Postoperative Study of Bone Gain in Mandibular Alveolar Bone Reconstructed with Screw-Guided Bone Regeneration Technique and Porcine-Derived Xenograft in 42 Edentulous Patient Candidates for Implant-Prosthetic Therapy
Abstract
:1. Introduction
2. Materials and Method
2.1. Patients Selection Study Design
- Test group (n = 20; 36 implants sites): implant sites augmentation with S-GBR technique and porcine-derived xenograft THE Graft (Purgo Biologics, Seongnam-si, Korea);
- Control group (n = 22; 50 implants sites): implant sites augmentation with S-GBR technique and bovine-derived xenograft CompactBone B (Dentegris GmBH, Rheinberg, Germany).
2.2. Description of Surgical Protocol
- (a)
- Dental implants placement simultaneously with alveolar augmentation by S-GBR technique (mixture of autogenous bone with autogenous bone 90:10; porcine pericardial collagen membrane) (test group);
- (b)
- Dental implants placement simultaneously with alveolar augmentation by S-GBR technique (mixture of bovine xenograft with autogenous bone 90:10; porcine pericardial collagen membrane) (control group).
2.3. Evaluation of Clinical and Bone Parameters
2.4. Statistical Analysis
3. Results
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guillaume, B. Dental implants: A review. Morphologie 2016, 100, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Mittal, Y.; Jindal, G.; Garg, S. Bone manipulation procedures in dental implants. Indian J. Dent. 2016, 7, 86–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Liu, X.; Shang, Y.; Qiao, F.; Chen, G. Current trends in research on bone regeneration: A bibliometric analysis. BioMed Res. Int. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, R.; Cooper, P.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone grafts and substitutes in dentistry: A review of current trends and developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.-S.; Kim, J.-W.; Hwang, J.-H.; Ahn, K.-M. Frequency of bone graft in implant surgery. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Törok, B.; Törok, R.; Forna, N. Study regarding share of peri-implant bone augmentation techniques and materials. Rom. J. Oral Rehab. 2020, 12, 88–96. [Google Scholar]
- Mitrea, M.; Niculescu, S.; Dmor, A.; Walid, E.A.H.; Florea, C.; Săveanu, I.C.; Balcos, C.; Forna, N.C. Esthetic rehabilitation with implants-supported fixed dentures after periodontitis. Rom. J. Oral Rehab. 2021, 13, 102–113. [Google Scholar]
- Sammartino, G.; Bernard, J.P. A clinical round table about the treatment of the severely resorbed posterior mandible. Part 1: Challenges, endeavor and perspectives. POSEIDO 2013, 1, 65–67. [Google Scholar]
- Moy, P.K.; Aghaloo, T. Risk factors in bone augmentation procedures. Periodontol. 2000 2019, 81, 76–90. [Google Scholar] [CrossRef]
- Tolstunov, L.; Hamrick, J.F.E.; Broumand, V.; Shilo, D.; Rachmiel, A. Bone augmentation techniques for horizontal and vertical alveolar ridge deficiency in oral implantology. Oral Maxillofac. Surg. Clin. North. Am. 2019, 31, 163–191. [Google Scholar] [CrossRef]
- Chiapasco, M.; Casentini, P.; Zaniboni, M. Bone augmentation procedures in implant dentistry. Int. J. Oral Maxillofac. Implant. 2009, 24, 237–259. [Google Scholar]
- Toeroek, R.; Dohan Ehrenfest, D.M. The concept of Screw-Guided Bone Regeneration (S-GBR). Part 2: S-GBR in the severely resorbed preimplant posterior mandible using bone xenograft and Leukocyte and Platelet-Rich Fibrin (L-PRF): A 5-year follow-up. POSEIDO 2013, 1, 85–92. [Google Scholar]
- Török, B.; Török, R.; Ehrenfest, D.D.; Agop-Forna, D.; Dascălu, C.; Forna, N. Study of immediate implants placed in mandibular alveolar bone reconstructed with screw-guided bone regeneration technique: A 24-months follow-up. Appl. Sci. 2021, 11, 6054. [Google Scholar] [CrossRef]
- Kim, Y.; Rodriguez, A.E.; Nowzari, H. The risk of prion infection through bovine grafting materials. Clin. Implant Dent. Relat. Res. 2016, 18, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.E.; Nowzari, H. The long-term risks and complications of bovine-derived xenografts: A case series. J. Indian Soc. Periodontol. 2019, 23, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Salamanca, E.; Lee, W.-F.; Lin, C.-Y.; Huang, H.-M.; Lin, C.-T.; Feng, S.-W.; Chang, W.-J. A novel porcine graft for regeneration of bone defects. Materials 2015, 8, 2523–2536. [Google Scholar] [CrossRef] [Green Version]
- Bracey, D.N.; Seyler, T.M.; Jinnah, A.H.; Lively, M.O.; Willey, J.S.; Smith, T.L.; Van Dyke, M.E.; Whitlock, P.W. A Decellularized porcine xenograft-derived bone scaffold for clinical use as a bone graft substitute: A critical evaluation of processing and structure. J. Funct. Biomater. 2018, 9, 45. [Google Scholar] [CrossRef] [Green Version]
- Buser, D.; Mericske-Stern, R.; Dula, K.; Lang, N.P. Clinical experience with one-stage, non-submerged dental implants. Adv. Dent. Res. 1999, 13, 153–161. [Google Scholar] [CrossRef]
- Esposito, M.; Grusovin, M.G.; Felice, P.; Karatzopoulos, G.; Worthington, H.; Coulthard, P. The efficacy of horizontal and vertical bone augmentation procedures for dental implants: A cochrane systematic review. Evid.-Based Pract. Towar. Optim. Clin. Outcomes 2010, 2, 195–218. [Google Scholar] [CrossRef]
- Wessing, B.; Lettner, S.; Zechner, W. Guided bone regeneration with collagen membranes and particulate graft materials: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, 87–100. [Google Scholar] [CrossRef]
- Elnayef, B.; Porta, C.; del Amo, F.S.L.; Mordini, L.; Gargallo-Albiol, J.; Hernández-Alfaro, F. The fate of lateral ridge augmentation: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, 622–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elakkiya, S.; Ramesh, A.S.; Prabhu, K. Systematic analysis on the efficacy of bone enhancement methods used for success in dental implants. J. Indian Prosthodont. Soc. 2017, 17, 219–225. [Google Scholar] [CrossRef]
- Herford, A.S.; Nguyen, K. Complex bone augmentation in alveolar ridge defects. Oral Maxillofac. Surg. Clin. North. Am. 2015, 27, 227–244. [Google Scholar] [CrossRef] [PubMed]
- Chavda, S.; Levin, L. Human studies of vertical and horizontal alveolar ridge augmentation comparing different types of bone graft materials: A systematic review. J. Oral Implant. 2018, 44, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xuan, F.; Choi, B.H.; Jeong, S.M. Minimally invasive ridge augmentation using xenogenous bone blocks in an atrophied posterior mandible: A clinical and histological study. Implantol. Dent. 2013, 22, 112–116. [Google Scholar] [CrossRef]
- Falacho, R.; Palma, P.; Marques, J.; Figueiredo, M.; Caramelo, F.; Dias, I.; Viegas, C.; Guerra, F. Collagenated porcine heterologous bone grafts: Histomorphometric evaluation of bone formation using different physical forms in a rabbit cancellous bone model. Molecules 2021, 26, 1339. [Google Scholar] [CrossRef]
- Salamanca, E.; Hsu, C.-C.; Huang, H.-M.; Teng, N.-C.; Lin, C.-T.; Pan, Y.-H.; Chang, W.-J. Bone regeneration using a porcine bone substitute collagen composite in vitro and in vivo. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.S.; Pai, Y.; Chang, S. Physicochemical characterization of InterOss® and Bio-Oss® anorganic bovine bone grafting ma-terial for oral surgery: A comparative study. Mater. Chem. Phys. 2014, 146, 99–104. [Google Scholar] [CrossRef]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Siegel, R.W.; Bizios, R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials 2000, 21, 1803–1810. [Google Scholar] [CrossRef]
- Kubies, D.; Himmlová, L.; Riedel, T.; Chánová, E.; Balík, K.; Douděrová, M.; Bártová, J.; Pesakova, V.J.P.R. The interaction of osteoblasts with bone-implant materials: The effect of physicochemical surface properties of implant materials. Physiol. Res. 2011, 60, 95–111. [Google Scholar] [CrossRef]
- Rupp, F.; Gittens, R.A.; Scheideler, L.; Marmur, A.; Boyan, B.D.; Schwartz, Z.; Geis-Gerstorfer, J. A review on the wettability of dental implant surfaces I: Theoretical and experimental aspects. Acta Biomater. 2014, 10, 2894–2906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troeltzsch, M.; Troeltzsch, M.; Kauffmann, P.; Gruber, R.; Brockmeyer, P.; Moser, N.; Rau, A.; Schliephake, H. Clinical efficacy of grafting materials in alveolar ridge augmentation: A systematic review. J. Cranio-Maxillofac. Surg. 2016, 44, 1618–1629. [Google Scholar] [CrossRef] [PubMed]
- Urban, I.A.; Nagursky, H.; Lozada, J.L.; Nagy, K. Horizontal ridge augmentation with a collagen membrane and a combination of particulated autogenous bone and anorganic bovine bone-derived mineral: A prospective case series in 25 patients. Int. J. Periodontics Restor. Dent. 2013, 33, 299–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mordenfeld, A.; Johansson, C.B.; Albrektsson, T.; Hallman, M. A randomized and controlled clinical trial of two different compositions of deproteinized bovine bone and autogenous bone used for lateral ridge augmentation. Clin. Oral Implant. Res. 2014, 25, 310–320. [Google Scholar] [CrossRef]
- De Santis, D.; Gelpi, F.; Verlato, G.; Luciano, U.; Torroni, L.; Antonucci, N.; Bernardello, F.; Zarantonello, M.; Nocini, P. Digital customized titanium mesh for bone regeneration of vertical, horizontal and combined defects: A case series. Medicine 2021, 57, 60. [Google Scholar] [CrossRef]
- Işık, G.; Yüce, M.Ö.; Koçak-Topbaş, N.; Günbay, T. Guided bone regeneration simultaneous with implant placement using bovine-derived xenograft with and without liquid platelet-rich fibrin: A randomized controlled clinical trial. Clin. Oral Investig. 2021, 25, 5563–5575. [Google Scholar] [CrossRef]
- Uzbek, U.H.; Rahman, S.A.; Alam, M.K.; Gillani, S.W. Bone forming potential of an-organic bovine bone graft: A cone beam CT study. J. Clin. Diagn Res. 2014, 8, 73–76. [Google Scholar] [CrossRef]
- Papageorgiou, S.N.; Papageorgiou, P.; Deschner, J.; Götz, W. Comparative effectiveness of natural and synthetic bone grafts in oral and maxillofacial surgery prior to insertion of dental implants: Systematic review and network meta-analysis of parallel and cluster randomized controlled trials. J. Dent. 2016, 48, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zaki, J.; Yusuf, N.; El-Khadem, A.; Scholten, R.J.P.M.; Jenniskens, K. Efficacy of bone-substitute materials use in immediate dental implant placement: A systematic review and meta-analysis. Clin. Implant Dent. Relat. Res. 2021, 23, 506–519. [Google Scholar] [CrossRef]
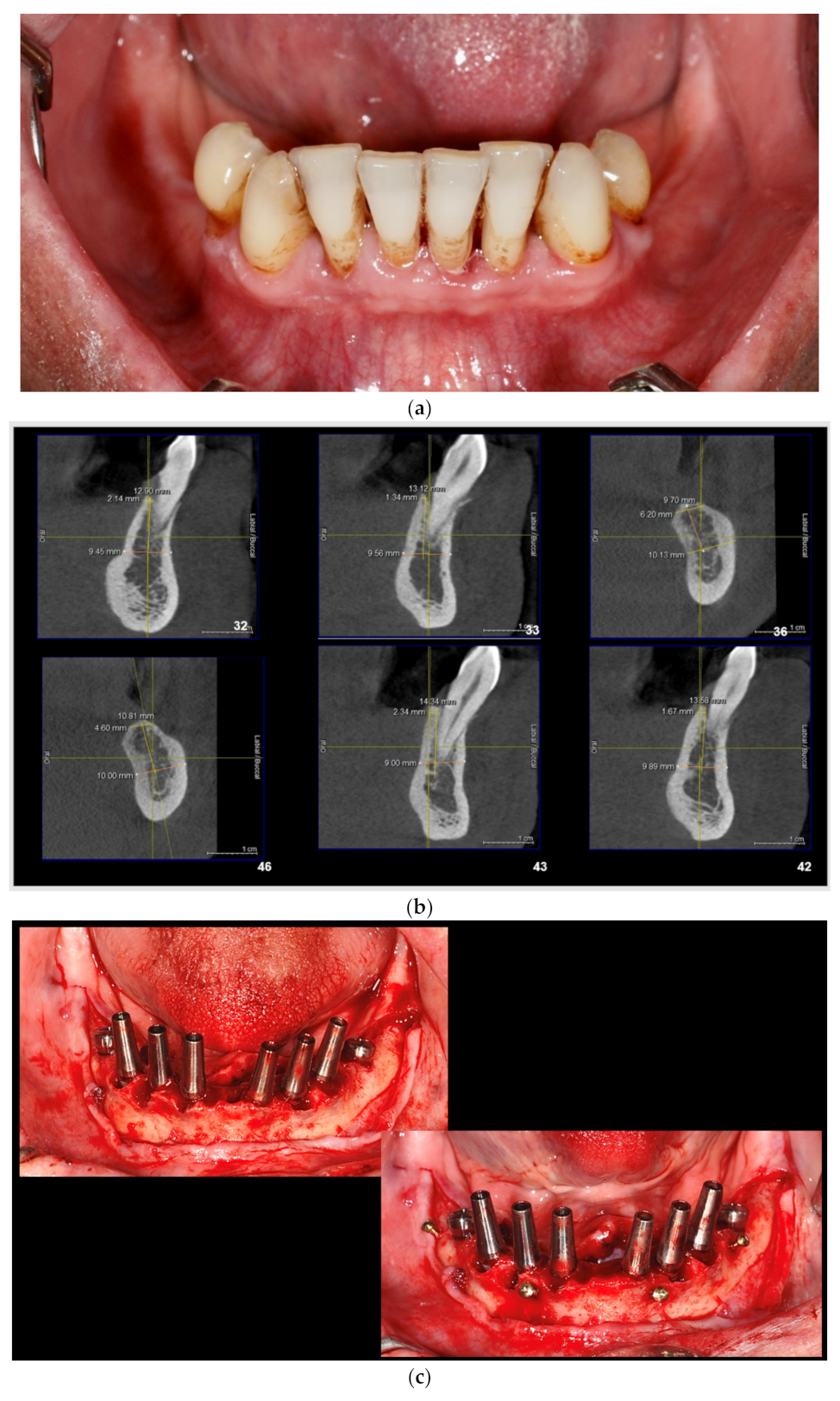
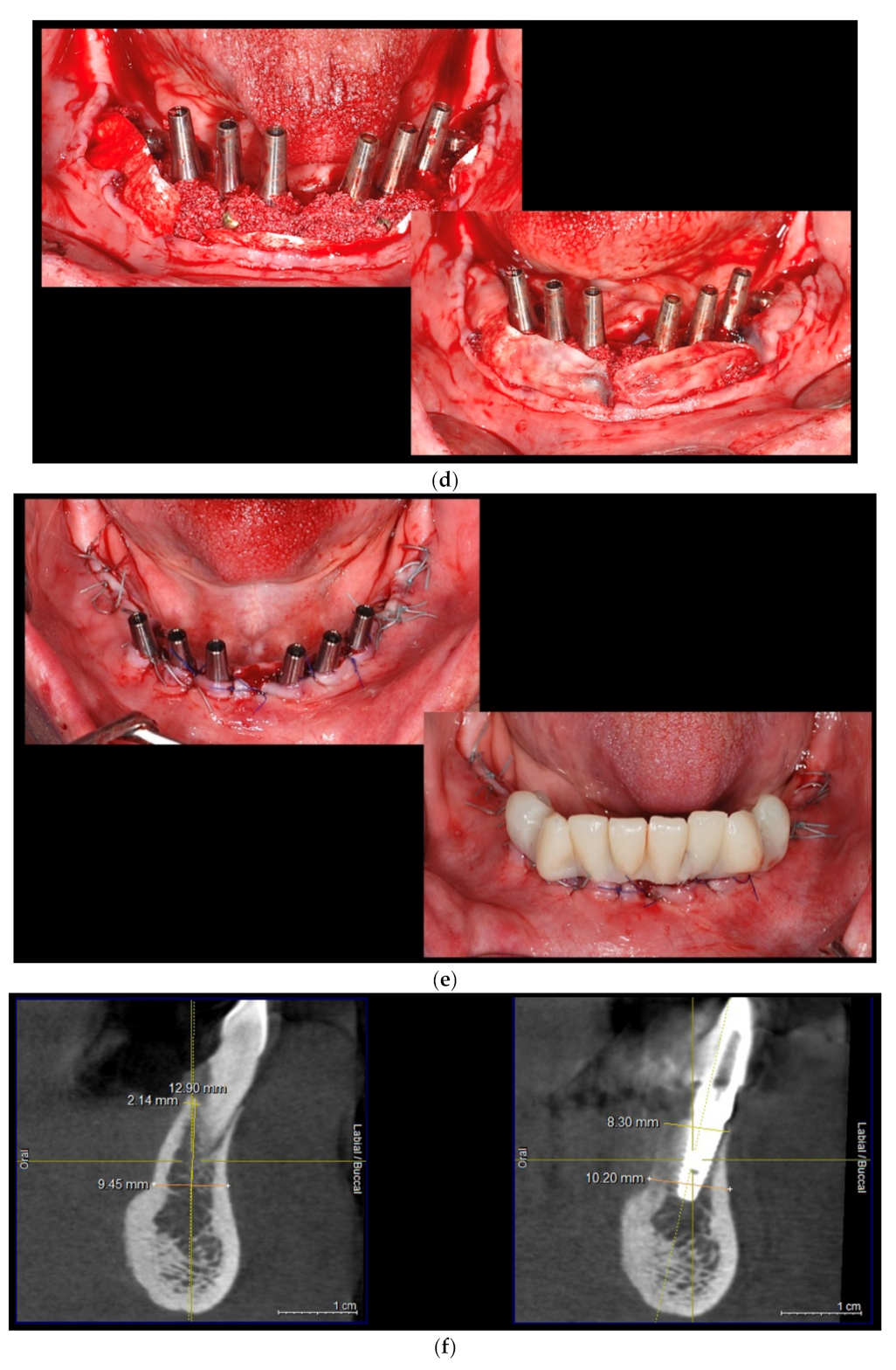
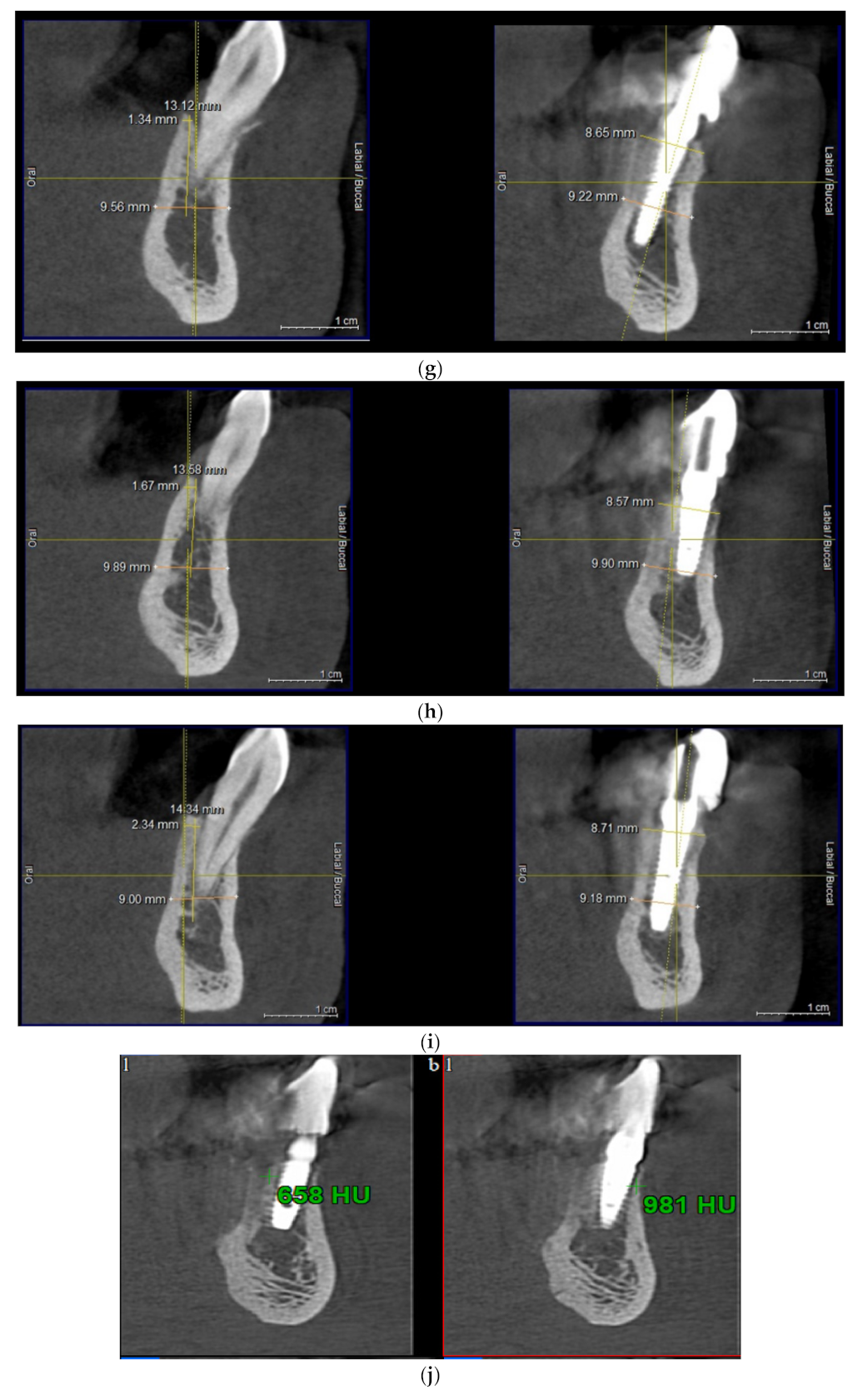
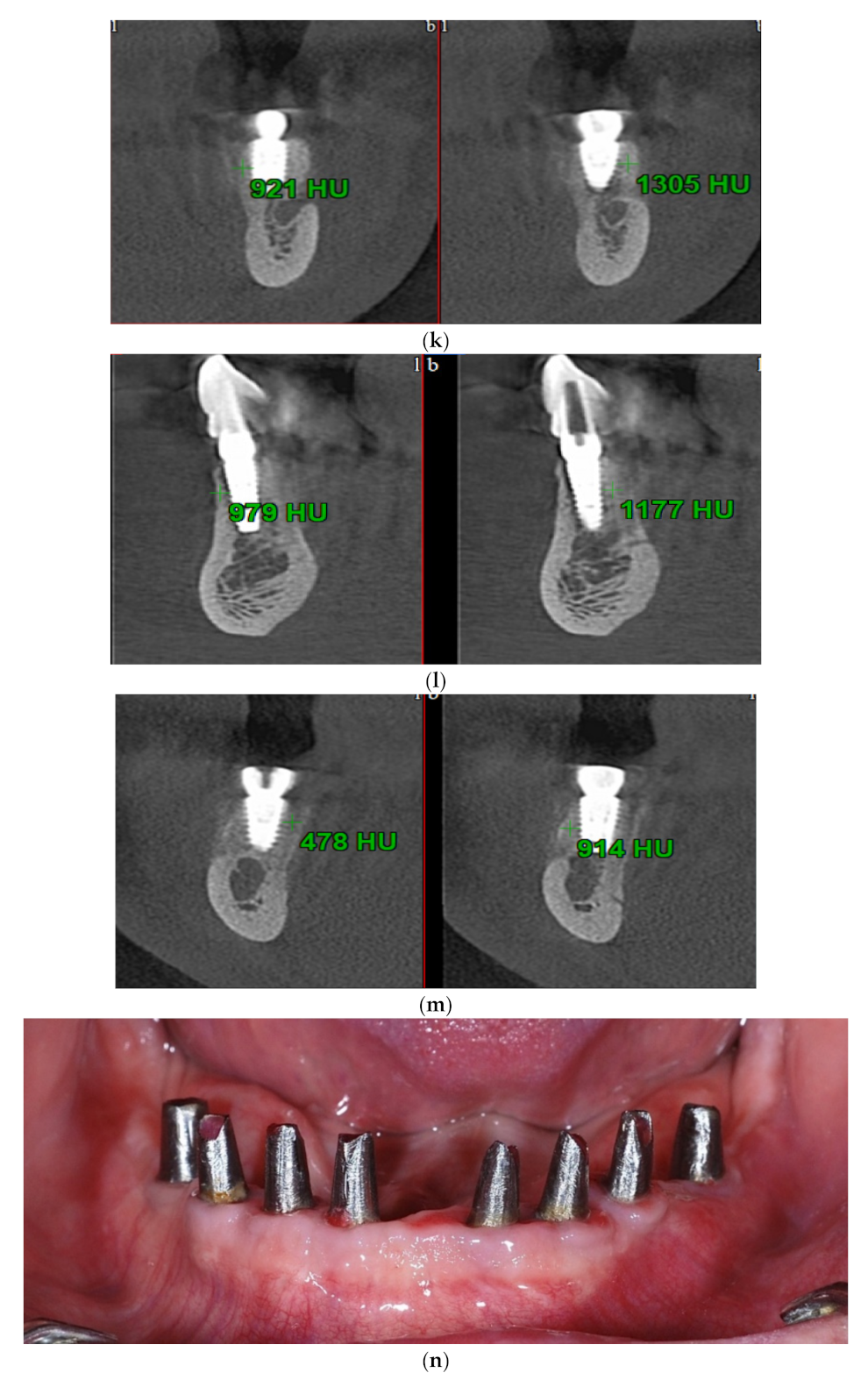


| Test Group (S-GBR + Bovine-Derived Xenograft) | Control Group (S-GBR + Porcine-Derived Xenograft) | Total | |
|---|---|---|---|
| N of subjects (Ns) N of sites (Ni) | 20 (47.6%) 36 (41.9%) | 22 (52.4%) 50 (58.1%) | 42 86 (100.0%) |
| Age groups | |||
| age 40–59, Ni (%) | 16 (44.4%) | 24 (48.0%) | 40 (46.5%) |
| age 60–79, Ni (%) | 20 (55.6%) | 26 (52.0%) | 46 (53.5%) |
| Gender | |||
| M, Ni (%) | 16 (44.4%) | 24 (48.0%) | 40 (46.5%) |
| F, Ni (%) | 20 (55.6%) | 26 (52.0%) | 46 (53.5%) |
| Dental group | |||
| Anterior, Ni (%) | 10 (27.8%) | 16 (32.0%) | 26 (30.2%) |
| Posterior, Ni (%) | 26 (72.2%) | 34 (68.0%) | 60 (69.8%) |
| Component | Description |
|---|---|
| Population (P) | Mandibular edentulous patients requiring alveolar bone grafting and implant-prosthetic therapy |
| Intervention (I) |
|
| Comparison (C) |
|
| Outcome (O) | Bone gain at 6 months follow-up (width and osteodensity) |
| No. | Procedure | Instruments and Materials | Role |
|---|---|---|---|
| 1. | Local anesthesia | Ultracain DS-Forte (Sanofi, Germany) | Ensure patient and operator comfort |
| 2. | Full-thickness flaps in the alveolar surgical areas | DeveMed GmbH, (Tuttlingen, Germany) | Opened surgical field |
| 3. | Immediate implant placement (length 10–13 mm, diameter 3.5–4.5 mm) | BioSTI implants (Tafers, Swiss) Dentium implants (South Korea) | |
| 4. | Periosteal incisions in flaps areas | Aesculap AG (Tuttlingen, Germany) | The closure of the flaps without tension |
| 5. | Osteosynthesis screws (1.5 mm diameter, 8 mm length) insertion on the buccal face of the bone areas (in 45° angle to the alveolar ridge) | Implantology kits Synthes GmbH, (Zuchwil, Switzerland) DeveMed GmbH, (Tuttlingen, Germany) | Maintain the space for the bone regeneration compartment |
| 6. | The exposed bone surface area will be covered with a small layer of autologous bone, followed by the porcine/bovine xenograft near to the osteosynthesis screw head. The grafted area will be covered with resorbable porcine pericardial membrane | Autogenous bone Bovine xenograft CompactBone, (Dentegris GmbH, Germany) Porcine xenograft Purgo (Purgos Biologics, Seongnam-si, Korea) Porcine pericardial tissue membrane BoneProtect Membrane (Dentegris GmbH, Germany) | Isolated and protected graft in front of the cells from the gingival tissue. Promotion of the gingival healing. Protect the surgical site from gingival dehiscence in the next 3–4 months. Reconstruction of the lateral alveolar bone area |
| 7. | Surgical site suture | Nonresorbable sutures (polypropylene 5.0, Hu-Friedy, USA) | Protection for the reconstructed bone and peri-implant soft tissues |
| 8. | Postoperative care (7 days): antibiotherapy, analgesics, oral cavity rinsing with chlorhexidine 0.5% | Augmentin 625 mg, GalaxoSmithKline Pharma, Viena, Austria); Ibuflam 600 (Zentiva, Lichtenstein) | Postoperative control of the pain and inflammatory processes |
| 9. | Removal of the sutures at 9–11 days postoperatively | ||
| 10. | Second stage surgery: osteosynthesis screws removal at 4 months postoperative |
| Group | Shapiro–Wilk Test | Mann–Whitney/ t-Student | |||
|---|---|---|---|---|---|
| Statistics | Sig. p | U/t Student | Sig. p | ||
| WIDTH– Bone gain | Control | 0.938 | 0.045 * | 738,000 | 0.156 |
| Test | 0.890 | 0.000 ** | |||
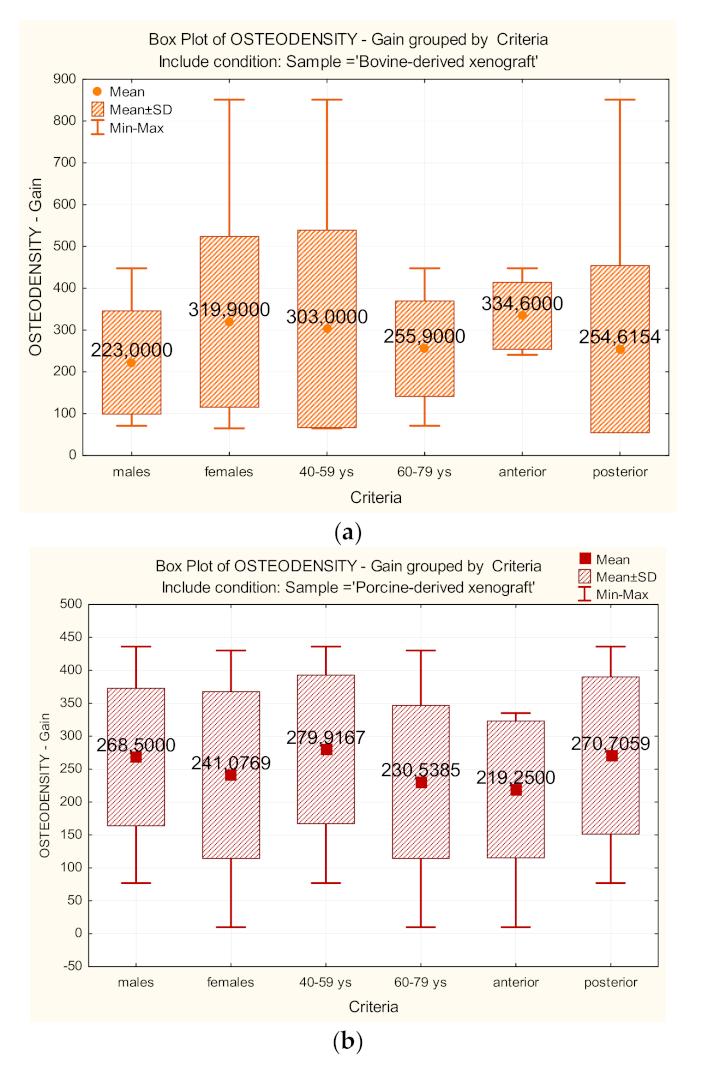
| OSTEODENSITY– Bone gain | Control | 0.823 | 0.000 ** | 896,000 | 0.972 |
| Test | 0.964 | 0.125 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agop-Forna, D.; Törok, R.; Törok, B.; Dragomir, R.; Ehrenfest, D.M.D.; Dascălu, C.; Stelea, C.G. Postoperative Study of Bone Gain in Mandibular Alveolar Bone Reconstructed with Screw-Guided Bone Regeneration Technique and Porcine-Derived Xenograft in 42 Edentulous Patient Candidates for Implant-Prosthetic Therapy. Appl. Sci. 2021, 11, 9826. https://doi.org/10.3390/app11219826
Agop-Forna D, Törok R, Törok B, Dragomir R, Ehrenfest DMD, Dascălu C, Stelea CG. Postoperative Study of Bone Gain in Mandibular Alveolar Bone Reconstructed with Screw-Guided Bone Regeneration Technique and Porcine-Derived Xenograft in 42 Edentulous Patient Candidates for Implant-Prosthetic Therapy. Applied Sciences. 2021; 11(21):9826. https://doi.org/10.3390/app11219826
Chicago/Turabian StyleAgop-Forna, Doriana, Roland Törok, Bianca Törok, Raluca Dragomir, David M. Dohan Ehrenfest, Cristina Dascălu, and Carmen Gabriela Stelea. 2021. "Postoperative Study of Bone Gain in Mandibular Alveolar Bone Reconstructed with Screw-Guided Bone Regeneration Technique and Porcine-Derived Xenograft in 42 Edentulous Patient Candidates for Implant-Prosthetic Therapy" Applied Sciences 11, no. 21: 9826. https://doi.org/10.3390/app11219826





