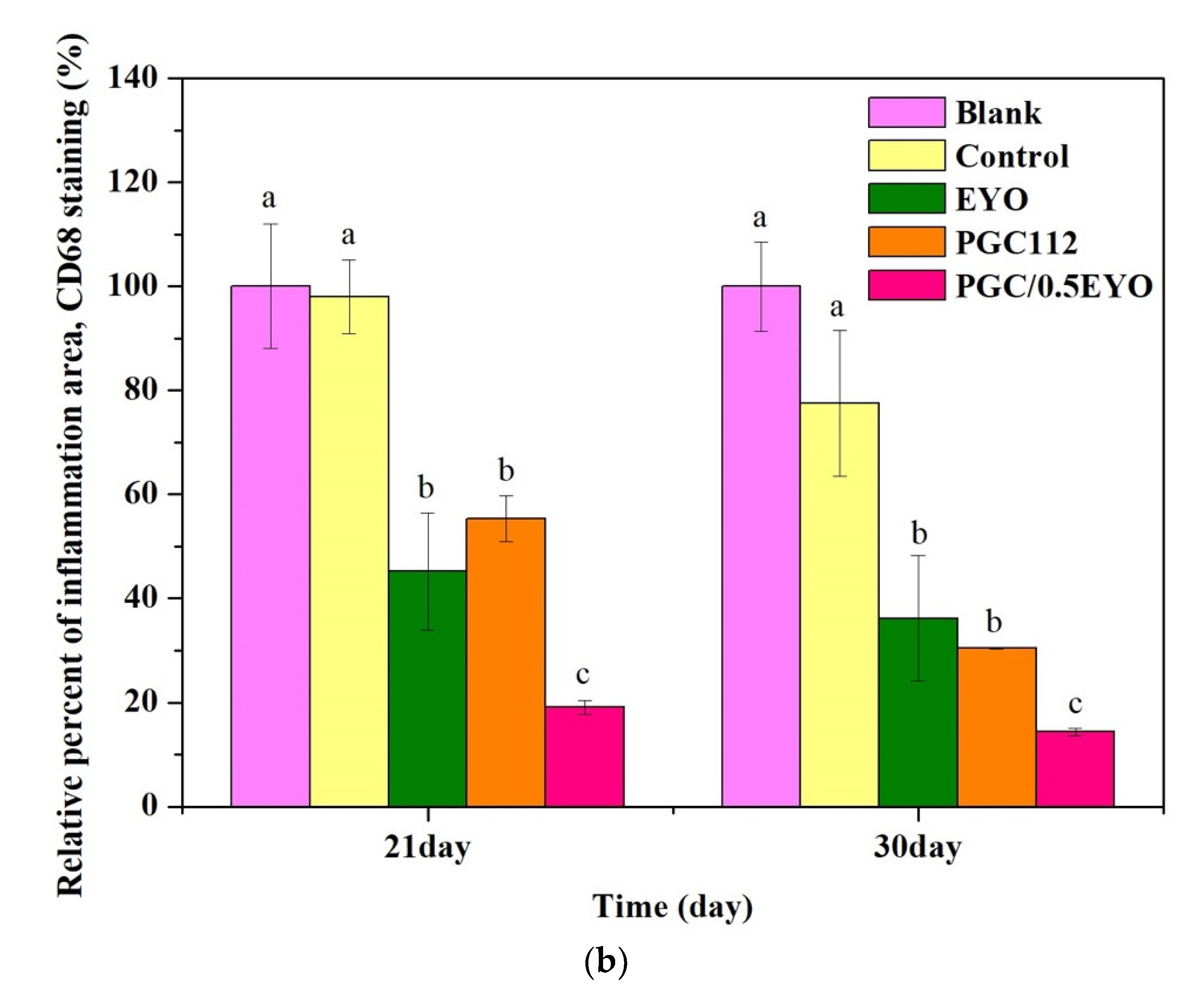
3.4.2. Immunochemical Staining Observation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, M.; Alavi, A.; Wong, R.; Akita, S. Radiodermatitis: A review of our current understanding. Am. J. Clin. Dermatol. 2016, 17, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.; Scott, C.; Stevens, R.; Marconi, B.; Champion, L.; Freedman, G.M.; Asrari, F.; Pilepich, M.; Gagnon, J.D.; Wong, G. Randomized phase III study comparing Best Supportive Care to Biafine as a prophylactic agent for radiation-induced skin toxicity for women undergoing breast irradiation: Radiation Therapy Oncology Group (RTOG) 97-13. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 1307–1310. [Google Scholar] [CrossRef]
- D’haese, S.; Van Roy, M.; Bate, T.; Bijdekerke, P.; Vinh-Hung, V. Management of skin reactions during radiotherapy in Flanders (Belgium): A study of nursing practice before and after the introduction of a skin care protocol. Eur. J. Oncol. Nurs. 2010, 14, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.; Smith, J.E.; McIntyre, M.; Thomas, R.; Pilkington, K. Aloe vera for preventing radiation-induced skin reactions: A systematic literature review. Clin. Oncol. 2005, 17, 478–484. [Google Scholar] [CrossRef]
- Momm, F.; Weißenberger, C.; Bartelt, S.; Henke, M. Moist skin care can diminish acute radiation-induced skin toxicity. Strahlenther. Onkol. 2003, 179, 708–712. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Gao, Y.; Xie, Z.; Zhou, M.; He, Y.; Wu, H.; Zhou, W.; Dong, X.; Yang, Z. Cinnamon oil-loaded composite emulsion hydrogels with antibacterial activity prepared using concentrated emulsion templates. Ind. Crop. Prod. 2018, 112, 281–289. [Google Scholar] [CrossRef]
- Yogi, V.; Singh, O.; Mandloi, V.; Ahirwar, M. Role of topical aloe vera gel in the recovery of high-grade, radiation-induced dermatitis. Clin. Cancer Investig. J. 2018, 7, 167. [Google Scholar] [CrossRef]
- Heggie, S.; Bryant, G.P.; Tripcony, L.; Keller, J.; Rose, P.; Glendenning, M.; Heath, J. A phase III study on the efficacy of topical aloe vera gel on irradiated breast tissue. Cancer Nurs. 2002, 25, 442–451. [Google Scholar] [CrossRef]
- Rashid, R.; Lim, N.S.J.; Chee, S.M.L.; Png, S.N.; Wohland, T.; Raghunath, M. Novel use for polyvinylpyrrolidone as a macromolecular crowder for enhanced extracellular matrix deposition and cell proliferation. Tissue Eng. Part C Methods 2014, 20, 994–1002. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zivanovic, S.; Davidson, P.A.; Kit, K. Characterization and comparison of chitosan/PVP and chitosan/PEO blend films. Carbohydr. Polym. 2010, 79, 786–791. [Google Scholar] [CrossRef]
- Salles, T.H.C.; Lombello, C.B.; d’Ávila, M.A. Electrospinning of gelatin/poly (vinyl pyrrolidone) blends from water/acetic acid solutions. Mater. Res. 2015, 18, 509–518. [Google Scholar] [CrossRef] [Green Version]
- Serafim, A.; Petre, D.G.; Moraru, L.; Cioflan, H.E.; Vasile, E.; Mastalier-Manolescu, B.; Petrutescu, M.; Stancu, I.C. Gelatin-PVP hydrogels with potential skin grafts applications. Key Eng. Mater. 2015, 638, 38–46. [Google Scholar] [CrossRef]
- Ran, W.; Ma, H.; Li, M. In Vitro and In Vivo Studies of Polyvinyl Pyrrolidone–Coated Sparfloxacin-Loaded Ring Contact Lens to Treat Conjunctivitis. J. Pharm. Sci. 2020, 109, 1951–1957. [Google Scholar] [CrossRef]
- El-Aassar, M.; Ibrahim, O.M.; Fouda, M.M.; Fakhry, H.; Ajarem, J.; Maodaa, S.N.; Allam, A.A.; Hafez, E.E. Wound dressing of chitosan-based-crosslinked gelatin/polyvinyl pyrrolidone embedded silver nanoparticles, for targeting multidrug resistance microbes. Carbohydr. Polym. 2021, 255, 117484. [Google Scholar] [CrossRef]
- Risbud, M.; Hardikar, A.; Bhonde, R. Growth modulation of fibroblasts by chitosan-polyvinyl pyrrolidone hydrogel: Implications for wound management? J. Biosci. 2000, 25, 25–30. [Google Scholar] [CrossRef]
- Janvikul, W.; Uppanan, P.; Thavornyutikarn, B.; Krewraing, J.; Prateepasen, R. In vitro comparative hemostatic studies of chitin, chitosan, and their derivatives. J. Appl. Polym. Sci. 2006, 102, 445–451. [Google Scholar] [CrossRef]
- Neumann, P.; Zur, B.; Ehrenreich, Y. Gelatin-based sprayable foam as a skin substitute. J. Biomed. Mater. Res. 1981, 15, 9–18. [Google Scholar] [CrossRef]
- Lan, G.; Lu, B.; Wang, T.; Wang, L.; Chen, J.; Yu, K.; Liu, J.; Dai, F.; Wu, D. Chitosan/gelatin composite sponge is an absorbable surgical hemostatic agent. Colloids Surf. B Biointerfaces 2015, 136, 1026–1034. [Google Scholar] [CrossRef]
- Xiong, S.; Gong, Q.-F.; Ning, X.-X. The research progress of egg yolk. J. Gannan Med Univ. 2014, 34, 313–320. [Google Scholar]
- Rastegar, F.; Azarpira, N.; Amiri, M.; Azarpira, A. The effect of egg yolk oil in the healing of third degree burn wound in rats. Iran. Red Crescent Med J. 2011, 13, 739. [Google Scholar]
- Zhao, Y.; Zhang, Y.; Liu, X.; Kong, H.; Wang, Y.; Qin, G.; Cao, P.; Song, X.; Yan, X.; Wang, Q. Novel carbon quantum dots from egg yolk oil and their haemostatic effects. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Klinkesorn, U. The role of chitosan in emulsion formation and stabilization. Food Rev. Int. 2013, 29, 371–393. [Google Scholar] [CrossRef]
- Yenilmez, E.; Başaran, E.; Arslan, R.; Berkman, M.; Güven, U.; Baycu, C.; Yazan, Y. Chitosan gel formulations containing egg yolk oil and epidermal growth factor for dermal burn treatment. Die Pharm. Int. J. Pharm. Sci. 2015, 70, 67–73. [Google Scholar]
- Rodil, A.; Laca, A.; Paredes, B.; Rendueles, M.; Meana, Á.; Díaz, M. Gels prepared from egg yolk and its fractions for tissue engineering. Biotechnol. Prog. 2016, 32, 1577–1583. [Google Scholar] [CrossRef]
- El Achaby, M.; Essamlali, Y.; El Miri, N.; Snik, A.; Abdelouahdi, K.; Fihri, A.; Zahouily, M.; Solhy, A. Graphene oxide reinforced chitosan/polyvinylpyrrolidone polymer bio-nanocomposites. J. Appl. Polym. Sci. 2014, 131, 41042. [Google Scholar] [CrossRef]
- Wahba, M.I. Enhancement of the mechanical properties of chitosan. J. Biomater. Sci. Polym. Ed. 2020, 31, 350–375. [Google Scholar] [CrossRef]
- Huang, M.-Y.; Huang, J.-J.; Chai, C.-Y.; Chen, S.-H.; Kuo, M.-P.; Lin, C.-C. The reduction effect of extracts of soybean seeds on acute radiation dermatitis. Fooyin J. Health Sci. 2010, 2, 21–25. [Google Scholar] [CrossRef] [Green Version]
- Al-Abayaji, M.A. Extraction and Determination of Iraqi Boiled Egg Yolk Constituents, and Characterization of Lecithin. J. Coll. Basic Educ. 2015, 20, 49–56. [Google Scholar]
- Bereiter-Hahn, J.; Bernd, A.; Beschmann, H.; Eberle, I.; Kippenberger, S.; Rossberg, M.; Strecker, V.; Zöller, N. Cellular responses to egg-oil (charismon©). Acta Med. 2015, 57, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Shaw, S.-Z.; Nien, H.-H.; Wu, C.-J.; Lui, L.T.; Su, J.-F.; Lang, C.-H. 3M Cavilon No-Sting Barrier Film or topical corticosteroid (mometasone furoate) for protection against radiation dermatitis: A clinical trial. J. Formos. Med Assoc. 2015, 114, 407–414. [Google Scholar] [CrossRef] [Green Version]
- Dai, T.; Tanaka, M.; Huang, Y.-Y.; Hamblin, M.R. Chitosan preparations for wounds and burns: Antimicrobial and wound-healing effects. Expert Rev. Anti-Infect. Ther. 2011, 9, 857–879. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, X.-K.; Xue, X.-T.; Wu, D.-Y. Hydrogel sheets of chitosan, honey and gelatin as burn wound dressings. Carbohydr. Polym. 2012, 88, 75–83. [Google Scholar] [CrossRef]
- Kumar, P.S.; Praveen, G.; Raj, M.; Chennazhi, K.; Jayakumar, R. Flexible, micro-porous chitosan–gelatin hydrogel/nanofibrin composite bandages for treating burn wounds. RSC Adv. 2014, 4, 65081–65087. [Google Scholar] [CrossRef]
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef] [Green Version]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef]
- Ertekin, M.V.; Tekin, S.B.; Erdoğan, F.; Karslioğlu, I.; Gepdiremen, A.; Sezen, O.; Balci, E.; Gündoğdu, C. The effect of zinc sulphate in the prevention of radiation-induced dermatitis. J. Radiat. Res. 2004, 45, 543–548. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Tu, W.; Tang, Y.; Zhang, S. Prevention and treatment for radiation-induced skin injury during radiotherapy. Radiat. Med. Prot. 2020, 1, 60–68. [Google Scholar] [CrossRef]
- Doctrow, S.R.; Lopez, A.; Schock, A.M.; Duncan, N.E.; Jourdan, M.M.; Olasz, E.B.; Moulder, J.E.; Fish, B.L.; Mäder, M.; Lazar, J. A synthetic superoxide dismutase/catalase mimetic EUK-207 mitigates radiation dermatitis and promotes wound healing in irradiated rat skin. J. Investig. Dermatol. 2013, 133, 1088–1096. [Google Scholar] [CrossRef] [Green Version]
| Group | Description |
|---|---|
| Blank | No treatment |
| Control | The dressing and, from inside to outside, 3M™ Tegaderm™ transparent film dressing and sterilized breathable dressing, gauze, elastic bandage, and mesh bandage, respectively. |
| EYO | Wrap EYO and, from inside to outside, 3M™ Tegaderm™ transparent film dressing and sterilized breathable dressing, gauze, elastic bandage, and mesh bandage, respectively. |
| PGC112 | Wrap PGC112 film and, from inside to outside, 3M™ Tegaderm™ transparent film dressing and sterilized breathable dressing, gauze, elastic bandage, and mesh bandage, respectively. |
| PGC/0.5EYO | Wrap PGC/0.5EYO film and, from inside to outside, 3M™ Tegaderm™ transparent film dressing and sterilized breathable dressing, gauze, elastic bandage, and mesh bandage, respectively. |
| Sample | PVP | Gelatin | Chitosan | Water Content (%) | Young’s Modulus (MPa) | Tensile Strength (MPa) |
|---|---|---|---|---|---|---|
| PGC112 | 1 | 1 | 2 | 92.8 ± 0.1 | 927 ± 13 | 62.3 ± 2.2 |
| PGC122 | 1 | 2 | 2 | 91.8 ± 0.3 | 907 ± 24 | 51.8 ± 1.7 * |
| Sample | EYO (wt%) | Water Content (%) | Young’s Modulus (MPa) | Tensile Strength (MPa) |
|---|---|---|---|---|
| PGC112 | 0 | 92.8 ± 0.1 | 927 ± 13 | 62.3 ± 2.2 |
| PGC/0.25EYO | 0.25 | 92.9 ± 0.3 | 878 ± 26 | 66.8 ± 2.6 |
| PGC/0.5EYO | 0.5 | 93.8 ± 0.9 | 963± 105 | 67.2 ± 4.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, Y.-C.; Hsieh, S.-C.; Hou, S.-R.; Kung, J.-Y.; Tang, C.-M.; Chang, C.-J. In Vivo Evaluation of PVP-Gelatin-Chitosan Composite Blended with Egg-Yolk Oil for Radiodermatitis. Appl. Sci. 2021, 11, 10290. https://doi.org/10.3390/app112110290
Hung Y-C, Hsieh S-C, Hou S-R, Kung J-Y, Tang C-M, Chang C-J. In Vivo Evaluation of PVP-Gelatin-Chitosan Composite Blended with Egg-Yolk Oil for Radiodermatitis. Applied Sciences. 2021; 11(21):10290. https://doi.org/10.3390/app112110290
Chicago/Turabian StyleHung, Yi-Chi, Shu-Chih Hsieh, Syuan-Ren Hou, Jui-Yin Kung, Cheng-Ming Tang, and Chen-Jung Chang. 2021. "In Vivo Evaluation of PVP-Gelatin-Chitosan Composite Blended with Egg-Yolk Oil for Radiodermatitis" Applied Sciences 11, no. 21: 10290. https://doi.org/10.3390/app112110290
APA StyleHung, Y.-C., Hsieh, S.-C., Hou, S.-R., Kung, J.-Y., Tang, C.-M., & Chang, C.-J. (2021). In Vivo Evaluation of PVP-Gelatin-Chitosan Composite Blended with Egg-Yolk Oil for Radiodermatitis. Applied Sciences, 11(21), 10290. https://doi.org/10.3390/app112110290





