Abstract
Dental pulp inflammation, caused by the evolution of caries, involves numerous interrelated activities at a cellular and molecular level. Cytokines, proteases, growth factors, and other biomarkers of the host response may take part in dental pulp’s immune defense. The aim of this pilot study was to determine the levels of inflammation, oxidative stress, and extracellular matrix degradation biomarkers in healthy and symptomatic irreversibly inflamed dental pulp samples from children and adolescents. Twenty-three dental pulp samples were collected from permanent teeth with irreversible inflammation, while nineteen healthy dental pulp samples were obtained from teeth extracted for orthodontic reasons. Pulp lysates were obtained and the levels of IL-2, IL-17, TNF-α, SOD3, TGF-β1, catalase, osteocalcin, MMP-7, and MMP-9 were determined using the enzyme-linked immunosorbent assay (ELISA) technique. We detected significantly higher levels (p < 0.001) of IL-2, IL-17, TNF-α, SOD3, osteocalcin, and TGF-β1 in pulp samples with irreversible inflammation than in controls. Catalase and MMP-7 showed higher levels in the experimental group, while MMP-9 showed slightly increased levels in the control group, but none of these differences were statistically significant (p = 0.064/p = 0.061/p = 0.625). Inflamed dental pulp samples showed an up-regulation of IL-2, IL-17, TNF-α, SOD3, osteocalcin, and TGF-β1. These biomarkers appear to have a powerful role in the inflammation process of human dental pulp.
1. Introduction
Approximately 500 million children suffer from tooth decay in their primary dentition, while Global Burden of Disease (2017) reports that untreated tooth decay in permanent teeth is the most common health condition [1]. Dental caries is a chronic infectious disease and the most common cause of pulpal pathology, such as pulp inflammation (or pulpitis) [2]. Dental pulp inflammation can occur before bacteria take over the pulp tissue. Bacteria and their metabolic products, such as lipoteichoic acid (LTA), can diffuse through dentinal tubules and may initiate the mechanisms of the immune response in the dental pulp [3].
Dental pulp is a loose connective tissue located in a low-compliance environment. Bacterial invasion is accompanied by macroscopic changes, such as vasodilatation and a decrease in the blood flow resistance [4]. Intravascular pressure and capillary blood flow increases, accompanied by leukocyte extravasation and an accumulation of serum proteins. These vascular alterations are followed by the recruitment of immune cells, which tend to eliminate the pathogens and their metabolites. If microbial irritation continues, exceeding the pulp’s immune capacity, local hypoxia is installed by compression of the venules, metabolic waste products and CO2 increase, and further vasodilatation occurs. All of these lead to the spreading of inflammation. Considering this, if the edema from inflammation is not resolved by removing the insult, tooth pain is the first and main sign of pulp pathology [3].
An initial invasion of microbes activates an innate immunity of the dentine/pulp complex. Innate immunity, as a response to caries, is provided by the action of odontoblasts; the effects of neuropeptides and neurogenic inflammation; an increased outflow of the dentinal fluid and intratubular deposition of immunoglobulins; the functioning of innate immune cells, such as immature and pulpal dendritic cells (DCs); monocytes and macrophages; lymphocytes, such as natural killer (NK) cells and T cells, and their cytokines and chemokines [5]. All of these components stop bacterial penetration and abolish the invading bacteria by recognizing them or their components [6]. Adaptative immunity with cellular and specific antibody responses is activated when an innate response can’t eliminate the pulp injury. The transition from innate to adaptive immunity is believed to take place in irreversibly inflamed pulp tissue, located at less than 2 mm from the deep carious lesion [6].
LTA is produced by cariogenic bacteria after fermenting sucrose, and is attached to the cell membranes of streptococci. LTA can initiate the innate immune system by binding to CD14, by activating the signaling with Toll-like receptors (TLRs), and by stimulating the release of the following cytokines from innate immune cells: tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-10 (IL-10), interleukin-12 (IL-12), interleukin-18 (IL-18), and IFN-γ [3].
TNF-α and IL-1 promote the expression of adhesion molecules, which induce extravasation of phagocytes during inflammation, while IFN-γ, produced by NK cells, activates phagocytes and enhances TNF-α actions on endothelial cells. Macrophages produce IL-10, which has a crucial role in the control of innate and cell-mediated immunity by inhibiting macrophages and DCs’s functions. In response to bacteria or cytokines, various cell types, such as IL-1 and TNF-α, secrete IL-6, which intensifies the production of neutrophils and acute-phase proteins from bone marrow progenitors. IL-12 and IL-18 produced by resting T cells can direct the following adaptative cellular immune response [6].
Matrix metalloproteinases (MMPs) can degrade extracellular matrix (ECM) proteins and participate in tissue homeostasis [4,7]. MMPs take part in inflammation pathogenesis; the stimulation of protective innate and adaptative immune response; tissue destruction; and the spread of inflammation if they have uncontrolled activity. The degenerative potential of MMPs is balanced by tissue inhibitors of metalloproteinases (TIMPs) and nonspecific protein inhibitors (α-2 macroglobulin) [7]. MMP-9 is produced by PMNs, which are numerous in inflamed dental pulps, and is considered a marker of pulp tissue breakdown [8].
Neutrophils invade the pulp near the infected dentin and release MMPs to recruit immune cells to the dental pulp and also release reactive oxygen intermediates (ROI), such as hydrogen peroxide, superoxide anions, and hydroxyl radicals. ROI are oxidizing agents released in high amounts during bacterial inflammation, which can be toxic to cells, especially to hydrogen peroxide. Aerobic cells’ defense equipment includes enzymes, such as superoxide dismutase (SOD), catalase, and glutathione peroxidase, which are able to inactivate the free radicals. An increased production of ROI, or decreased removal of these, can damage the cellular and extracellular structures [9]. TNF-α can stimulate neutrophils to secrete massive quantities of ROI and trigger neutrophil degranulation [4]. An interconnection between inflammation, tissue degradation, and oxidative stress in human dental pulp is thus seen [5].
Reactionary/reparative dentine formation is desired for the protection of the underlying pulp from the dentin infection. A rapid dentinogenesis initiation can promote dental pulp healing and a regaining of health [10]. From a clinical perspective, it is critical to understand the molecular and cellular pathways able to reduce inflammatory reactions within pulp tissue, and to encourage fast installation of tissue homeostasis, once the bacterial infection is eliminated. The aim of the present pilot study is to evaluate the levels of molecular biomarkers involved in the inflammatory process (IL-2, IL-17, TNF-α, TGF-β1, and osteocalcin), ECM degradation (MMP-7 and MMP-9), and oxidative stress (SOD3 and catalase) mechanisms in clinically healthy and symptomatic irreversibly inflamed dental pulp samples, in children and adolescents.
2. Materials and Methods
2.1. Patients and Sample Collection
The study group consisted of 42 children aged from 6- to 18-years who sought treatment in the Pedodontics clinic of the Carol Davila University of Medicine and Pharmacy in Bucharest. Dental pulps (n = 42) were collected from the permanent teeth of these patients (one tooth/patient). All parents/caregivers gave their informed consent for the collection of dental pulp from their children, according to the protocol approved by the ethics committee of Carol Davila University of Medicine and Pharmacy, document No. 5751/05.03.2021. Only healthy cooperative patients without any medical conditions were included in the study. Uncooperative subjects, or subjects whose caregiver did not sign the informed consent, were excluded. From each patient included in the study, dental pulp from a single tooth was collected. Clinically symptomatic, irreversibly inflamed dental pulp samples (experimental group, n = 23) were collected from teeth with a history of prolonged or spontaneous lingering pain, extreme pain response to thermal stimuli (cold), sensibility on percussion, caries, and/or restorations on the tooth surface, from patients who sought emergency treatment. Radiographic examination did not reveal periapical pathosis, or periodontal diseases. Normal, healthy pulp samples (control group, n = 19) were collected from clinically healthy permanent teeth extracted for orthodontic reasons. Anamnesis, clinical, and radiographic examinations were performed for the exclusion of pain history, caries, restorations, or any type of fracture.
All dental pulp samples were collected in aseptic conditions. Before infiltration for local anaesthesia, all teeth were professionally cleaned of dental plaque, and teeth surfaces were also cleaned with 70% alcohol. A pulpectomy of teeth with irreversible pulpitis was performed only after the placement of a rubber dam. Caries and the roof of the pulp chamber were removed, and inflamed dental pulp tissues were collected using barbed broaches (VDW, GmbH, Munich, Germany). Irrigation of the endodontic space during pulp collection was performed only with sterile saline solution. Healthy teeth extracted for orthodontic reasons were grooved longitudinally using a #557 diamond bur and split using an elevator. Dental pulp was removed using a spoon excavator (LM Dental, Planmeca Group, Parainen, Finland). Only dental pulp samples that were removed in a single piece were used. All pulp samples were placed in Eppendorf Tubes containing 5 mL of saline solution and were stored at −70 °C until use.
2.2. Pulp Lysates
All frozen pulp samples were thawed for 30 min and 1 mL of phosphate-buffered saline (PBS) solution, pH 7.0, was added to each sample. PBS solution was prepared by dissolving 1 PBS tablet (Invitrogen/GIBCO) in 50 mL of distilled water. Pulp tissue was crushed using a glass rod followed by sonication for 3 min. Homogenized samples were centrifuged at 4000 rpm for 10 min. Pulp supernatants were collected for the ELISA determination.
During the preparation of the pulp lysates, all samples were placed on ice to prevent protein denaturation.
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
Levels of IL-2, IL-17, TNF-α, MMP-7, MMP-9, catalase, SOD3, osteocalcin, and TGF-β1 were measured in dental pulp lysates using commercially available ELISA kits from Elabscience (Houston, TX, USA) and a semiautomatic ELISA analyzer STAT FAX 303-PLUS from Awareness Technologies (Palm City, FL, USA. Determinations were made according to the kit manufacturer’s recommendations using the sandwich ELISA technique. All samples were analyzed in duplicate.
2.4. Statistical Analysis
Data analysis was performed using IBM Statistics 25 and Microsoft Office Excel/Word 2013. Quantitative variables were tested using the Shapiro–Wilk test. The Mann–Whitney U test was used for non-parametric distributions, while a Student/Welch t-test was employed for parametric distributions. Statistical significance of differences in the expression of protein levels was set for p-values < 0.05.
3. Results
The mean age of the patients who participated in our research was 10.98 ± 3.65 years old; children in the experimental group were 10.91 ± 3.18 years old, while the mean age of those in the control group was 11.06 ± 4.25 years old. The mean values of each biomarker in irreversibly inflamed and healthy dental pulps, and the statistical analysis, are presented in Table 1. All mediators were detected in both the experimental and control group of pulp samples.

Table 1.
Mean levels of IL-2, IL-17, TNF-α, MMP-7, MMP-9, SOD3, catalase, osteocalcin, and TGF-β1 in healthy and irreversibly inflamed dental pulp samples.
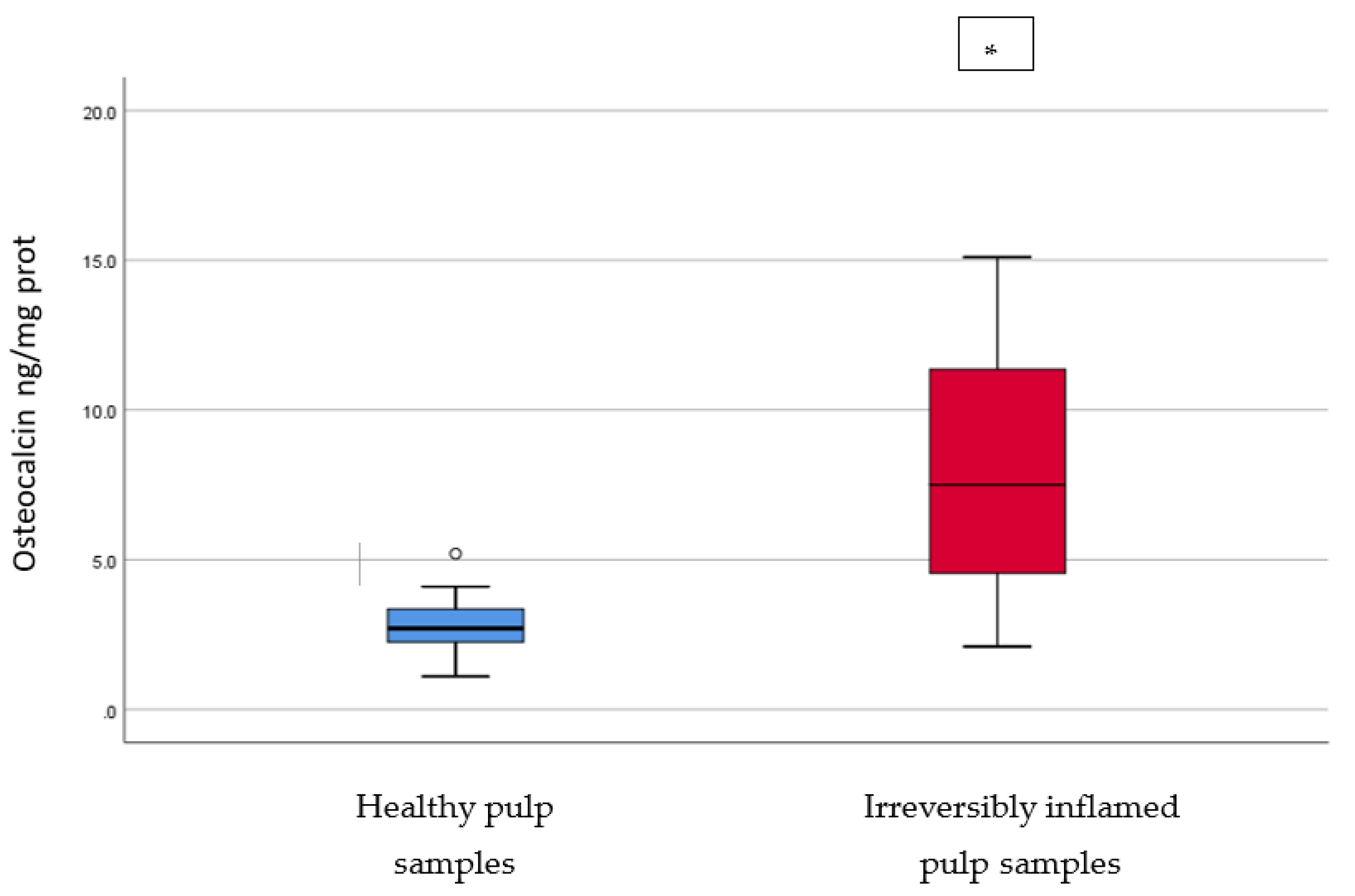
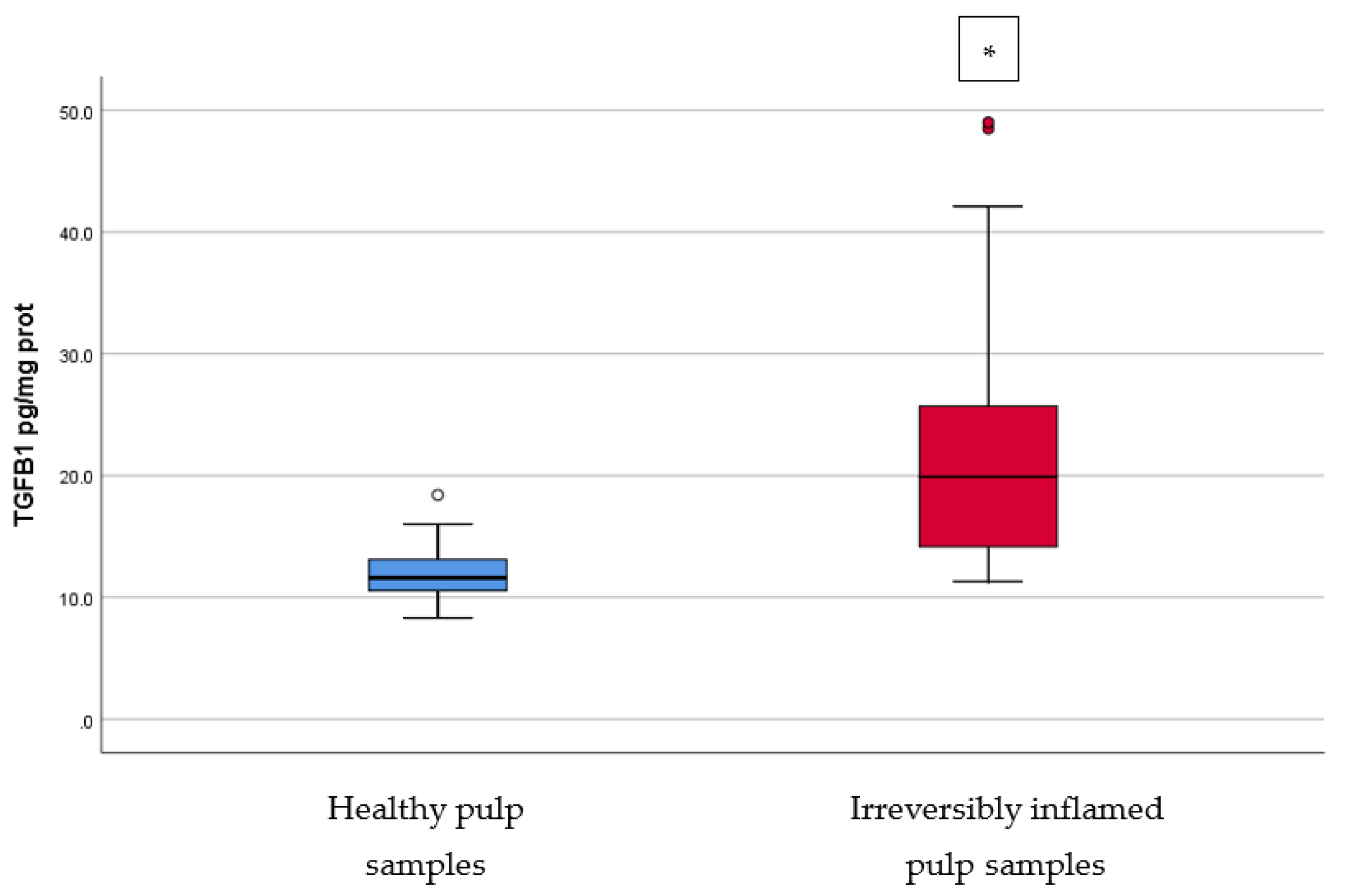
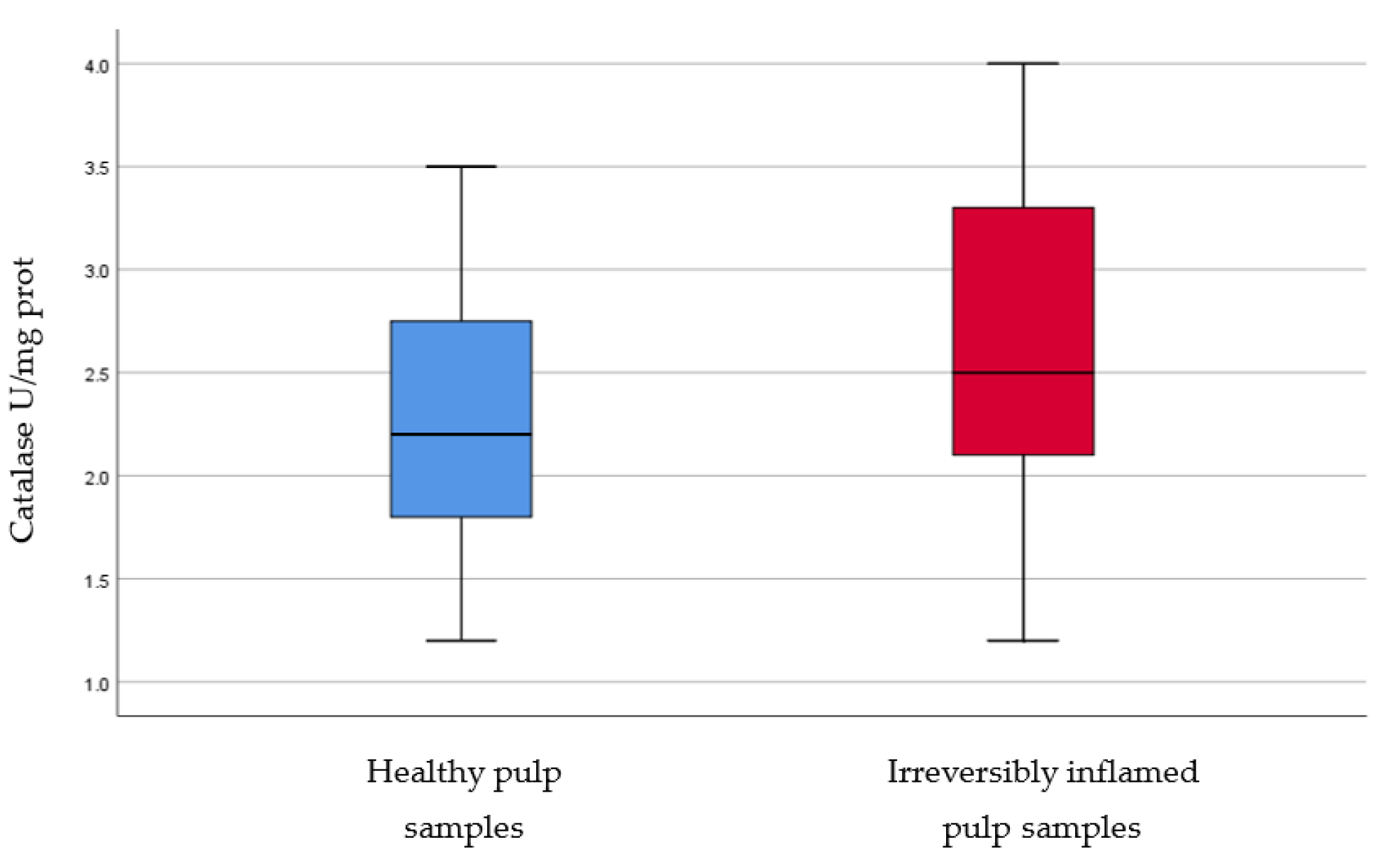
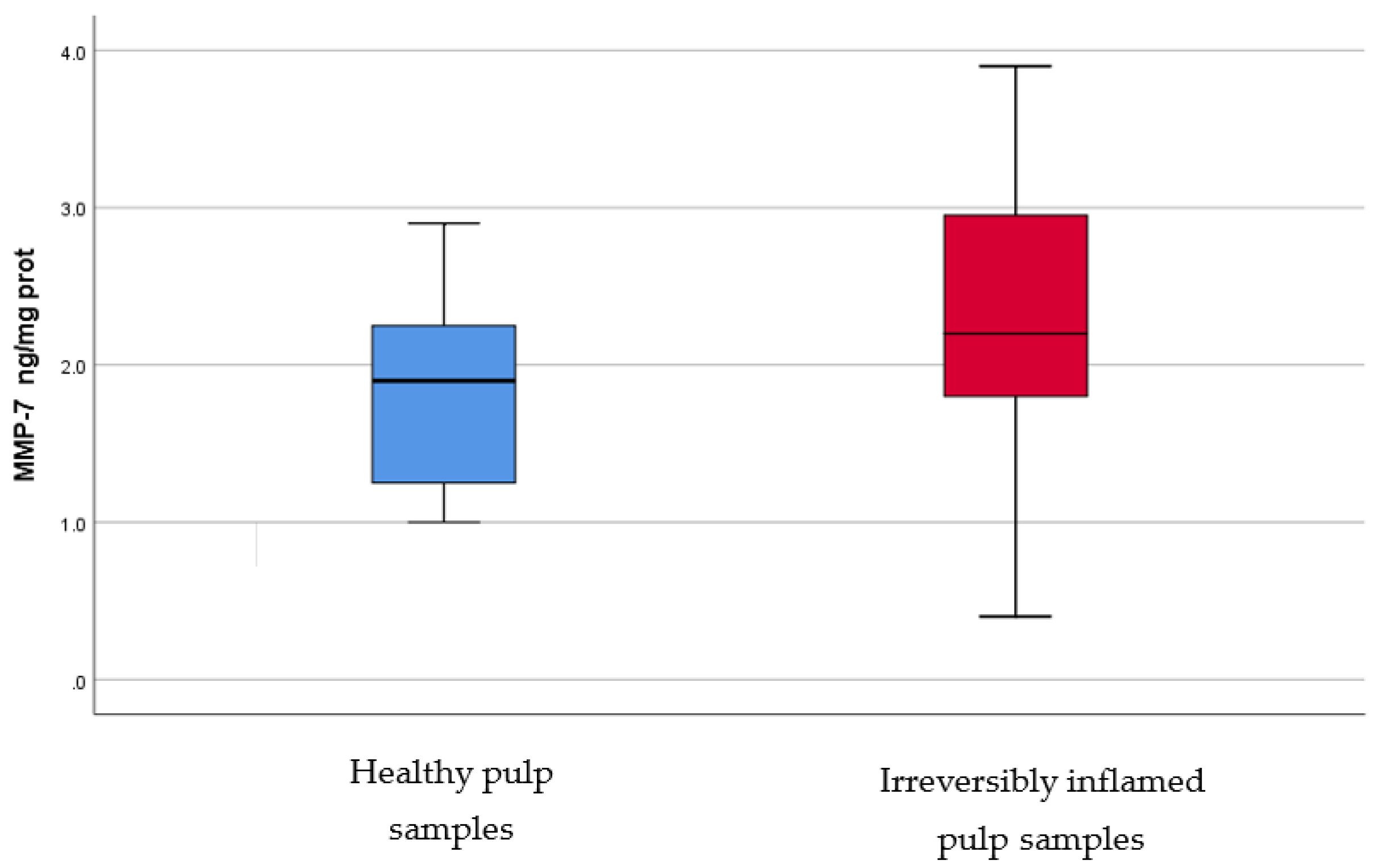
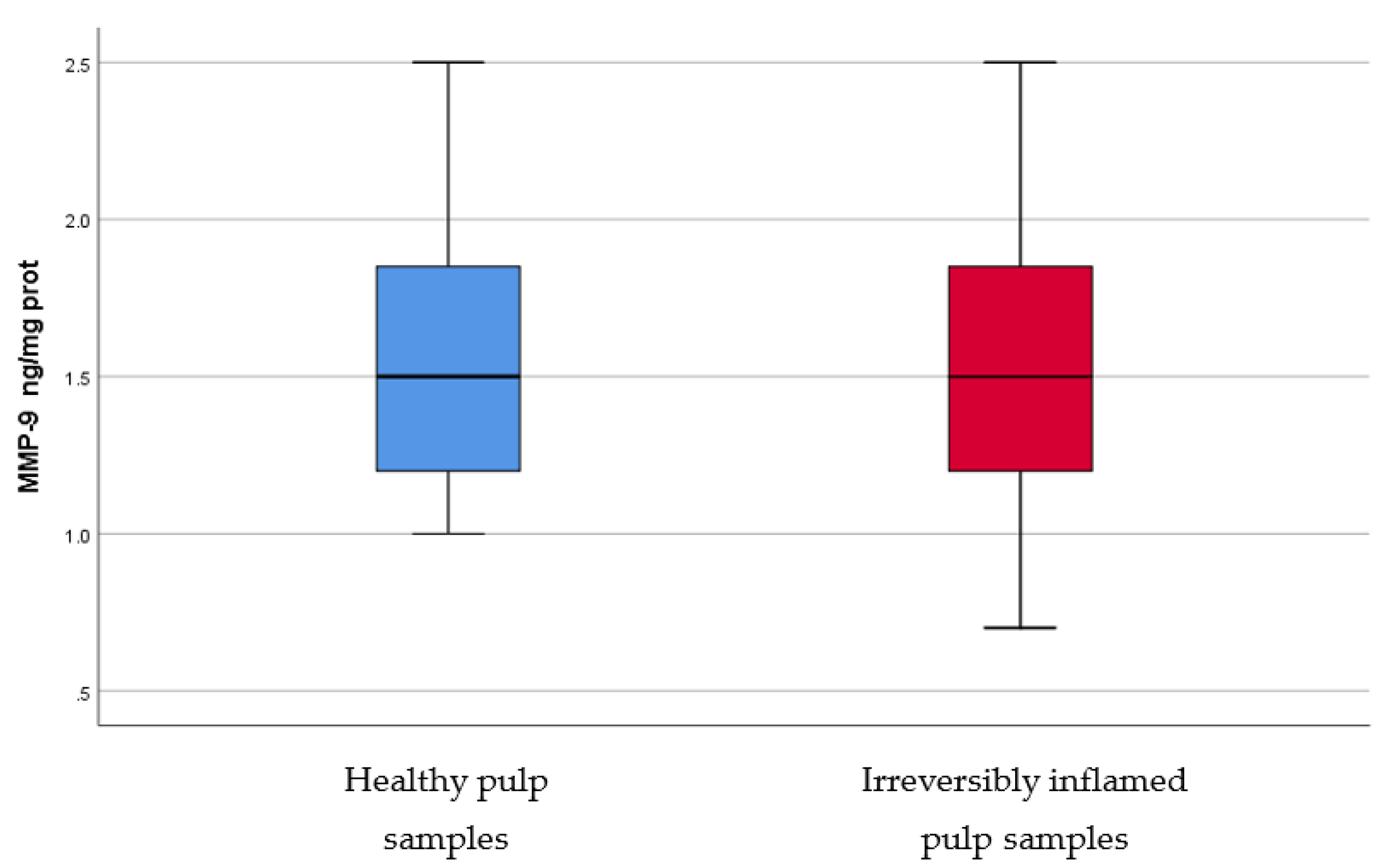
IL-2, IL-17, TNF-α, SOD3, osteocalcin, and TGF-β1 were significantly increased in irreversibly inflamed dental pulp samples compared to the controls (p < 0.001) (Table 1; Figure 1, Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6). Catalase presented higher levels in the irreversible stage of pulp inflammation when compared to healthy pulp samples, but the differences were not statistically significant (p = 0.064) (Table 1, Figure 7). Regarding the MMPs levels, MMP-7 showed higher levels in the experimental group compared to the control group (p = 0.061), while MMP-9 levels were increased in healthy pulp samples (p = 0.625). The MMPs results revealed no significant differences between the irreversibly inflamed and normal dental pulp samples (Table 1; Figure 8 and Figure 9).
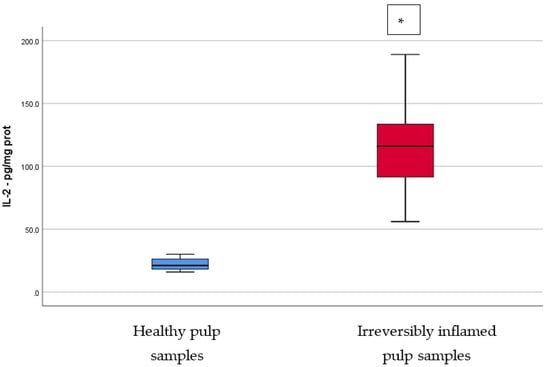
Figure 1.
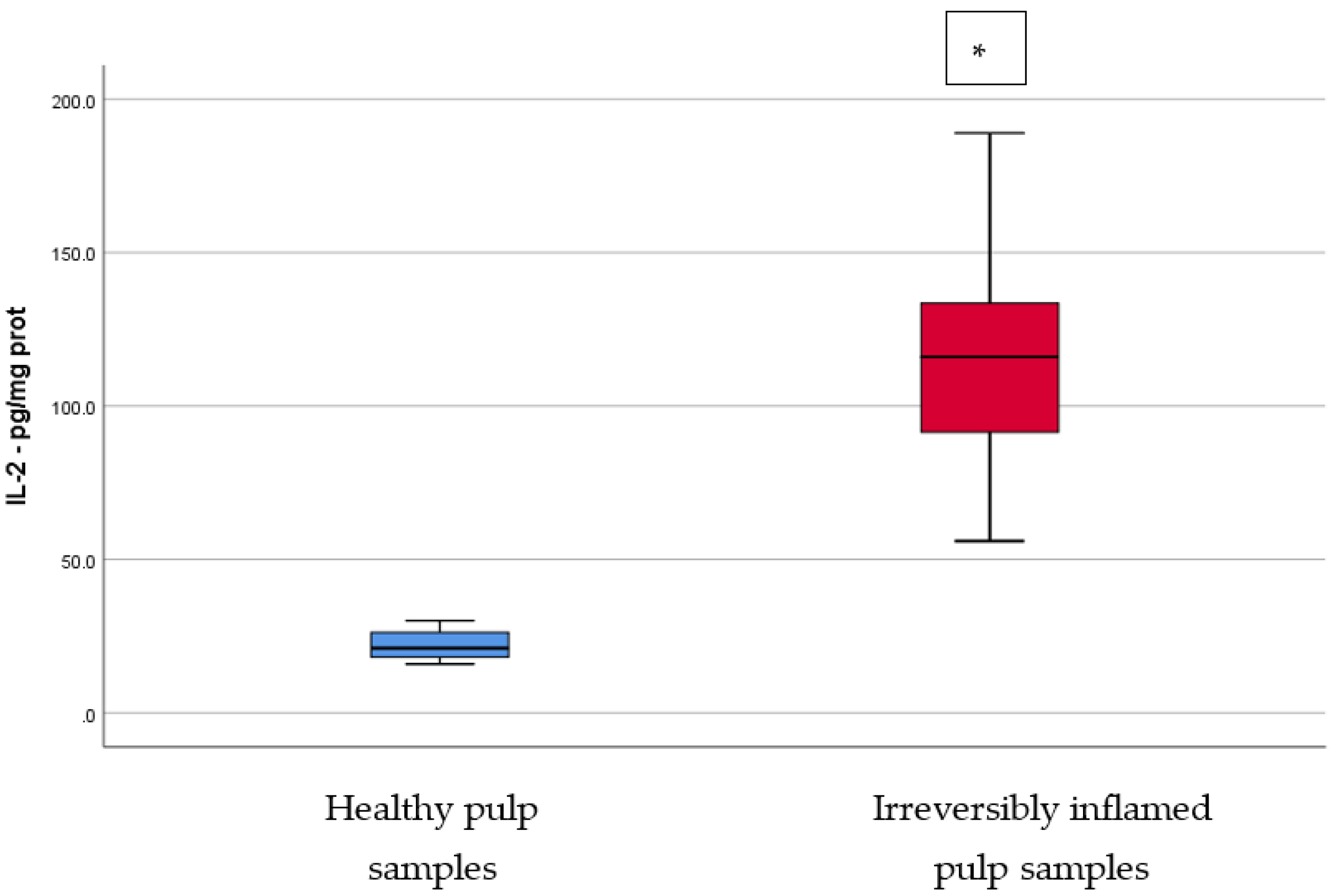
Comparison of IL-2 levels (pg/mg protein) in healthy and irreversibly inflamed dental pulp samples, respectively. The distribution of variables is parametric in both groups according to the Shapiro–Wilk test (p > 0.05). Welch’s t-test shows that the differences between the two groups are statistically significant (p < 0.001). (* indicates a statistically significant difference).
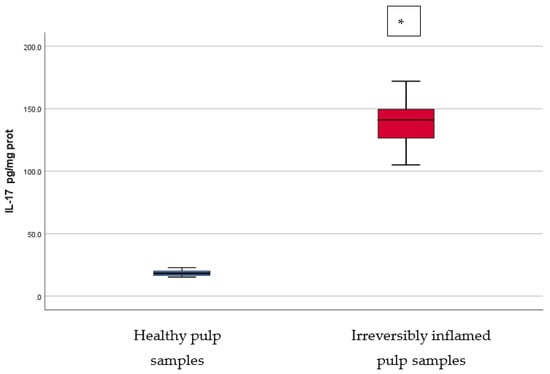
Figure 2.
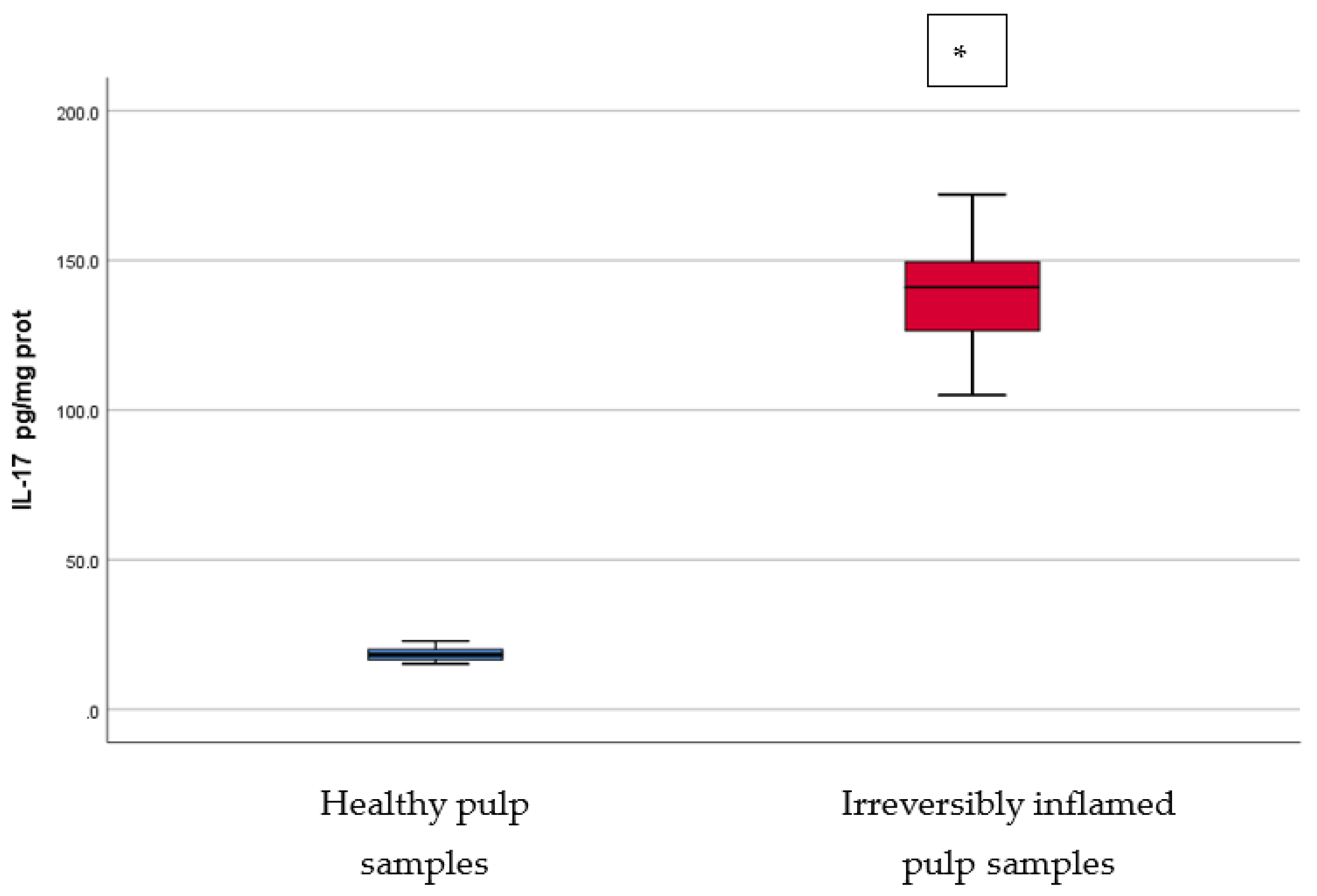
Comparison of IL-17 levels (pg/mg protein) in healthy and irreversibly inflamed dental pulp samples, respectively. The distribution of variables is parametric in both groups according to the Shapiro–Wilk test (p > 0.05). Welch’s t-test shows that the differences between the two groups are statistically significant (p < 0.001). (* indicates a statistically significant difference).
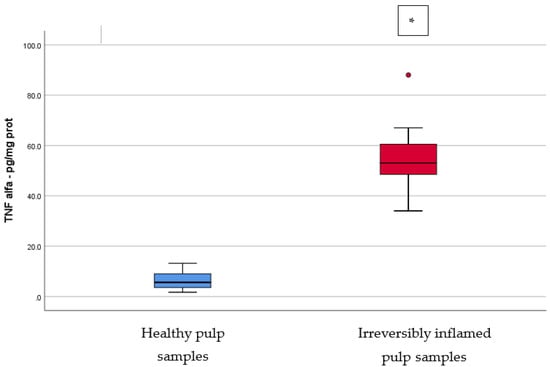
Figure 3.
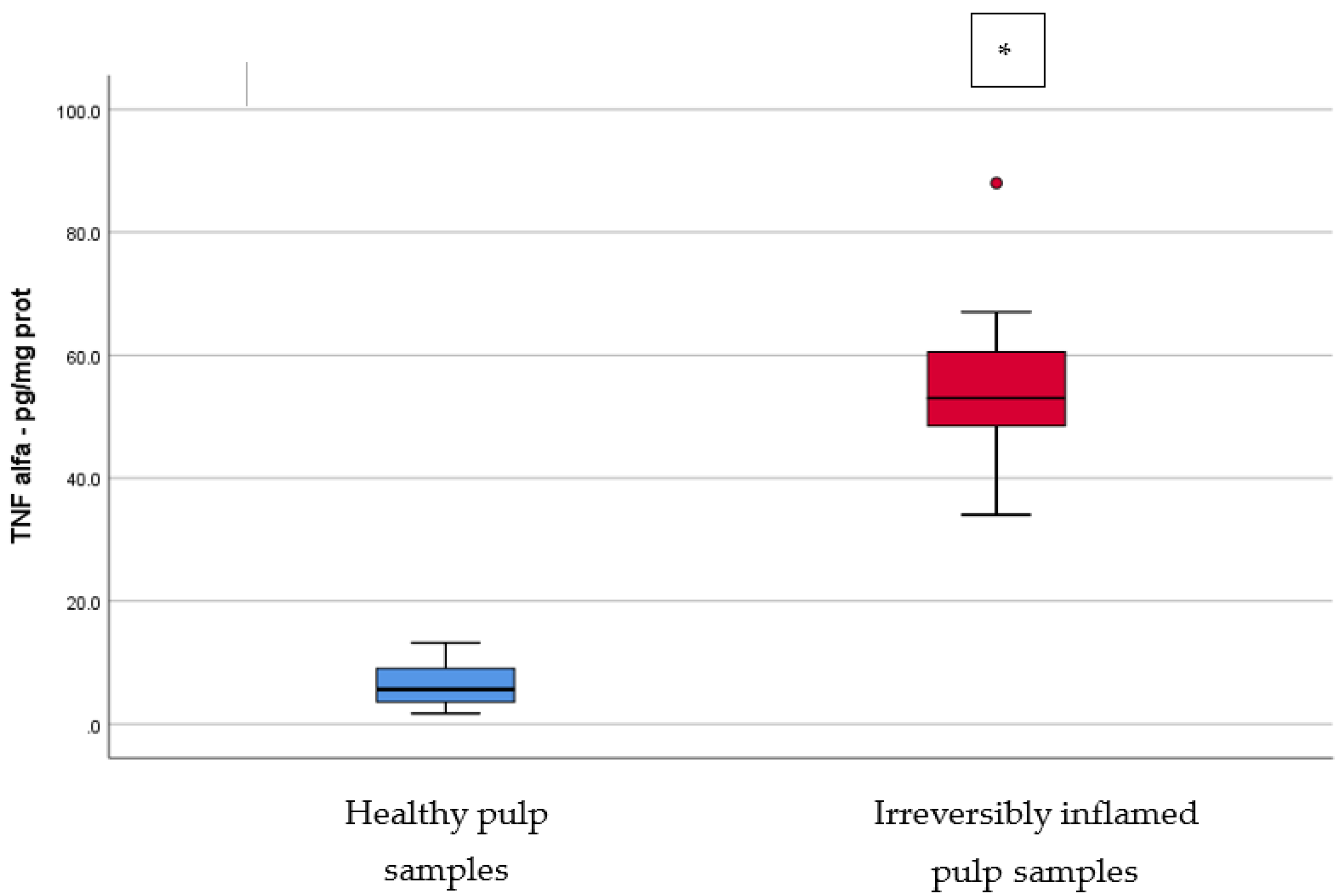
Comparison of TNF-α levels (pg/mg protein) in healthy and irreversibly inflamed dental pulp samples, respectively. The distribution of variables is parametric in both groups according to the Shapiro–Wilk test (p > 0.05). Welch’s t-test shows that the differences between the two groups are statistically significant (p < 0.001). (* indicates a statistically significant difference).
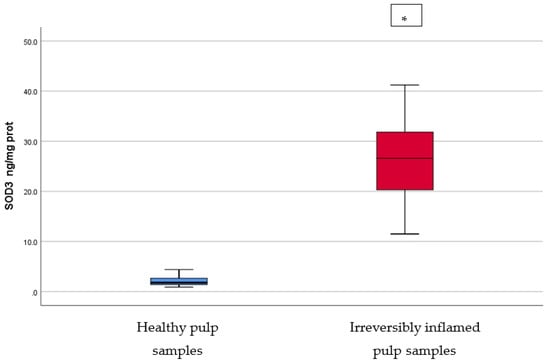
Figure 4.
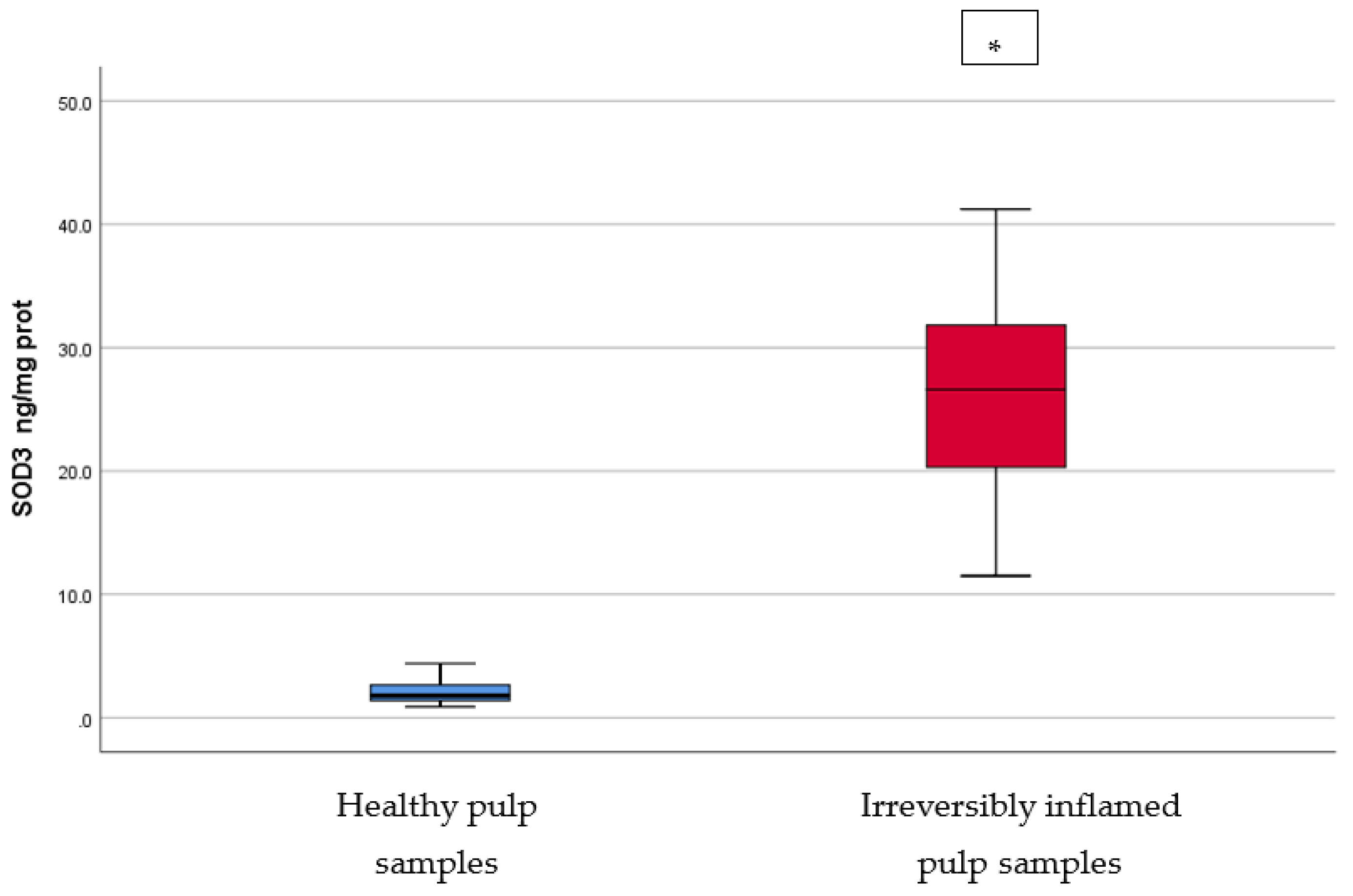
Comparison of SOD3 levels (ng/mg protein) in healthy and irreversibly inflamed dental pulp samples, respectively. The distribution of variables is parametric in both groups according to the Shapiro–Wilk test (p > 0.05). Welch’s t-test shows that the differences between the two groups are statistically significant (p < 0.001). (* indicates a statistically significant difference).
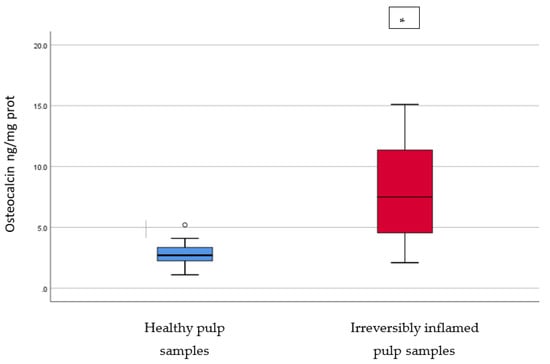
Figure 5.
Comparison of Osteocalcin levels (ng/mg protein) in healthy and irreversibly inflamed dental pulp samples, respectively. The distribution of variables is parametric in both groups according to the Shapiro–Wilk test (p > 0.05). Welch’s t-test shows that the differences between the two groups are statistically significant (p < 0.001). (* indicates a statistically significant difference).
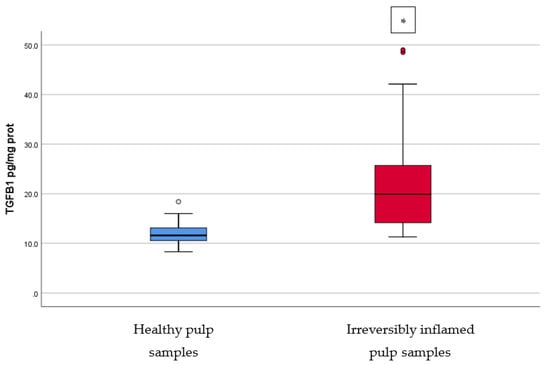
Figure 6.
Comparison of TGF-β1 levels (pg/mg protein) in healthy and irreversibly inflamed dental pulp samples, respectively. The distribution of variables is non-parametric in the irreversibly inflamed pulp samples group according to the Shapiro–Wilk test (p = 0.001). Mann–Whitney’s U test shows that the differences between the two groups are statistically significant (p < 0.001). (* indicates a statistically significant difference).
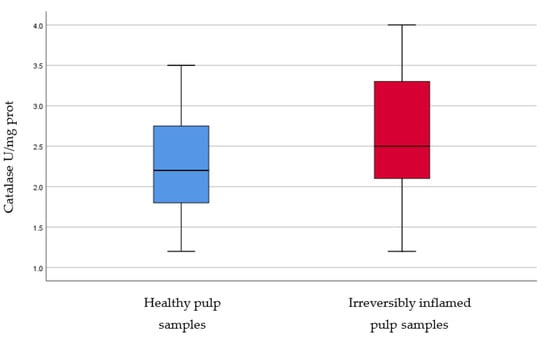
Figure 7.
Comparison of Catalase levels (U/mg protein) in healthy and irreversibly inflamed dental pulp samples, respectively. The distribution of variables is parametric in both groups according to the Shapiro–Wilk test (p > 0.05). Student’s t-test shows that the differences between the two groups are not statistically significant (p = 0.064), although irreversibly inflamed pulp samples presented increased catalase levels compared to the control group.
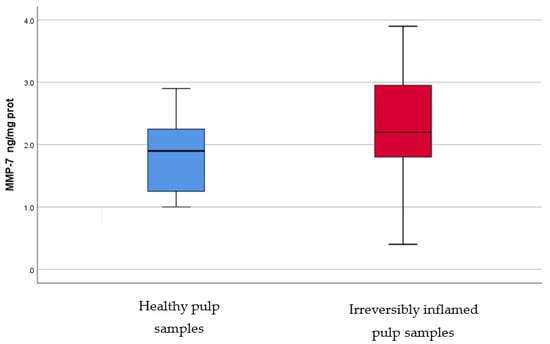
Figure 8.
Comparison of MMP-7 levels (ng/mg protein) in healthy and irreversibly inflamed dental pulp samples, respectively. The distribution of variables is parametric in both groups according to the Shapiro–Wilk test (p > 0.05). Student’s t-test shows that the differences between the two groups are not statistically significant (p = 0.061), although irreversibly inflamed pulp samples presented higher MMP-7 levels compared to the control group.
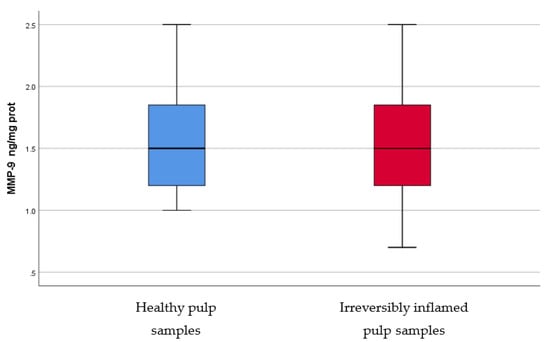
Figure 9.
Comparison of MMP-9 levels (ng/mg protein) in healthy and irreversibly inflamed dental pulp samples, respectively. The distribution of variables is parametric in both groups according to the Shapiro–Wilk test (p > 0.05). The control group showed higher levels of MMP-9 compared to the experimental group, but, according to Student’s t-test, differences between the two groups are not statistically significant (p = 0.625).
4. Discussion
The inflammatory status of the dental pulp is triggered and maintained by secreted molecules and cellular responses. Dental pulp inflammation is characterized by the presence of a massive T cell infiltration, which is acting by secreting numerous cytokines [11]. Cytokines present in inflamed dental pulps are interconnected with other biomarkers, such as those that participate in extracellular matrix degradation and oxidative stress. The current study has shown that most of the studied biomarkers (IL-2, IL-17, TNF-α, SOD3, TGF-β1, and osteocalcin) present statistically significant increased levels in irreversibly inflamed dental pulp samples, as compared to healthy pulp samples (Table 1, Figure 1, Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6), while others (catalase, MMP-7, and MMP-9) have not expressed significant differences (Table 1, Figure 7, Figure 8 and Figure 9).
IL-2 has an important role in the pathogenesis or progression of inflammation. This cytokine is mainly secreted by CD4+ T helper cells (Th), which are subsequently regulated by IL-2 itself. IL-2 also has the ability to stimulate clonal expansion and proliferation of T cells, which are the predominant lymphocytes in inflammatory lesions [12]. In addition to the effects on T cells, IL-2 can increase antibody secretion from B cells. IL-2 can activate the transcription of other inflammatory cytokines, such as TNF-α, IFN-γ, and granulocyte-macrophage colony-stimulating factor (GM-CSF) [13]. Our results are similar to those reported by Rauschenberger et al. [14], revealing statistically significant elevated IL-2 levels in inflamed pulp samples clinically diagnosed with irreversible pulpitis (p < 0.05). Anderson et al. [15] also studied the presence of IL-2 in normal and irreversibly inflamed dental pulp samples, reporting no statistically significant differences. Similarly, ElSalhy et al. [16] did not find statistically significant differences between the irreversible pulpitis group and the normal group (p > 0.05). IL-2 levels were significantly higher in the caries exposure group when compared to both the normal group (p < 0.01) and the irreversible pulpitis group (p > 0.05). Although all studies used the same analyzing method (ELISA technique), the results are in confliction. This could be due the use of different types of samples, for example, ElSalhy et al. [16] used blood obtained from pulp exposure sites, while Anderson et al. [15] reported a pipetting error during the preparation of the samples.
IL-17 can be produced by activated CD4+ Th17 cells, but can also be secreted by CD8+ T cells, innate lymphoid cells, γδ cells, NK cells, and neutrophils. IL-17 plays an important role in various autoimmune and inflammatory pathologies [17]. TGF-β signaling contributes to the production of IL-17 from Th17 [18]. IL-17 can be characterized as an inflammatory cytokine that stimulates the release of other cytokines, such as CXC-chemokine ligand-1 (CXCL1), CXCL2, IL-8, and CXCL6, inducing neutrophil recruitment in inflammatory areas [19]. Furthermore, IL-17 enhances neutrophil elastase and myeloperoxidase (MPO) activities. In addition, IL-17 can trigger fibroblasts, endothelial cells, epithelial cells, and osteoblasts to produce proinflammatory cytokines, such as IL-6, IL-8, GM-CSF, and MMPs [19]. Significantly higher levels (p < 0.05) of IL-17 were identified in chronic periodontitis lesions compared to healthy periodontal ligament samples [18,20,21,22,23]. Cytokine IL-17 mRNA expression in the pulp tissue was found to be increased in inflamed dental pulp samples (p < 0.05) [24], the results being in accordance with our findings. The results of the present study are also in agreement with those of Liu et al. [25], who found higher levels of IL-17 in pulp samples of symptomatic teeth using immunohistochemical staining.
TNF-α is a proinflammatory cytokine produced mainly in response to infection, and is released by various cells that take part in the immune-inflammatory process [26]. It can also trigger the formation of the acute-phase inflammation proteins, enhance the toxicity of leucocytes, and stimulate the expression of other proinflammatory cytokines [26]. TNF-α, and other cytokines, can stimulate the formation and secretion of proteolytic enzymes, such as MMPs, which destroy the ECM [27]. TNF-α can cause vasodilatation and increased permeability of blood vessels with leukocytes extravasation in infected areas [28]. Our results are similar to those of Pezelj-Ribaric et al. [28], who reported significant higher levels (p = 0.000) of TNF-α protein in pulp samples from teeth clinically diagnosed with irreversible pulp inflammation compared to healthy pulp samples, using the ELISA technique. TNF-α gene expression was also studied, showing statistically significant increased levels (p = 0.002) in the study group consisting of irreversible pulpitis samples, as compared to healthy pulps [26]. Another study with similar results was conducted by Abd-Elmeguid et al. [29], which reported a significant increase in TNF-α protein levels in the irreversible stages of pulp inflammation when compared to the control group, using a Multiplex assay (p < 0.01). Although ElSalhy et al. [16] investigated the levels of TNF-α, using ELISA, in pulpal blood obtained from irreversible pulpitis and normal pulps, our results are in accordance with their study.
SOD is one of the most important antioxidant enzymes in controlling the concentrations of ROI and, as a result, preventing excessive tissue damage under oxidative stress conditions [30]. Cellular and extracellular components are damaged if a higher production, or lesser removal, of free radicals occurs. Macrophages can produce increased levels of ROI during inflammation, which inactivate the bacteria, but, also, can damage these cells and the nearby tissue, including ECM [9]. SOD acts against free radicals by catalyzing the dismutation of the superoxide ion into oxygen and hydrogen peroxide. SOD exists in 3 isoenzymes: cytosolic Cu, Zn-SOD, mitochondrial Mn-SOD, and extracellular EC-SOD [9]. Conflicting results have been published throughout the years regarding the activity of the SOD enzyme (total, or isoenzymes Cu, Zn-SOD, Mn-SOD) in normal and inflamed dental pulps. Our results are in accordance with the reports of Davis et al. [31], who found significantly elevated levels (p = 0.0001) of Cu, Zn-SOD in inflamed dental pulp samples compared to healthy pulp samples obtained from adult patients. On the other hand, Tulunoglu et al. [32] reported no differences in the total activity of SOD in irreversibly inflamed versus healthy pulp samples from children’s permanent teeth. The observations of the present study are also similar to those of Bodor et al. [30], who found a significant increase (p < 0.05) in both Mn-SOD and Cu, Zn-SOD mRNA expression in cases of irreversible pulpitis, and Baumgardner & Sulfaro [33], who also reported an increased immunoreactivity in both SOD isoenzymes in inflamed dental pulp samples using immunohistochemistry. In contrast to these results, Varvara et al. [34] observed significantly lower (p < 0.001) Cu, Zn-SOD activity in irreversibly inflamed pulp samples, as compared to healthy dental pulp samples. The decreased Cu, Zn-SOD activity may be attributed to the progression of inflammation and depletion, or destruction, of the enzyme in oxidative stress conditions.
Osteocalcin is a protein produced by osteoblasts during the matrix mineralization phase. At bone level it is assumed to be incorporated into the bone matrix and to be involved in calcium binding [35]. It is a major non-collagenous protein of the dentine matrix and is regarded as a reparative molecule, expressed as a response to an offence of the dental pulp. Osteocalcin is regarded as a late differentiation marker of odontoblasts [29,36]. Our results are similar to those reported by Abd-Elmeguid et al. [29], who measured osteocalcin levels in dental pulp using a multiplex assay and found significantly higher levels (p < 0.05) of this protein in irreversibly inflamed human dental pulps compared to healthy pulp samples. Cells that are dominant in inflamed dental pulps, such as macrophages, may express osteocalcin in their transdifferentiation to osteoblasts [29]. These findings may indicate that large amounts of osteocalcin are secreted from odontoblasts to form the predentin of reactionary dentin in response to the pulp inflammation [37]. However, it can be speculated that the elevated expression of other inflammation biomarkers impedes the limitation of inflammation and the reactionary dentinogenesis initiation.
Transforming growth factor-beta (TGF-β1) is produced by odontoblasts in normal pulps, and promotes matrix metalloproteinase secretion and dentin mineralization, playing an important role in dentinogenesis and repair. TGF-β molecules have mitogenic effects and chemotactic properties for fibroblasts, macrophages, neutrophils, and monocytes [38]. In vivo studies showed that the use of TGF-β1 in wound areas may expand the synthesis of ECM proteins [39,40]. Our results are in accordance with those of previous studies, reporting a significantly higher TGF-β1 expression in irreversibly inflamed dental pulps [41,42]. In vitro studies reported a downregulation of TGF-β1 gene expression [43]. TGF-β1 can also increase the local predentin secretion [44]. The role of TGF-β in dentinogenesis could be attributed to its involvement in odontoblast differentiation [45], and other activities mentioned above; it may take part in reparative processes by regulating cell proliferation, migration, and ECM production in dental pulp [46].
Catalase is also an enzyme (like SOD3) that scavenges hydrogen peroxide released during, both, normal aerobic and pathogenic metabolism (inflammation) from host cells [47]. In our study, the determination of catalase levels in irreversibly inflamed and healthy dental pulp samples showed non-significant differences (p = 0.064). Esposito et al. [48] also evaluated catalase activity in irreversibly inflamed and healthy pulp samples. Their study showed a significant increase (p < 0.001) in the inflamed pulp tissues in comparison to the controls. The differences between our results and the reports of Esposito et al. [48], may be caused by the different manner in which catalase’s presence was determined in dental pulp samples: our study used the ELISA technique for the determination of protein expression, while Esposito et al. [48] evaluated catalase activity using spectrophotometric methods.
MMPs are zinc-dependent proteases with endopeptidase activity, which can degrade ECM, contributing to the inflammation pathogenesis [49]. MMPs play an important role in physiological and pathological conditions, such as pulpal and periapical tissue destruction caused by inflammation, and wound healing [11,49]. These enzymes can be upregulated in different pathologies, such as inflammation [11,49]. MMP-7, or matrilysin, has the ability to cleave entactin, a basement membrane protein, which links laminin and collagen type IV, triggering the basement membrane disruption [50]. To the best of our knowledge, there are no studies regarding MMP-7 levels in healthy versus inflamed dental pulp in humans, however, significantly elevated mRNA levels and protein expression of MMP-7 were found in inflammatory lesion tissue samples of chronic apical abscesses, as compared to control samples of healthy periodontal ligament tissue. In our study, irreversibly inflamed pulp samples showed higher levels (1.853 ± 0.59 ng/mg protein) of MMP-7 than healthy pulp samples (2.3 ± 0.85 ng/mg protein), but differences were not statistically significant (p > 0.05). During the inflammatory process, MMP-9, or gelatinase, or type IV collagenase, is mainly secreted by PMNs. We found slightly higher levels of MMP-9 in the control group (1.568 ± 0.44 ng/mg protein) than in irreversibly inflamed dental pulp samples (1.496 ± 0.5 ng/mg protein), but the results did not differ significantly (p = 0.625). In contrast with these findings, previous studies using various methodologies (ELISA, RT-PCR, Western blot, Zymography assay), reported elevated levels of MMP-9 in irreversibly inflamed dental pulp samples, as compared to healthy pulp samples [51,52,53,54]. A possible explanation for our findings regarding MMPs levels, could be that a symptomatic pulpal condition may not always be associated with microabscesses, or severe tissue degradation [51,55]. Another possible explanation is that some dental pulps were less inflamed than they appeared clinically (children may tend to overreact to painful stimuli, or a child could fail to understand the tests used for diagnosis); moreover, for similar clinical symptoms, the amount, or intensity, of the trigger that determines MMPs production may differ [54].
Dental pulp collection, either by pulpectomy, for the irreversibly inflamed dental pulp tissues, or by removing the pulp tissue after extraction of the teeth, was conducted carefully. We tried to remove the pulp tissue gently, in a single piece, to prevent its destruction. All dental treatments (the removal of caries and pulp chambers and the splitting of the extracted teeth) were performed under continuous cooling water, avoiding excessive heat in the pulp chamber and the possibility of protein denaturation, which could affect the results of the study. Furthermore, all dental pulp samples were collected in aseptic conditions, while infected dentine, from the teeth with irreversible inflammation, was thoroughly removed before pulp extraction to avoid further bacteria contamination.
The ability of the dental pulp to respond to an injury depends on the severity of the impact. Dental pulp can respond by initiating an inflammatory reaction, which, at the beginning, is limited to an area. At the same time, reactionary, or reparative, dentin is secreted for reparation. If the impact is mild and the odontoblasts survive, deposition of reactionary dentin occurs [45]. On the other hand, when the intensity of the injury is higher, this results in odontoblast death, and new odontoblast-like cells are required to produce dentine (reparative dentinogenesis). In irreversible inflammation, vital inflamed pulp is unable to heal and total pulpal necrosis may occur, triggering the periapical pathology with local bone involvement [56]. Less severe cases of pulp inflammation are represented by reversible pulpitis, clinically accompanied by hypersensitivity, or short-term pain [57]. In reversible pulpitis, once the inflammation resolves, the dental pulp has the potential to return to normal [56].
Vital pulp treatment should be the golden standard in case of reversible pulpitis, mainly in children with immature permanent teeth. This kind of treatment should promote pulpal healing of the remaining dental pulp, leading to apexogenesis with root development and apical closure. On the other hand, treatment of an irreversible pulp inflammation should aim to eliminate pulp infection and to induce the formation of a hard tissue barrier in the apical portion of the root of immature permanent teeth by apexification procedures.
This study’s hypothesis was to determine if there are any differences in the levels of various biomarkers of inflammation, oxidative stress, and ECM degradation in irreversibly inflamed, compared to healthy, dental pulp samples, collected from young permanent teeth. The hypothesis was partially confirmed once the concentrations of IL-2, IL-17, TNF-α, SOD3, osteocalcin, and TGF-β1 were significantly increased in the experimental group compared to the control group.
5. Conclusions
Inflamed dental pulp showed an upregulation of IL-2, IL-17, TNF-α, SOD3, osteocalcin, and TGF-β1. Molecular procedures quantifying these biomarkers, which appear to play a powerful role in the inflammation process of human dental pulp, may be helpful for accurate assessment of the inflammation status, especially in permanent teeth when differential diagnosis between reversible and irreversible pulp inflammation is mandatory. Further developments in this field are required in order to put these findings to work in a practical way.
Author Contributions
Conceptualization, M.G., M.I., K.K., and, A.R.T.; methodology, K.K., A.R.T., D.M., and, T.-C.S.; software, R.I., T.-C.S., and, I.-I.S.-S.; validation, M.G., M.T., and, M.I.; formal analysis, R.I., and A.V.; investigation, M.T., A.V., and, D.M.; resources, T.-C.S., I.-I.S.-S., R.I., A.V., and, D.M.; data curation, A.R.T., K.K., D.M., and, M.T.; writing—original draft preparation, K.K., T.-C.S., and, I.-I.S.-S.; writing—review and editing I.-I.S.-S., and A.R.T.; visualization, T.-C.S., I.-I.S.-S., and, R.I.; supervision, M.I.; project administration, M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Carol Davila University of Medicine and Pharmacy (5751/05.03.2021).
Informed Consent Statement
Informed consent was obtained from the caregivers of all minor subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy reasons.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 23 September 2021).
- Zero, D.T.; Zandona, A.F.; Vail, M.M.; Spolnik, K.J. Dental caries and pulpal disease. Dent. Clin. N. Am. 2011, 55, 29–46. [Google Scholar] [CrossRef]
- Hahn, C.L.; Liewehr, F.R. Relationships between caries bacteria, host responses, and clinical signs and symptoms of pulpitis. J. Endod. 2007, 33, 213–219. [Google Scholar] [CrossRef]
- Zanini, M.; Meyer, E.; Simon, S. Pulp Inflammation Diagnosis from Clinical to Inflammatory Mediators: A Systematic Review. J. Endod. 2017, 43, 1033–1051. [Google Scholar] [CrossRef]
- Conrads, G.; About, I. Pathophysiology of Dental Caries. Monogr. Oral Sci. 2018, 27, 1–10. [Google Scholar] [CrossRef]
- Hahn, C.L.; Liewehr, F.R. Innate immune responses of the dental pulp to caries. J. Endod. 2007, 33, 643–651. [Google Scholar] [CrossRef]
- Kritikou, K.; Greabu, M.; Imre, M.; Miricescu, D.; Ripszky Totan, A.; Burcea, M.; Stanescu, S., II; Spinu, T. ILs and MMPs Levels in Inflamed Human Dental Pulp: A Systematic Review. Molecules 2021, 26, 4129. [Google Scholar] [CrossRef]
- Okamoto, T.; Takahashi, S.; Nakamura, E.; Nagaya, K.; Hayashi, T.; Shirai, M.; Fujieda, K. Increased expression of matrix metalloproteinase-9 and hepatocyte growth factor in the cerebrospinal fluid of infants with posthemorrhagic hydrocephalus. Early Hum. Dev. 2010, 86, 251–254. [Google Scholar] [CrossRef]
- Grossi, G.B.; Borrello, S.; Giuliani, M.; Galeotti, T.; Miani, C. Copper-zinc superoxide dismutase in human and animal dental pulp. J. Dent. 1991, 19, 319–321. [Google Scholar] [CrossRef]
- Farges, J.C.; Alliot-Licht, B.; Renard, E.; Ducret, M.; Gaudin, A.; Smith, A.J.; Cooper, P.R. Dental Pulp Defence and Repair Mechanisms in Dental Caries. Mediat. Inflamm. 2015, 2015, 230251. [Google Scholar] [CrossRef] [Green Version]
- Leone, A.; Angelova Volponi, A.; Uzzo, M.L.; Spatola, G.F.; Jurjus, A.; Vandevska-Radunovic, V. Dental pulp in mature replanted human teeth: Morphological alterations and metalloproteineses-2 and -9, Annexin-5, BCL-2 and iNOS modulation. J. Biol. Regul. Homeost. Agents 2015, 29, 961–967. [Google Scholar]
- Yamasaki, M.; Morimoto, T.; Tsuji, M.; Akihiro, I.; Maekawa, Y.; Nakamura, H. Role of IL-2 and helper T-lymphocytes in limiting periapical pathosis. J. Endod. 2006, 32, 24–29. [Google Scholar] [CrossRef]
- Gaffen, S.L.; Liu, K.D. Overview of interleukin-2 function, production and clinical applications. Cytokine 2004, 28, 109–123. [Google Scholar] [CrossRef]
- Rauschenberger, C.R.; Bailey, J.C.; Cootauco, C.J. Detection of human IL-2 in normal and inflamed dental pulps. J. Endod. 1997, 23, 366–370. [Google Scholar] [CrossRef]
- Anderson, L.M.; Dumsha, T.C.; McDonald, N.J.; Spitznagel, J.K., Jr. Evaluating IL-2 levels in human pulp tissue. J. Endod. 2002, 28, 651–655. [Google Scholar] [CrossRef]
- Elsalhy, M.; Azizieh, F.; Raghupathy, R. Cytokines as diagnostic markers of pulpal inflammation. Int. Endod. J. 2013, 46, 573–580. [Google Scholar] [CrossRef]
- Wei, L.; Liu, M.; Xiong, H.; Peng, B. Up-regulation of IL-23 expression in human dental pulp fibroblasts by IL-17 via activation of the NF-kappaB and MAPK pathways. Int. Endod. J. 2018, 51, 622–631. [Google Scholar] [CrossRef]
- Cardoso, C.R.; Garlet, G.P.; Crippa, G.E.; Rosa, A.L.; Junior, W.M.; Rossi, M.A.; Silva, J.S. Evidence of the presence of T helper type 17 cells in chronic lesions of human periodontal disease. Oral Microbiol. Immunol. 2009, 24, 1–6. [Google Scholar] [CrossRef]
- Yu, J.J.; Gaffen, S.L. Interleukin-17: A novel inflammatory cytokine that bridges innate and adaptive immunity. Front. Biosci. 2008, 13, 170–177. [Google Scholar] [CrossRef]
- Johnson, R.B.; Wood, N.; Serio, F.G. Interleukin-11 and IL-17 and the pathogenesis of periodontal disease. J. Periodontol. 2004, 75, 37–43. [Google Scholar] [CrossRef]
- Colic, M.; Vasilijic, S.; Gazivoda, D.; Vucevic, D.; Marjanovic, M.; Lukic, A. Interleukin-17 plays a role in exacerbation of inflammation within chronic periapical lesions. Eur. J. Oral Sci. 2007, 115, 315–320. [Google Scholar] [CrossRef]
- Xiong, H.; Wei, L.; Peng, B. Immunohistochemical localization of IL-17 in induced rat periapical lesions. J. Endod. 2009, 35, 216–220. [Google Scholar] [CrossRef]
- Marcal, J.R.; Samuel, R.O.; Fernandes, D.; de Araujo, M.S.; Napimoga, M.H.; Pereira, S.A.; Clemente-Napimoga, J.T.; Alves, P.M.; Mattar, R.; Rodrigues, V., Jr.; et al. T-helper cell type 17/regulatory T-cell immunoregulatory balance in human radicular cysts and periapical granulomas. J. Endod. 2010, 36, 995–999. [Google Scholar] [CrossRef]
- Xiong, H.; Wei, L.; Peng, B. IL-17 stimulates the production of the inflammatory chemokines IL-6 and IL-8 in human dental pulp fibroblasts. Int. Endod. J. 2015, 48, 505–511. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, Y.; Wang, C.; Luo, H.; Peng, A.; Ye, L. Interleukin-17 plays a role in pulp inflammation partly by WNT5A protein induction. Arch. Oral Biol. 2019, 103, 33–39. [Google Scholar] [CrossRef]
- Kokkas, A.B.; Goulas, A.; Varsamidis, K.; Mirtsou, V.; Tziafas, D. Irreversible but not reversible pulpitis is associated with up-regulation of tumour necrosis factor-alpha gene expression in human pulp. Int. Endod. J. 2007, 40, 198–203. [Google Scholar] [CrossRef] [PubMed]
- O’Boskey, F.J., Jr.; Panagakos, F.S. Cytokines stimulate matrix metalloproteinase production by human pulp cells during long-term culture. J. Endod. 1998, 24, 7–10. [Google Scholar] [CrossRef]
- Pezelj-Ribaric, S.; Anic, I.; Brekalo, I.; Miletic, I.; Hasan, M.; Simunovic-Soskic, M. Detection of tumor necrosis factor alpha in normal and inflamed human dental pulps. Arch. Med. Res. 2002, 33, 482–484. [Google Scholar] [CrossRef]
- Abd-Elmeguid, A.; Abdeldayem, M.; Kline, L.W.; Moqbel, R.; Vliagoftis, H.; Yu, D.C. Osteocalcin expression in pulp inflammation. J. Endod. 2013, 39, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Bodor, C.; Matolcsy, A.; Bernath, M. Elevated expression of Cu, Zn-SOD and Mn-SOD mRNA in inflamed dental pulp tissue. Int. Endod. J. 2007, 40, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Davis, W.L.; Jacoby, B.H.; Craig, K.R.; Wagner, G.; Harrison, J.W. Copper-zinc superoxide dismutase activity in normal and inflamed human dental pulp tissue. J. Endod. 1991, 17, 316–318. [Google Scholar] [CrossRef]
- Tulunoglu, O.; Alacam, A.; Bastug, M.; Yavuzer, S. Superoxide dismutase activity in healthy and inflamed pulp tissues of permanent teeth in children. J. Clin. Pediatric Dent. 1998, 22, 341–345. [Google Scholar]
- Baumgardner, K.R.; Sulfaro, M.A. The anti-inflammatory effects of human recombinant copper-zinc superoxide dismutase on pulp inflammation. J. Endod. 2001, 27, 190–195. [Google Scholar] [CrossRef]
- Varvara, G.; Traini, T.; Esposito, P.; Caputi, S.; Perinetti, G. Copper-zinc superoxide dismutase activity in healthy and inflamed human dental pulp. Int. Endod. J. 2005, 38, 195–199. [Google Scholar] [CrossRef]
- Cantatore, F.P.; Crivellato, E.; Nico, B.; Ribatti, D. Osteocalcin is angiogenic in vivo. Cell Biol. Int. 2005, 29, 583–585. [Google Scholar] [CrossRef]
- Wei, X.; Ling, J.; Wu, L.; Liu, L.; Xiao, Y. Expression of mineralization markers in dental pulp cells. J. Endod. 2007, 33, 703–708. [Google Scholar] [CrossRef]
- Hirata, M.; Yamaza, T.; Mei, Y.F.; Akamine, A. Expression of osteocalcin and Jun D in the early period during reactionary dentin formation after tooth preparation in rat molars. Cell Tissue Res. 2005, 319, 455–465. [Google Scholar] [CrossRef]
- Tziafas, D.; Papadimitriou, S. Role of exogenous TGF-beta in induction of reparative dentinogenesis in vivo. Eur. J. Oral Sci. 1998, 106 (Suppl. 1), 192–196. [Google Scholar] [CrossRef]
- Sporn, M.B.; Roberts, A.B. A major advance in the use of growth factors to enhance wound healing. J Clin. Investig. 1993, 92, 2565–2566. [Google Scholar] [CrossRef] [Green Version]
- Pierce, G.F.; Tarpley, J.E.; Yanagihara, D.; Mustoe, T.A.; Fox, G.M.; Thomason, A. Platelet-derived growth factor (BB homodimer), transforming growth factor-beta 1, and basic fibroblast growth factor in dermal wound healing. Neovessel and matrix formation and cessation of repair. Am. J. Pathol. 1992, 140, 1375–1388. [Google Scholar]
- Sloan, A.J.; Perry, H.; Matthews, J.B.; Smith, A.J. Transforming growth factor-beta isoform expression in mature human healthy and carious molar teeth. Histochem. J. 2000, 32, 247–252. [Google Scholar] [CrossRef]
- Piattelli, A.; Rubini, C.; Fioroni, M.; Tripodi, D.; Strocchi, R. Transforming growth factor-beta 1 (TGF-beta 1) expression in normal healthy pulps and in those with irreversible pulpitis. Int. Endod. J. 2004, 37, 114–119. [Google Scholar] [CrossRef]
- Durand, S.H.; Flacher, V.; Romeas, A.; Carrouel, F.; Colomb, E.; Vincent, C.; Magloire, H.; Couble, M.L.; Bleicher, F.; Staquet, M.J.; et al. Lipoteichoic acid increases TLR and functional chemokine expression while reducing dentin formation in in vitro differentiated human odontoblasts. J. Immunol. 2006, 176, 2880–2887. [Google Scholar] [CrossRef]
- Sloan, A.J.; Matthews, J.B.; Smith, A.J. TGF-beta receptor expression in human odontoblasts and pulpal cells. Histochem. J. 1999, 31, 565–569. [Google Scholar] [CrossRef]
- Goldberg, M.; Smith, A.J. Cells and Extracellular Matrices of Dentin and Pulp: A Biological Basis for Repair and Tissue Engineering. Crit. Rev. Oral Biol. Med. 2004, 15, 13–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melin, M.; Joffre-Romeas, A.; Farges, J.C.; Couble, M.L.; Magloire, H.; Bleicher, F. Effects of TGFbeta1 on dental pulp cells in cultured human tooth slices. J. Dent. Res. 2000, 79, 1689–1696. [Google Scholar] [CrossRef]
- Forman, H.J.; Torres, M. Redox signaling in macrophages. Mol. Asp. Med. 2001, 22, 189–216. [Google Scholar] [CrossRef]
- Esposito, P.; Varvara, G.; Caputi, S.; Perinetti, G. Catalase activity in human healthy and inflamed dental pulps. Int. Endod. J. 2003, 36, 599–603. [Google Scholar] [CrossRef]
- He, J.; Qin, M.; Chen, Y.; Hu, Z.; Xie, F.; Ye, L.; Hui, T. Epigenetic regulation of matrix metalloproteinases in inflammatory diseases: A narrative review. Cell Biosci. 2020, 10, 86. [Google Scholar] [CrossRef]
- Letra, A.; Ghaneh, G.; Zhao, M.; Ray, H., Jr.; Francisconi, C.F.; Garlet, G.P.; Silva, R.M. MMP-7 and TIMP-1, new targets in predicting poor wound healing in apical periodontitis. J. Endod. 2013, 39, 1141–1146. [Google Scholar] [CrossRef]
- Gusman, H.; Santana, R.B.; Zehnder, M. Matrix metalloproteinase levels and gelatinolytic activity in clinically healthy and inflamed human dental pulps. Eur. J. Oral Sci. 2002, 110, 353–357. [Google Scholar] [CrossRef]
- Tsai, C.H.; Chen, Y.J.; Huang, F.M.; Su, Y.F.; Chang, Y.C. The upregulation of matrix metalloproteinase-9 in inflamed human dental pulps. J. Endod. 2005, 31, 860–862. [Google Scholar] [CrossRef] [PubMed]
- Suwanchai, A.; Theerapiboon, U.; Chattipakorn, N.; Chattipakorn, S.C. NaV 1.8, but not NaV 1.9, is upregulated in the inflamed dental pulp tissue of human primary teeth. Int. Endod. J. 2012, 45, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Accorsi-Mendonca, T.; Silva, E.J.; Marcaccini, A.M.; Gerlach, R.F.; Duarte, K.M.; Pardo, A.P.; Line, S.R.; Zaia, A.A. Evaluation of gelatinases, tissue inhibitor of matrix metalloproteinase-2, and myeloperoxidase protein in healthy and inflamed human dental pulp tissue. J. Endod. 2013, 39, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Seltzer, S.; Bender, I.B.; Ziontz, M. The Dynamics of Pulp Inflammation: Correlations between Diagnostic Data and Actual Histologic Findings in the Pulp. Oral Surg. Oral Med. Oral Pathol. 1963, 16, 969–977. [Google Scholar] [CrossRef]
- American Association of Endodontists. Glossary of Endodontic Terms; 180 N. Stetson Ave. Suite 1500; AAE: Chicago, IL, USA, 2020. [Google Scholar]
- Murray, P.E.; Smith, A.J. Saving pulps—a biological basis. An overview. Prim. Dent. Care 2002, 9, 21–26. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).