Comparison of the Efficacy of Dextrose Prolotherapy and Ozone in Patients with Knee Osteoarthritis: A Randomized Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Size Calculation
2.2. Patient Selection
2.3. Ethical Issues
2.4. Study Design
2.4.1. Dextrose Prolotherapy Group
2.4.2. Ozone Therapy Group
2.4.3. Home-Based Exercise Group
- Sitting on a chair, stretch your legs and place a rolled towel under your right knee. Straighten your leg by stretching your knee, pressing your knee down.
- Sitting on a chair, stretch your legs and place a rolled towel between your knees, count to 10, then relax for a few seconds.
- In the supine position, with the knee straight, raise your right leg 15–30 cm, count to 10, then relax for a few seconds.
- In the supine position, straighten your legs, and pull your right leg towards you for a count of 10, then relax.
- Lie face down and bend your right knee (pull it towards you), count to 10, then relax for a few seconds
- Lie on your side, bend your right leg and hip towards you, and count to 10. Then straighten your leg and extend your back as far as you can, then relax for a few seconds.
2.5. Outcome Assessment
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Villafañe, J.H.; Valdes, K.; Pedersini, P.; Berjano, P. Osteoarthritis: A call for research on central pain mechanism and personalized prevention strategies. Clin. Rheumatol. 2019, 38, 583–584. [Google Scholar] [CrossRef]
- Villafañe, J.H.; Bissolotti, L.; La Touche, R.; Pedersini, P.; Negrini, S. Effect of muscle strengthening on perceived pain and static knee angles in young subjects with patellofemoral pain syndrome. J. Exerc. Rehabil. 2019, 15, 454–459. [Google Scholar] [CrossRef] [Green Version]
- Sánchez Romero, E.A.; Fernández Carnero, J.; Villafañe, J.H.; Calvo-Lobo, C.; Ochoa Sáez, V.; Burgos Caballero, V.; Laguarta Val, S.; Pedersini, P.; Pecos Martín, D. Prevalence of Myofascial Trigger Points in Patients with Mild to Moderate Painful Knee Osteoarthritis: A Secondary Analysis. J. Clin. Med. 2020, 9, 2561. [Google Scholar] [CrossRef] [PubMed]
- Vora, A.; Borg-Stein, J.; Nguyen, R.T. Regenerative injection therapy for osteoarthritis: Fundamental concepts and evidence-based review. PM&R 2012, 4, S104–S109. [Google Scholar]
- Im, G.-I.; Kim, T.-K. Regenerative therapy for osteoarthritis: A perspective. Int. J. Stem Cells 2020, 13, 177. [Google Scholar] [CrossRef] [PubMed]
- Hemshekhar, M.; Thushara, R.M.; Chandranayaka, S.; Sherman, L.S.; Kemparaju, K.; Girish, K.S. Emerging roles of hyaluronic acid bioscaffolds in tissue engineering and regenerative medicine. Int. J. Biol. Macromol. 2016, 86, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Scaturro, D.; Vitagliani, F.; Terrana, P.; Cuntrera, D.; Falco, V.; Tomasello, S.; Letizia Mauro, G. Intra-Articular Hybrid Hyaluronic Acid Injection Treatment in Overweight Patients with Knee Osteoarthritis: A Single-Center, Open-Label, Prospective Study. Appl. Sci. 2021, 11, 8711. [Google Scholar] [CrossRef]
- Rabago, D.; Slattengren, A.; Zgierska, A. Prolotherapy in primary care practice. Prim. Care Clin. Off. Pract. 2010, 37, 65–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manoto, S.L.; Maepa, M.J.; Motaung, S.K. Medical ozone therapy as a potential treatment modality for regeneration of damaged articular cartilage in osteoarthritis. Saudi J. Biol. Sci. 2018, 25, 672–679. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, E. Pharmacologic and non-pharmacologic treatment of osteoarthritis. Curr. Treat. Options Rheumatol. 2016, 2, 111–125. [Google Scholar] [CrossRef] [Green Version]
- Derogatis, M.; Anis, H.K.; Sodhi, N.; Ehiorobo, J.O.; Chughtai, M.; Bhave, A.; Mont, M.A. Non-operative treatment options for knee osteoarthritis. Ann. Transl. Med. 2019, 7 (Suppl. 7), S245. [Google Scholar] [CrossRef]
- Goswami, A. Prolotherapy. J. Pain Palliat. Care Pharmacother. 2012, 26, 376–378. [Google Scholar] [CrossRef] [PubMed]
- Distel, L.M.; Best, T.M. Best, Prolotherapy: A clinical review of its role in treating chronic musculoskeletal pain. PM&R 2011, 3, S78–S81. [Google Scholar]
- Hauser, R.A.; Lackner, J.B.; Steilen-Matias, D.; Harris, D.K. A systematic review of dextrose prolotherapy for chronic musculoskeletal pain. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2016, 9, 139–159. [Google Scholar] [CrossRef] [Green Version]
- Clarkson, M.R.; Murphy, M.; Gupta, S.; Lambe, T.; Mackenzie, H.S.; Godson, C.; Martin, F.; Brady, H.R. High glucose-altered gene expression in mesangial cells: Actin-regulatory protein gene expression is triggered by oxidative stress and cytoskeletal disassembly. J. Biol. Chem. 2002, 277, 9707–9712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabago, D.; Nourani, B. Prolotherapy for osteoarthritis and tendinopathy: A descriptive review. Curr. Rheumatol. Rep. 2017, 19, 34. [Google Scholar] [CrossRef]
- Wee, T.C.; Neo, E.J.R.; Tan, Y.L. Dextrose prolotherapy in knee osteoarthritis: A systematic review and meta-analysis. J. Clin. Orthop. Trauma 2021, 19, 108–117. [Google Scholar] [CrossRef]
- Sit, R.W.; Chung, V.C.; Reeves, K.D.; Rabago, D.; Chan, K.K.; Chan, D.C.; Wu, X.; Ho, R.S.; Wong, S.Y. Hypertonic dextrose injections (prolotherapy) in the treatment of symptomatic knee osteoarthritis: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 2020, 72, 220–233. [Google Scholar] [CrossRef]
- Calunga, J.L.; Menéndez, S.; León, R.; Chang, S.; Guanche, D.; Balbín, A.; Zayas, J.; García, P. Application of ozone therapy in patients with knee osteoarthritis. Ozone Sci. Eng. Eng. 2012, 34, 469–475. [Google Scholar] [CrossRef]
- Sconza, C.; Respizzi, S.; Virelli, L.; Vandenbulcke, F.; Iacono, F.; Kon, E.; Di Matteo, B. Oxygen–ozone therapy for the treatment of knee osteoarthritis: A systematic review of randomized controlled trials. Arthrosc. J. Arthrosc. Relat. Surg. 2020, 36, 277–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bocci, V.A. Scientific and medical aspects of ozone therapy. State of the art. Arch. Med. Res. 2006, 37, 425–435. [Google Scholar] [CrossRef]
- Felson, D.T. The sources of pain in knee osteoarthritis. Curr. Opin. Rheumatol. 2005, 17, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Peat, G.; McCarney, R.; Croft, P. Croft, Knee pain and osteoarthritis in older adults: A review of community burden and current use of primary health care. Ann. Rheum. Dis. 2001, 60, 91–97. [Google Scholar] [CrossRef]
- Sit, R.W.S.; Wu, R.W.K.; Reeves, K.D.; Rabago, D.; Chan, D.C.C.; Yip, B.H.K.; Chung, V.C.H.; Wong, S.Y.-S. Efficacy of intra-articular hypertonic dextrose prolotherapy versus normal saline for knee osteoarthritis: A protocol for a triple-blinded randomized controlled trial. BMC Complementary Altern. Med. 2018, 18, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eroğlu, A.; Aylin, S.; Durmuş, B. Platelet-rich plasma vs prolotherapy in the management of knee osteoarthritis: Randomized placebo-controlled trial. Spor Hekim. Derg. 2016, 51, 34–43. [Google Scholar]
- Rabago, D.; Patterson, J.J.; Mundt, M.; Kijowski, R.; Grettie, J.; Segal, N.A.; Zgierska, A. Dextrose prolotherapy for knee osteoarthritis: A randomized controlled trial. Ann. Fam. Med. 2013, 11, 229–237. [Google Scholar] [CrossRef]
- Sert, A.T.; Sen, E.I.; Esmaeilzadeh, S.; Ozcan, E. The Effects of Dextrose Prolotherapy in Symptomatic Knee Osteoarthritis: A Randomized Controlled Study. J. Altern. Complement. Med. 2020, 26, 409–417. [Google Scholar] [CrossRef]
- Tüzün, E.; Eker, L.; Aytar, A.; Daşkapan, A.; Bayramoğlu, M. Acceptability, reliability, validity and responsiveness of the Turkish version of WOMAC osteoarthritis index. Osteoarthr. Cartil. 2005, 13, 28–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raposo, F.; Ramos, M.; Cruz, A.L. Effects of exercise on knee osteoarthritis: A systematic review. Musculoskelet. Care 2021, 5, 1–37. [Google Scholar]
- Rabago, D.; Mundt, M.; Zgierska, A.; Grettie, J. Hypertonic dextrose injection (prolotherapy) for knee osteoarthritis: Long term outcomes. Complement. Ther. Med. 2015, 23, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, B.; Taheri, M.; Ardekani, R.P.; Moradi, S.; Mofrad, M.K. Periarticular hypertonic dextrose vs. intraarticular hyaluronic acid injections: A comparison of two minimally invasive techniques in the treatment of symptomatic knee osteoarthritis. Open Access Rheumatol. Res. Rev. 2019, 11, 269. [Google Scholar] [CrossRef] [Green Version]
- Alexandre, E.B.A.; Alexandre, E.I.A.; Borrelli, E.; Iliakis, E.; Bocci, A.A.A.V. Disc herniation and knee arthritis as chronic oxidative stress diseases: The therapeutic role of oxygen ozone therapy. J. Arthritis 2015, 4, 161. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, X. Study on treatment for knee osteoarthritis by medical ozone. Gansu Med. J. 2010, 1, 10–11. [Google Scholar]
- Scassellati, C.; Galoforo, A.C.; Bonvicini, C.; Esposito, C.; Ricevuti, G. Ozone: A natural bioactive molecule with antioxidant property as potential new strategy in aging and in neurodegenerative disorders. Ageing Res. Rev. 2020, 63, 101138. [Google Scholar] [CrossRef]
- Goyal, N. Intraarticular Ozone Therapy for Knee Osteoarthritis: A Single Centre Experience. Call Editor. Board Memb. 2019, 6, 1387–1398. [Google Scholar] [CrossRef]
- Raeissadat, S.A.; Rayegani, S.M.; Forogh, B.; Abadi, P.H.; Moridnia, M.; Dehgolan, S.R. Intra-articular ozone or hyaluronic acid injection: Which one is superior in patients with knee osteoarthritis? A 6-month randomized clinical trial. J. Pain Res. 2018, 11, 111. [Google Scholar] [CrossRef] [Green Version]
- Hashemi, M.; Jalili, P.; Mennati, S.; Koosha, A.; Rohanifar, R.; Madadi, F.; Razavi, S.S.; Taheri, F. The effects of prolotherapy with hypertonic dextrose versus prolozone (intraarticular ozone) in patients with knee osteoarthritis. Anesthesiol. Pain Med. 2015, 5, e27585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, S.K.; Pramanik, R.; Das, P.; Das, P.P.; Palit, A.K.; Roy, J.; Halder, R.N. Role of intra-articular ozone in osteo-arthritis of knee for functional and symptomatic improvement. Ind. J. Phys. Med. Rehabilit 2011, 22, 65–69. [Google Scholar]
- Lopes de Jesus, C.C.; Dos Santos, F.C.; de Jesus, L.M.O.B.; Monteiro, I.; Sant’Ana, M.S.S.C.; Trevisani, V.F.M. Comparison between intra-articular ozone and placebo in the treatment of knee osteoarthritis: A randomized, double-blinded, placebo-controlled study. PLoS ONE 2017, 12, e0179185. [Google Scholar] [CrossRef] [Green Version]
- Duymus, T.M.; Mutlu, D.T.; Dernek, B.; Komur, B.; Aydogmus, S.; Kesiktas, F.N. Choice of intra-articular injection in treatment of knee osteoarthritis: Platelet-rich plasma, hyaluronic acid or ozone options. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, M.; Stagno, D.; Carda, S.; Grana, E.; Picelli, A.; Smania, N.; Cisari, C.; Baricich, A. Safety of intra-articular oxygen-ozone therapy compared to intra-articular sodium hyaluronate in knee osteoarthritis: A randomized single blind pilot study. Int. J. Phys. Med. Rehabil. 2017, 5, 2. [Google Scholar]



| Characteristics and Clinical Features | Total Patients with KOA (n = 75) | Dextrose Prolotherapy (n = 25) | Ozone Therapy (n = 25) | Home-Based Exercise Therapy (n = 25) | p |
|---|---|---|---|---|---|
| Age (years) | 56.7 ± 7.3 | 56.6 ± 7.1 | 57.0 ± 7.6 | 56.5 ± 7.4 | 0.967 |
| Female sex—n (%) | 64 (%85.3) | 21 (%84) | 22 (%88) | 21 (%84) | 0.899 |
| Smoker—n (%) | 6 (%8) | 2 (%8) | 1 (%4) | 3 (%12) | 0.600 |
| BMI (kg/m2) | 33.10 ± 4.98 | 34.3 ± 4.6 | 33.2 ± 4.4 | 32.2 ± 5.4 | 0.245 |
| Duration of clinical disease (months) | 33.43 ± 29.47 | 35.1 ± 29.6 | 34.3 ± 27.6 | 30.8 ± 31.9 | 0.864 |
| More symptomatic knee, n (%) | 0.288 | ||||
| Right | 51 (%68) | 18 (%72) | 17 (%68) | 16 (%64) | |
| Left | 24 (%32) | 7 (%28) | 8 (%32) | 9 (%36) | |
| Grade of KOA, n (%) | 0.958 | ||||
| Grade II | 46 (%61.3) | 16 (%64) | 15 (%60) | 15 (%60) | |
| Grade III | 29 (%38.7) | 9 (%36) | 10 (%40) | 10 (%40) | |
| VAS-Rest | Groups | p | η2 | ||
|---|---|---|---|---|---|
| Dextrose Prolotherapy (n = 25) | Ozone Therapy (n = 25) | Home-Based Exercise Therapy (n = 25) | |||
| Baseline value | 5.08 ± 2.06 | 9.71 ± 0.55 | 5.84 ± 2.70 | <0.01 ** a 0.376 b <0.01 ** c <0.01 ** | 0.513 |
| 6th week | −1.44 ± 1.73 | −7.08 ± 2.01 | −0.56 ± 1.66 | <0.01 ** a 0.216 b <0.01 ** c <0.01 ** | 0.217 |
| 12th week | −2.08 ± 2.04 | −7.08 ± 2.01 | −1.68 ± 1.70 | 0.045 * a 0.744 b <0.01 ** c <0.01 ** | 0.108 |
| p | <0.01 ** | <0.01 ** | <0.01 ** | ||
| Baseline-6th week | d 0.01 ** | d <0.01 ** | d 0.314 | ||
| Baseline-12th week | d <0.01 ** | d <0.01 ** | d <0.01 ** | ||
| VAS-movement | |||||
| Baseline | 7.92 ± 1.77 | 9.75 ± 0.53 | 8.20 ± 1.32 | <0.01 ** a 0.733 b <0.01 ** c <0.01 ** | 0.276 |
| 6th week | −2.48 ± 1.71 | −5.96 ± 1.96 | −1.60 ± 1.97 | <0.01 ** a 0.233 b <0.01 ** c <0.01 ** | 0.296 |
| 12th week | −4.00 ± 2.27 | −5.88 ± 1.98 | −2.12 ± 1.59 | <0.01 ** a 0.003 ** b <0.01 ** c 0.003 ** | 0.288 |
| p | <0.01 ** | <0.01 ** | <0.01 ** | ||
| Baseline-6th week | d 0.01 ** | d 0.01 ** | d 0.01 ** | ||
| Baseline-12th week | d <0.01 ** | d <0.01 ** | d <0.01 ** | ||
| WOMAC-Stiffness | |||||
| Baseline | 4.20 ± 1.80 | 5.21 ± 1.81 | 4.72 ± 2.01 | 0.120 | 0.047 |
| 6th week | −1.52 ± 1.63 | −2.96 ± 1.45 | −0.40 ± 1.44 | <0.01 ** a 0.029 * b <0.01 ** c 0.004 ** | 0.204 |
| 12th week | −1.80 ± 1.75 | −2.96 ± 1.45 | −1.12 ± 1.61 | 0.001 ** a 0.302 b <0.01 ** c 0.035 * | 0.128 |
| p | <0.01 ** | <0.01 ** | <0.003 ** | ||
| Baseline-6th week | d <0.01 ** | d <0.01 ** | d <0.536 | ||
| Baseline-12th week | d <0.01 ** | d <0.01 ** | d <0.006 ** | ||
| WOMAC-function | |||||
| Baseline | 38.60 ± 11.81 | 39.50 ± 6.69 | 40.00 ± 15.33 | 0.898 | 0.003 |
| 6th week | −12.64 ± 11.79 | −17.56 ± 5.03 | −6.48 ± 9.88 | <0.01 ** a 0.058 b <0.01 ** c 0.158 | 0.148 |
| 12th week | −18.64 ± 13.13 | −17.56 ± 5.02 | −10.04 ± 9.26 | 0.005 ** a 0.007 ** b 0.021 * c 0.919 | 0.158 |
| p | <0.01 ** | <0.01 ** | <0.01 ** | ||
| Baseline-6th week | d <0.01 ** | d <0.01 ** | d 0.01 ** | ||
| Baseline-12th week | d <0.01 ** | d <0.01 ** | d <0.01 ** | ||
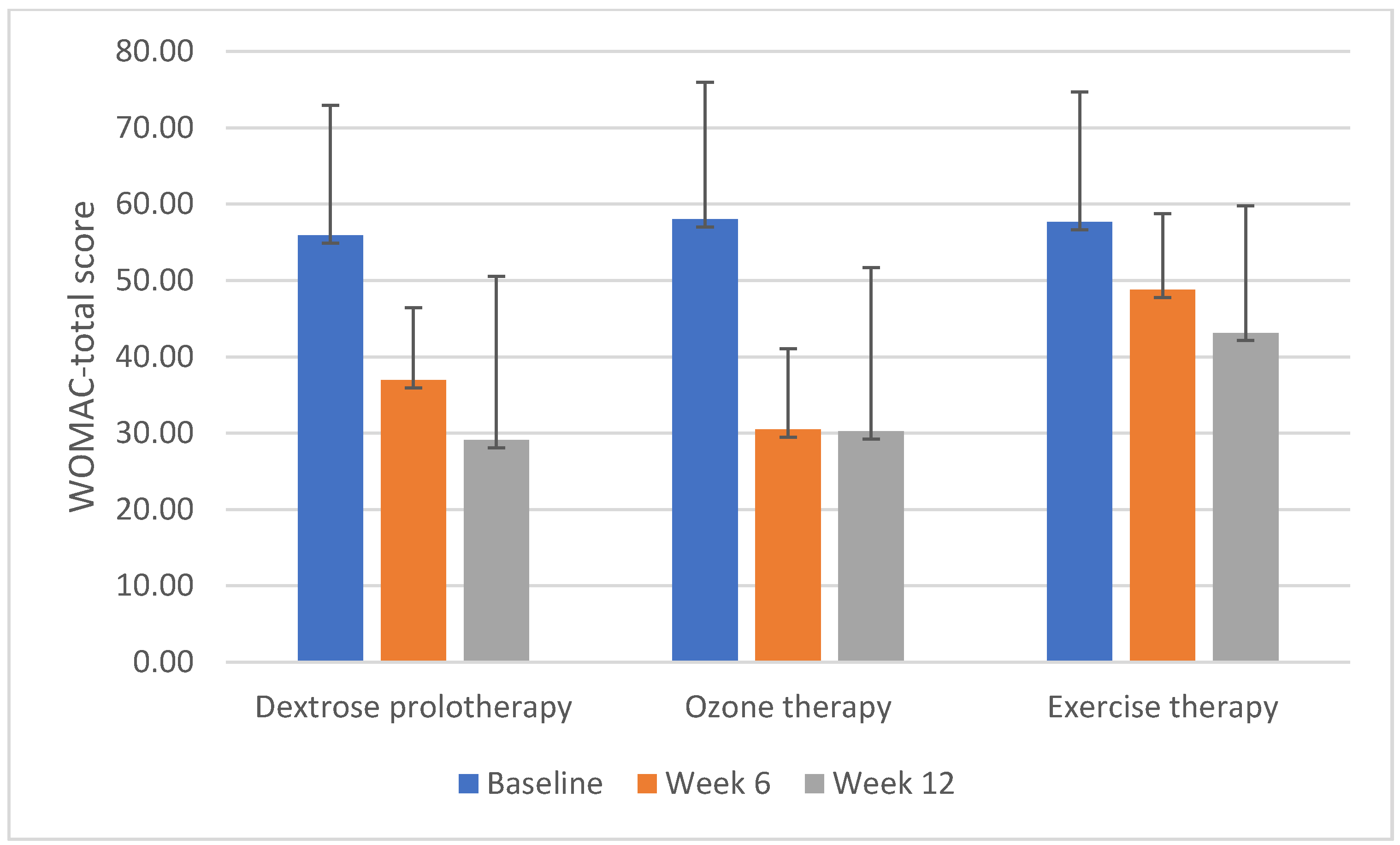
| WOMAC-total | |||||
| Baseline | 55.90 ± 17.02 | 58.00 ± 9.48 | 57.64 ± 21.45 | 0.844 | 0.003 |
| 6th week | −18.93 ± 15.71 | −26.80 ± 7.90 | −8.86 ± 14.60 | <0.01 ** a 0.053 b 0.003 ** c 0.562 | 0.166 |
| 12th week | −26.78 ± 17.91 | −27.04 ± 8.16 | −14.49 ± 13.78 | 0.002 ** a 0.023 * b <0.01 ** c 0.096 | 0.160 |
| p | <0.01 ** | <0.01 ** | <0.01 ** | ||
| Baseline-6th week | d <0.01 ** | d <0.01 ** | d 0.017 * | ||
| Baseline-12th week | d <0.01 ** | d <0.01 ** | d <0.01 ** |
| TUG | Groups | p | η2 | ||
|---|---|---|---|---|---|
| Dextrose Prolotherapy (n = 25) | Ozone Therapy (n = 25) | Home-Based Exercise Therapy (n = 25) | |||
| Baseline | 11.83 ± 2.28 | 13.82 ± 2.55 | 12.55 ± 2.91 | 0.019 * a 0.591 b 0.157 c 0.016 * | 0.094 |
| 6th week | −0.56 ± 0.90 | −0.79 ± 0.72 | −0.85 ± 1.36 | 0.588 | 0.082 |
| 12th week | −0.86 ± 1.19 | −1.31 ± 0.82 | −0.63 ± 1.30 | 0.102 | 0.067 |
| p | 0.030 * | <0.01 ** | 0.019 * | ||
| Baseline-6th week | d 0.013 * | d <0.01 ** | d 0.013 * | ||
| Baseline-12th week | d 0.004 ** | d <0.01 ** | d 0.067 | ||
| ROM-active | |||||
| Baseline | 126.0 ± 13.84 | 125.83 ± 9.96 | 129.80 ± 10.55 | 0.409 | 0.025 |
| 6th week | 6.40 ± 7.84 | 4.80 ± 7.42 | 2.28 ± 4.99 | 0.109 | 0.006 |
| 12th week | 9.40 ± 6.97 | 8.60 ± 6.04 | 3.80 ± 5.45 | 0.004 ** a 0.006 ** b 0.021 * c 0.891 | 0.009 |
| p | <0.01 ** | <0.01 ** | 0.006 ** | ||
| Baseline-6th week | d 0.01 ** | d 0.01 ** | d 0.095 | ||
| Baseline-12th week | d <0.01 ** | d <0.01 ** | d 0.006 ** | ||
| ROM-passive | |||||
| Baseline | 133.68 ± 10.83 | 132.92 ± 9.88 | 136.32 ± 5.97 | 0.366 | 0.026 |
| 6th week | 3.12 ± 5.66 | 3.20 ± 5.75 | 1.16 ± 3.81 | 0.291 | 0.006 |
| 12th week | 3.32 ± 5.82 | 4.80 ± 6.37 | 1.88 ± 3.76 | 0.172 | 0.009 |
| p | 0.024 * | 0.050* | 0.058 | ||
| Baseline-6th week | d 0.033 * | d 0.031 * | d 0.425 | ||
| Baseline-12th week | d 0.026 * | d 0.003 ** | d 0.060 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baygutalp, F.; Çelik, M.; Öztürk, M.U.; Yayık, A.M.; Ahıskalıoğlu, A. Comparison of the Efficacy of Dextrose Prolotherapy and Ozone in Patients with Knee Osteoarthritis: A Randomized Cross-Sectional Study. Appl. Sci. 2021, 11, 9991. https://doi.org/10.3390/app11219991
Baygutalp F, Çelik M, Öztürk MU, Yayık AM, Ahıskalıoğlu A. Comparison of the Efficacy of Dextrose Prolotherapy and Ozone in Patients with Knee Osteoarthritis: A Randomized Cross-Sectional Study. Applied Sciences. 2021; 11(21):9991. https://doi.org/10.3390/app11219991
Chicago/Turabian StyleBaygutalp, Fatih, Mine Çelik, Muhammet Uğur Öztürk, Ahmet Murat Yayık, and Ali Ahıskalıoğlu. 2021. "Comparison of the Efficacy of Dextrose Prolotherapy and Ozone in Patients with Knee Osteoarthritis: A Randomized Cross-Sectional Study" Applied Sciences 11, no. 21: 9991. https://doi.org/10.3390/app11219991





