The Use of Silicones as Extractants of Biologically Active Substances from Vegetable Raw Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Characterization of Silicones
2.2.2. Extraction Procedure
2.2.3. Characterization of the Extracts
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Coiai, S.; Campanella, B.; Paulert, R.; Cicogna, F.; Bramanti, E.; Lazzeri, A.; Pistelli, L.; Coltelli, M.B. Rosmarinic Acid and Ulvan from Terrestrial and Marine Sources in Anti-Microbial Bionanosystems and Biomaterials. Appl. Sci. 2021, 11, 9249. [Google Scholar] [CrossRef]
- Turrini, F.; Beruto, M.; Mela, L.; Curir, P.; Triglia, G.; Boggia, R.; Zunin, P.; Monroy, F. Ultrasound-Assisted Extraction of Lavender (Lavandula angustifolia Miller, Cultivar Rosa) Solid By-Products Remaining after the Distillation of the Essential Oil. Appl. Sci. 2021, 11, 5495. [Google Scholar] [CrossRef]
- Cavallaro, G.; Milioto, S.; Konnova, S.; Fakhrullina, G.; Akhatova, F.; Lazzara, G.; Fakhrullin, R.; Lvov, Y. Halloysite/Keratin Nanocomposite for Human Hair Photoprotection Coating. ACS Appl. Mater. Interfaces 2020, 12, 24348–24362. [Google Scholar] [CrossRef]
- Harhaun, R.; Kunik, O.; Saribekova, D.; Lazzara, G. Biologically active properties of plant extracts in cosmetic emulsions. Microchem. J. 2020, 154, 104543. [Google Scholar] [CrossRef] [Green Version]
- Lisuzzo, L.; Cavallaro, G.; Pasbakhsh, P.; Milioto, S.; Lazzara, G. Why does vacuum drive to the loading of halloysite nanotubes? The key role of water confinement. J. Colloid Interface Sci. 2019, 547, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Halloysite nanotubes filled with salicylic acid and sodium diclofenac: Effects of vacuum pumping on loading and release properties. J. Nanostruct. Chem. 2021. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Halloysite nanotubes coated by chitosan for the controlled release of khellin. Polymers 2020, 12, 1766. [Google Scholar] [CrossRef]
- Monsanto, M.; Mestrom, R.; Zondervan, E.; Bongers, P.; Meuldijk, J. Solvent swing adsorption for the recovery of polyphenols from Black Tea. Ind. Eng. Chem. Res. 2015, 54, 434–442. [Google Scholar] [CrossRef]
- Vergara-Salinas, J.R.; Pérez-Jiménez, J.; Torres, J.L.; Agosin, E.; Pérez-Correa, J.R. Effects of temperature and time on polyphenolic content and antioxidant activity in the pressurized hot water extraction of Deodorized Thyme (Thymus vulgaris). Agric. Food Chem. 2012, 60, 10920–10929. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Bergeron, C.; Gafner, S.; Clausen, E.; Carrier, D.J. Comparison of the chemical composition of extracts from Scutellaria lateriflora using accelerated solvent extraction and supercritical fluid extraction versus standard hot water or 70% ethanol extraction. J. Agric. Food Chem. 2005, 53, 3076–3080. [Google Scholar] [CrossRef]
- Gupta, R.; Badhe, R.B.Y.; Mitragotri, S. Molecular mechanism of the skin permeation enhancing effect of ethanol: A molecular dynamics study. RSC Adv. 2020, 10, 12234–12248. [Google Scholar] [CrossRef] [Green Version]
- Shanmugam, H.; Jayaprakasha, G.K.; Patil, B.S. Extraction and identification of health-promoting phytochemicals from Brussels Sprouts. Adv. Plant Phenolics Chem. Hum. Health 2018, 8, 151–174. [Google Scholar]
- Serna, J.; Narváez Rincón, P.C.; Falk, V.; Boly, V.; Camargo, M. Methodology for emulsion design based on emulsion science and expert knowledge. Part 2: An application in the cosmetics sector. Ind. Eng. Chem. Res. 2021, 60, 5220–5235. [Google Scholar] [CrossRef]
- Ferritto, M.S.; Owen, M.J. Silicone wettability and its significance in beauty products. Polym. Pers. Care Cosmet. 2013, 14, 219–232. [Google Scholar]
- Kunik, O.; Saribyekova, D.; Saleba, L.; Ivakhnenko, H.; Panchenko, Y. Research of physical and chemical properties of cosmetic emulsions of oil in water type based on polyorganosyloxanes and their alternative substitute. Chem. Chem. Technol. 2019, 13, 526–534. [Google Scholar] [CrossRef]
- Rücker, C.; Kümmerer, K. Environmental Chemistry of Organosiloxanes. Chem. Rev. 2015, 115, 466–524. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.; Génot, C.; Rodriguez, C.; Magniez, H.; Lacourt, S.; Fievez, A.; Len, C.; Pezron, I.; Luart, D.; Hecke, E. Emollients for cosmetic formulations: Towards relationships between physico-chemical properties and sensory perceptions. Colloids Surf. A Physicochem. Eng. Asp. 2018, 536, 156–164. [Google Scholar] [CrossRef]
- Bhagyaraj, S.; Oluwafemi, O.S.; Krupa, I. Chapter 13—Polymers in optics. In Polymer Science and Innovative Applications, Materials, Techniques, and Future Developments; Elsevier: Amsterdam, The Netherlands, 2020; pp. 423–455. [Google Scholar]
- Viana, M.; Jouannin, P.; Pontier, C.; Chulia, D. About pycnometric density measurements. Talanta 2002, 57, 583–593. [Google Scholar] [CrossRef]
- Koekemoer, L.R.; Badenhorst, M.J.G.; Everson, R.C. Determination of viscosity and density of di-(2-ethylhexyl) phosphoric acid + aliphatic kerosene. J. Chem. Eng. Data 2005, 50, 587–590. [Google Scholar] [CrossRef]
- Carvalho, T.C.; Horng, M.; McConville, J.T. Application of a pull on a disk method to measure surface tension of liquids. AAPS PharmSciTech 2012, 13, 305–312. [Google Scholar] [CrossRef] [Green Version]
- Bryk, P.; Korczeniewski, E.; Szyma’nski, G.S.; Kowalczyk, P.; Terpiłowski, K.; Terzyk, P. What is the value of water contact angle on silicon? Materials 2020, 13, 1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeidler, U.; Henkel, H.K. Über das spreiten von lipiden auf der haut. Eur. J. Lipid Sci. Technol. 1985, 87, 373–426. [Google Scholar] [CrossRef]
- Levine, V.E. The vitamins. I. The fat-soluble vitamins. J. Chem. Educ. 1935, 12, 357. [Google Scholar] [CrossRef]
- Spínola, V.; Mendes, B.; Câmara, J.S.; Castilho, P.C. Effect of time and temperature on vitamin C stability in horticultural extracts. UHPLC-PDA vs. iodometric titration as analytical methods. LWT–Food Sci. Technol. 2013, 50, 489–495. [Google Scholar] [CrossRef]
- The general methods of analysis: Determination of the content of tannins in medicinal plant raw materials. In The State Pharmacopoeia of the USSR, 11th ed.; Medicine: Moscow, Russia, 1987; Volume 1, p. 335.
- Faria, A.; Oliveira, J.; Neves, P.; Gameiro, P.; Santos-Buelga, C.; Freitas, V.; Mateus, N. Antioxidant properties of prepared blueberry (Vaccinium Myrtillus) extracts. J. Agric. Food Chem. 2005, 53, 6896–6902. [Google Scholar] [CrossRef]
- Pizzi, A. Tannins medical/pharmacological and related applications: A critical review. Sustain. Chem. Pharm. 2021, 22, 100481. [Google Scholar] [CrossRef]

| Chemical Structure | Chemical Structure |
|---|---|
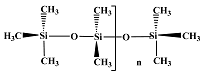 Silicone Oil 350 cst | 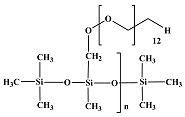 BRB 526 |
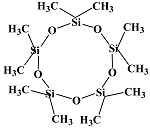 BRB CM 50 |  BRB 1834 |
 BRB PTM 20 | 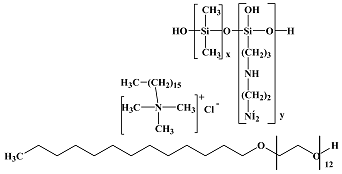 BRB 1288 |
| Indicator, Unit of Measurement | Traditional Extractants | Polyorganosiloxanes | ||||||
|---|---|---|---|---|---|---|---|---|
| Distilled Water | 70% Aqueous Alcoholic Solution | BRB CM 50 | BRB PTM 20 | Silicone Oil 350 cSt | BRB 526 | BRB 1834 | BRB 1288 | |
| Appearance | colorless, clear liquid | colorless, clear liquid | colorless, opaque, viscous liquid | white liquid | ||||
| Refractive index at 25 °C | 1.333 | 1.363 | 1.397 | 1.460 | 1.403 | 1.460 | 1.403 | 1.481 |
| Density at 25 °C, g/cm3 | 1.000 | 0.932 | 0.958 | 0.988 | 0.972 | 1.072 | 0.960 | 0.992 |
| Viscosity at 25 °C, mm2/s | 0.89 | 2.52 | 4.00 | 22.50 | 367.50 | 260.00 | 6000.00 | 5.00 |
| Surface tension at 22 °C, ×10−3 N/m | 72.40 | 30.96 | 18.54 | 21.89 | 21.10 | 18.81 | 19.10 | 27.12 |
| Cosine of the edge angle of wetting, cos θ | 0.121 (83°) | 0.648 (50°) | 0.971 (14°) | 0.891 (27°) | 0.945(19°) | 0.941 (19°) | 0.280 (73°) | 0.800 (36°) |
| Relative spreading, mm2/10 min | 70.85 | 86.55 | 660.19 | 580.77 | 482.81 | 103.82 | 195.97 | 298.50 |
| Substance | Polyorganosiloxanes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Silicone Oil 350 cSt | BRB CM 50 | BRB PTM 20 | BRB 526 | BRB 1834 | BRB 1288 | |||||||
| c | w | c | w | c | w | c | w | c | w | c | w | |
| B1, thiamine | + | + | + | + | + | + | + | + | + | + | + | + |
| B2, riboflavin | − | − | − | − | − | − | − | − | − | − | − | − |
| B5, pantothenic acid | + | + | + | + | + | + | + | + | + | + | + | + |
| B6, pyridoxine | − | − | − | − | − | − | − | − | − | − | − | − |
| PP, nicotinamide | + | + | + | + | + | + | + | + | + | + | + | + |
| P, routine | + | + | + | + | + | + | + | + | + | + | + | + |
| C, ascorbic acid | + | + | + | + | + | + | + | + | + | + | + | + |
| A, retinol | − | − | − | − | − | − | − | − | − | − | + | + |
| E, tocopherol | − | − | − | − | − | − | − | − | − | − | + | + |
| General reactions to: | ||||||||||||
| –flavonoids | + | + | + | + | + | + | + | + | + | + | + | |
| –tannins | + | + | + | + | + | + | + | + | + | + | + | + |
| –glycosides | − | − | + | + | − | + | + | + | + | + | + | + |
| Substance | Aqueous Extract | Polyorganosiloxane | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Silicone Oil 350 cSt | BRB CM 50 | BRB PTM 20 | BRB 526 | BRB 1834 | BRB 1288 | |||||||||
| c | w | c | w | c | w | c | w | c | w | c | w | c | w | |
| Vitamin C, g/L | 13.20 | 8.80 | 0.60 | 0.52 | 2.00 | 3.20 | 2.71 | 0.50 | 113.70 | 164.90 | 3.62 | 2.11 | 15.72 | 41.91 |
| The quantity of: flavonoids, % | 5.09 | 0.61 | 13.71 | 16.29 | 13.14 | 13.89 | 24.51 | 24.89 | 23.87 | 19.85 | 13.93 | 13.46 | 45.12 | 40.48 |
| Tannins, % | 0.73 | 1.45 | 0.52 | 0.34 | 0.31 | 0.15 | 0.26 | 0.24 | 1.69 | 1.39 | 0.68 | 0.25 | 0.59 | 0.65 |
| Extractives, % | 0.10 | 0.10 | 2.09 | 3.12 | 2.11 | 3.10 | 2.21 | 3.32 | 2.52 | 3.20 | 2.26 | 3.43 | 3.03 | 3.98 |
| Extract | Solution of Comparison | Aqueous Extract | Polyorganosiloxanes | |||||
|---|---|---|---|---|---|---|---|---|
| Silicone Oil 350 cSt | BRB CM 50 | BRB PTM 20 | BRB 526 | BRB 1834 | BRB 1288 | |||
| Optical Density, D | ||||||||
| Calendula | 0.770 | 0.711 | 0.457 | 0.221 | 0.218 | >3 | 1.507 | >3 |
| Wormwood | 0.657 | 0.451 | 0.254 | 0.204 | >3 | 1.111 | >3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saribekova, D.; Kunik, O.; Harhaun, R.; Saleba, L.; Cavallaro, G. The Use of Silicones as Extractants of Biologically Active Substances from Vegetable Raw Materials. Appl. Sci. 2021, 11, 10625. https://doi.org/10.3390/app112210625
Saribekova D, Kunik O, Harhaun R, Saleba L, Cavallaro G. The Use of Silicones as Extractants of Biologically Active Substances from Vegetable Raw Materials. Applied Sciences. 2021; 11(22):10625. https://doi.org/10.3390/app112210625
Chicago/Turabian StyleSaribekova, Diana, Oleksandra Kunik, Ruslana Harhaun, Ludmila Saleba, and Giuseppe Cavallaro. 2021. "The Use of Silicones as Extractants of Biologically Active Substances from Vegetable Raw Materials" Applied Sciences 11, no. 22: 10625. https://doi.org/10.3390/app112210625
APA StyleSaribekova, D., Kunik, O., Harhaun, R., Saleba, L., & Cavallaro, G. (2021). The Use of Silicones as Extractants of Biologically Active Substances from Vegetable Raw Materials. Applied Sciences, 11(22), 10625. https://doi.org/10.3390/app112210625






