Anti-Yeast Synergistic Effects and Mode of Action of Australian Native Plant Essential Oils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Essential Oils and Other Chemicals
2.2. Microorganisms
2.3. Determination of Antimicrobial Activity
2.4. Ergosterol Binding Assay
2.5. Sorbitol Osmotic Protection Assay
2.6. Leakage of Potassium and Magnesium Ions
2.7. Synergy Checkerboard (Interactions between EOs)
2.8. Fluorescence and Scanning Electron Microscopy
2.9. Fluorescence Microscopy and Yeast Cell Staining
2.10. Scanning Electron Microscopy
2.11. Gas Chromatography (GC)–Mass Spectrometry Analysis
2.12. Statistical Analysis
3. Results
3.1. Antimicrobial Properties
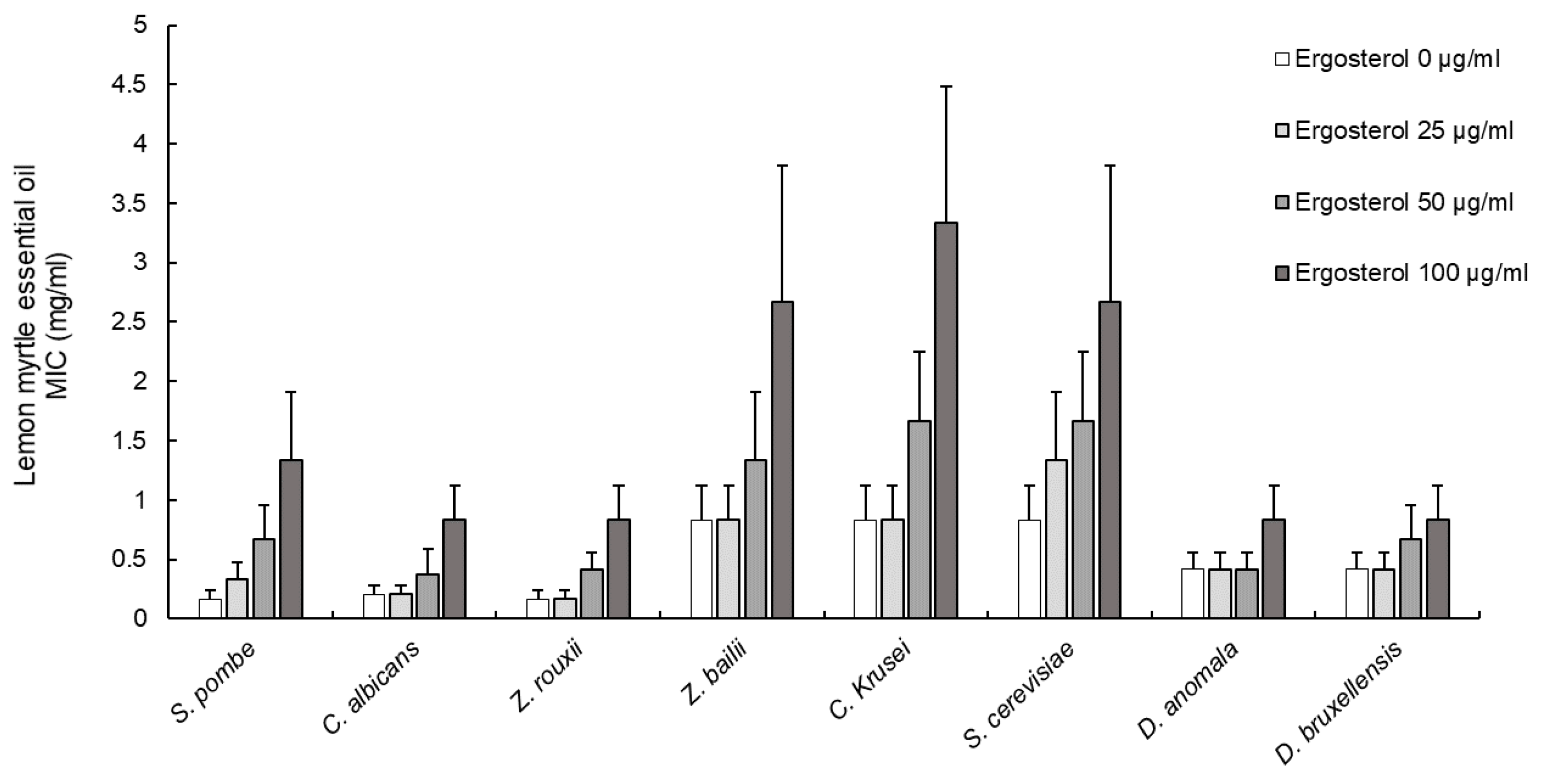
3.2. Effect of Ergosterol on Yeast Cell Membranes
3.3. Effect of Sorbitol on Yeast Cell Wall
3.4. Quantification of Cellular Potassium and Magnesium Ions
3.5. Synergic Effects of EOs
3.6. Microscopy of Yeast Cell Structure (Untreated and Treated)
3.7. Identification of the Main Bioactive Compounds in the Studied Eos
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Rhind, J. From Historical Origins to the Present Day: The Philosophies of the Pioneers and Influential Thinkers. In Essential Oils: A Handbook for Aromatherapy Practice; Singing Dragon: London, UK; Philadelphia, PA, USA, 2012; Volume 2, pp. 12–23. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, H.A.E.; Shibamoto, T.; El-Ghorab, A.H. Bioactivity of essential oils and their volatile aroma components: Review. J. Essent. Oil Res. 2012, 24, 203. [Google Scholar] [CrossRef]
- Van de Braak, S.; Leijten, G. Essential Oils and Oleoresins: A Survey in The Netherlands and Other Major Markets in the European Union; CBI, Centre for the Promotion of Imports from Developing Countries: Rotterdam, The Netherlands, 1994. [Google Scholar]
- Ndagijimana, M.; Belletti, N.; Lanciotti, R.; Guerzoni, M.; Ardini, F. Effect of Aroma Compounds on the Microbial Stabilization of Orange-based Soft Drinks. J. Food Sci. 2004, 69, SNQ20–SNQ24. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Valdramidis, V.P.; O’Donnell, C.P.; Muthukumarappan, K.; Bourke, P.; Cullen, P.J. Application of natural antimicrobials for food preservation. J. Agric. Food Chem. 2009, 57, 5987. [Google Scholar] [CrossRef] [Green Version]
- Tajkarimi, M.M.; Ibrahim, S.A.; Cliver, D.O. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Saad, N.Y.; Muller, C.D.; Lobstein, A. Major bioactivities and mechanism of action of essential oils and their components. Flavour Fragr. J. 2013, 28, 269–279. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Stratford, M.; James, S.A. Non-alcoholic beverages and yeasts. In Yeasts in Food: Beneficial and Detrimental Aspects; Boekhout, T., Robert, V., Eds.; Woodhead Publishing Ltd.: Cambridge, UK, 2003; pp. 309–345. [Google Scholar]
- Stratford, M.; Steels, H.; Nebe-von-Caron, G.; Novodvorska, M.; Hayer, K.; Archer, D.B. Extreme resistance to weak-acid preservatives in the spoilage yeast Zygosaccharomyces bailii. Int. J. Food Microbiol. 2013, 166, 126–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Divol, B.; du Toit, M.; Duckitt, E. Surviving in the presence of sulphur dioxide: Strategies developed by wine yeasts. Appl. Microbiol. Biotechnol. 2012, 95, 601–613. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Spoilage of stored, processed and preserved foods. In Fungi and Food Spoilage, 3rd ed.; Springer: New York, NY, USA, 2009; pp. 401–419. [Google Scholar]
- Hayashi, M.; Naknukool, S.; Hayakawa, S.; Ogawa, M.; Ni’matulah, A.-B.A. Enhancement of antimicrobial activity of a lactoperoxidase system by carrot extract and β-carotene. Food Chem. 2012, 130, 541–546. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [Green Version]
- Krisch, J.; Rentsenkhand, T.; Vágvölgyi, C. Essential oils against yeast and moulds causing food spoilage. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances Microbiology; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2011; pp. 1135–1142. [Google Scholar]
- Pengelly, A. Indigenous and naturalised herbs: Tasmannia lanceolata: Mountain pepper. Aust. J. Med. Herbal. 2002, 14, 71–74. [Google Scholar]
- Clarke, M. Australian Native Food Industry Stocktake; RIRDC Publication No. 12/066; Union Offset Printing: Canberra, Australia, 2012. [Google Scholar]
- Fujita, K.-I.; Kubo, I. Naturally occurring antifungal agents against Zygosaccharomyces bailii and their synergism. J. Agric. Food Chem. 2005, 53, 5187–5191. [Google Scholar] [CrossRef]
- Kubo, I.; Fujita, K.I.; Lee, S.H.; Ha, T.J. Antibacterial activity of polygodial. Phytother. Res. 2005, 19, 1013–1017. [Google Scholar] [CrossRef]
- Menary, R.C.; Dragar, V.A.; Thomas, S.; Read, C.D. Mountain Pepper Extract, Tasmannia Lanceolata: Quality Stabilisation and Registration: A Report for the Rural Industries Research and Development Corporation; RIRDC: Barton, Australia, 2003. [Google Scholar]
- Sultanbawa, Y. Tasmanian Pepper Leaf (Tasmannia lanceolata) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 819–823. [Google Scholar]
- Cock, I.E. The phytochemistry and chemotherapeutic potential of Tasmannia lanceolata (Tasmanian pepper): A review. Pharmacogn. Commun. 2013, 3, 13–25. [Google Scholar]
- Buchaillot, A.; Caffin, N.; Bhandari, B. Drying of lemon myrtle (Backhousia citriodora) leaves: Retention of volatiles and color. Dry. Technol. 2009, 27, 445–450. [Google Scholar] [CrossRef]
- Jones, G.L. Asia and Australia–Essentially a Well Oiled Connection. In Aromatic Plants from Asia, their Chemistry and Application in Food and Therapy; Jirovetz, L., Dung, N.X., Varshney, V.K., Eds.; Har Krishnan Bhalla: Dehradun, India, 2007; p. 247. [Google Scholar]
- Scheman, A.; Scheman, N.; Rakowski, E.-M. European directive fragrances in natural products. Dermatitis 2014, 25, 51–55. [Google Scholar] [CrossRef]
- Ress, N.; Hailey, J.; Maronpot, R.; Bucher, J.; Travlos, G.; Haseman, J.; Orzech, D.; Johnson, J.; Hejtmancik, M. Toxicology and carcinogenesis studies of microencapsulated citral in rats and mice. Toxicol. Sci. 2003, 71, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Sultanbawa, Y. Lemon Myrtle (Backhousia citriodora) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 517–520. [Google Scholar]
- Forbes-Smith, M.; Paton, J. Innovative products from Australian native foods. In Rural Industries Research and Development Corporation; RIRDC: Barton, Australia, 2002; pp. 1–78. [Google Scholar]
- Blewitt, M.; Southwell, I.A. Backhousia anisata Vickery, an alternative source of (E)-anethole. J. Essent. Oil Res. 2000, 12, 445–454. [Google Scholar] [CrossRef]
- Konczak, I.; Zabaras, D.; Dunstan, M.; Aguas, P. Antioxidant capacity and phenolic compounds in commercially grown native Australian herbs and spices. Food Chem. 2010, 122, 260–266. [Google Scholar] [CrossRef]
- Brophy, J.J.; Boland, D.J. The leaf essential oil of two chemotypes of Backhousia anisata Vickery. Flavour Fragr. J. 1991, 6, 187–188. [Google Scholar] [CrossRef]
- Ahmad, A.; Viljoen, A. The in vitro antimicrobial activity of Cymbopogon essential oil (lemon grass) and its interaction with silver ions. Phytomedicine 2015, 22, 657–665. [Google Scholar] [CrossRef]
- Lima, I.O.; Pereira, F.D.O.; Oliveira, W.A.D.; Lima, E.D.O.; Menezes, E.A.; Cunha, F.A.; Diniz, M.D.F.F.M. Antifungal activity and mode of action of carvacrol against Candida albicans strains. J. Essent. Oil Res. 2013, 25, 138–142. [Google Scholar] [CrossRef]
- Frost, D.J.; Brandt, K.D.; Cugier, D.; Goldman, R. A whole-cell Candida albicans assay for the detection of inhibitors towards fungal cell wall synthesis and assembly. J. Antibiot. 1995, 48, 306–310. [Google Scholar] [CrossRef] [Green Version]
- Prashar, A.; Hili, P.; Veness, R.G.; Evans, C.S. Antimicrobial action of palmarosa oil (Cymbopogon martinii) on Saccharomyces cerevisiae. Phytochemistry 2003, 63, 569–575. [Google Scholar] [CrossRef]
- Zengin, H.; Baysal, A.H. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef] [Green Version]
- Shimada, S.; Andou, M.; Naito, N.; Yamada, N.; Osumi, M.; Hayashi, R. Effects of hydrostatic pressure on the ultrastructure and leakage of internal substances in the yeast Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 1993, 40, 123–131. [Google Scholar] [CrossRef]
- Chaliha, M.; Cusack, A.; Currie, M.; Sultanbawa, Y.; Smyth, H. Effect of packaging materials and storage on major volatile compounds in three Australian native herbs. J. Agric. Food. Chem. 2013, 61, 5738–5745. [Google Scholar] [CrossRef] [PubMed]
- Karapinar, M.; Aktuǧ, Ş.E. Inhibition of foodborne pathogens by thymol, eugenol, menthol and anethole. Int. J. Food Microbiol. 1987, 4, 161–166. [Google Scholar] [CrossRef]
- Hançer Aydemir, D.; Cifci, G.; Aviyente, V.; Boşgelmez-Tinaz, G. Quorum-sensing inhibitor potential of trans-anethole aganist Pseudomonas aeruginosa. J. Appl. Microbiol. 2018, 125, 731–739. [Google Scholar] [CrossRef]
- Dąbrowska, M.; Zielińska-Bliźniewska, H.; Kwiatkowski, P.; Łopusiewicz, Ł.; Pruss, A.; Kostek, M.; Kochan, E.; Sienkiewicz, M. Inhibitory Effect of Eugenol and trans-Anethole Alone and in Combination with Antifungal Medicines on Candida albicans Clinical Isolates. Chem. Biodivers. 2021, 18, e2000843. [Google Scholar] [CrossRef]
- Kubo, I.; Fujita, K.I.; Nihei, K.I. Antimicrobial activity of anethole and related compounds from aniseed. J. Sci. Food Agric. 2008, 88, 242–247. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, J.; Zhou, L.; Wang, J.; Gong, Y.; Chen, X.; Guo, Z.; Wang, Q.; Jiang, W. Antifungal activity of the essential oil of Illicium verum fruit and its main component trans-anethole. Molecules 2010, 15, 7558–7569. [Google Scholar] [CrossRef] [PubMed]
- Naksang, P.; Tongchitpakdee, S.; Thumanu, K.; Oruna-Concha, M.J.; Niranjan, K.; Rachtanapun, C. Assessment of antimicrobial activity, mode of action and volatile compounds of Etlingera pavieana essential oil. Molecules 2020, 25, 3245. [Google Scholar] [CrossRef]
- Lis-Balchin, M.; Deans, S.G.; Eaglesham, E. Relationship between bioactivity and chemical composition of commercial essential oils. Flavour Fragr. J. 1998, 13, 98–104. [Google Scholar] [CrossRef]
- Kubo, I.; Himejima, M. Anethole, a synergist of polygodial against filamentous microorganisms. J. Agric. Food Chem. 1991, 39, 2290–2292. [Google Scholar] [CrossRef]
- Kubo, I.; Fujita, K.I.; Lee, S.H. Antifungal mechanism of polygodial. J. Agric. Food Chem. 2001, 49, 1607–1611. [Google Scholar] [CrossRef] [PubMed]
- Machida, K.; Tanaka, T.; Taniguchi, M. Depletion of glutathione as a cause of the promotive effects of polygodial, a sesquiterpene on the production of reactive oxygen species in Saccharomyces cerevisiae. J. Biosci. Bioeng. 1999, 88, 526–530. [Google Scholar] [CrossRef]
- Himejima, M.; Kubo, I. Fungicidal activity of polygodial in combination with anethole and indole against Candida albicans. J. Agric. Food Chem. 1993, 41, 1776–1779. [Google Scholar] [CrossRef]
- Fujita, K.-I.; Kubo, I. Multifunctional action of antifungal polygodial against Saccharomyces cerevisiae: Involvement of pyrrole formation on cell surface in antifungal action. Bioorg. Med. Chem. 2005, 13, 6742–6747. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Taniguchi, M. Polygodial, an antifungal potentiator. J. Nat. Prod. 1988, 51, 22–29. [Google Scholar] [CrossRef]
- Yano, Y.; Taniguchi, M.; Tanaka, T.; Oi, S.; Kubo, I. Protective effects of Ca2+ on cell membrane damage by polygodial in Saccharomyces cerevisiae. Agric. Biol. Chem. 1991, 55, 603–604. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Lamien-Meda, A.; Bayala, B.; Tirogo, S.; Franz, C.; Novak, J.; Nebié, R.C.; Dicko, M.H. Composition and Antimicrobial Activities of Lippia multiflora Moldenke, Mentha x piperita L. and Ocimum basilicum L. Essential Oils and Their Major Monoterpene Alcohols Alone and in Combination. Molecules 2010, 15, 7825. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [Green Version]
- Delaquis, P.J.; Stanich, K.; Girard, B.; Mazza, G. Antimicrobial activity of individual and mixed fractions of dill, cilantro, coriander and eucalyptus essential oils. Int. J. Food Microbiol. 2002, 74, 101–109. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Suzuki, H.; Matsumoto, K.; Wada, Y. Pheromone study on acarid mites. XI. Function of mite body as geometrical isomerization and reduction of citral (the alarm pheromone). Appl. Entomol. Zool. 1983, 18, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Southwell, I.A.; Russell, M.; Smith, R.L.; Archer, D.W. Backhousia citriodora F. Muell. (Myrtaceae), a superior source of citral. J. Essent. Oil Res. 2000, 12, 735–741. [Google Scholar] [CrossRef]
- Pengelly, A. Antimicrobial activity of lemon myrtle and tea tree oils. Aust. J. Med. Herbal. 2003, 15, 9. [Google Scholar]
- Wilkinson, J.M.; Cavanagh, H. Antibacterial activity of essential oils from Australian native plants. Phytother. Res. 2005, 19, 643–646. [Google Scholar] [CrossRef]
- Hood, J.R.; Wilkinson, J.M.; Cavanagh, H.M. Evaluation of common antibacterial screening methods utilized in essential oil research. J. Essent. Oil Res. 2003, 15, 428–433. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Mereddy, R.; Li, L.; Sultanbawa, Y. Formulation, characterisation and antibacterial activity of lemon myrtle and anise myrtle essential oil in water nanoemulsion. Food Chem. 2018, 254, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, J.M.; Hipwell, M.; Ryan, T.; Cavanagh, H.M. Bioactivity of Backhousia citriodora: Antibacterial and antifungal activity. J. Agric. Food Chem. 2003, 51, 76–81. [Google Scholar] [CrossRef]
- Andres, M.-I.; Forsby, A.; Walum, E. Polygodial-induced noradrenaline release in human neuroblastoma SH-SY5Y cells. Toxicol. Vitr. 1997, 11, 509–511. [Google Scholar] [CrossRef]
- Sultanbawa, Y.; Cusack, A.; Currie, M.; Davis, C. An innovative microplate assay to facilitate the detection of antimicrobial activity in plant extracts. J. Rapid Methods Autom. Microbiol. 2009, 17, 519–534. [Google Scholar] [CrossRef]
- Aoudou, Y.; Léopold, T.N.; Michel, J.D.P.; Franccedil, E.; Moses, M.C. Antifungal properties of essential oils and some constituents to reduce foodborne pathogen. J. Yeast Fungal Res. 2010, 1, 001–008. [Google Scholar]
- Wuryatmo, E.; Klieber, A.; Scott, E.S. Inhibition of citrus postharvest pathogens by vapor of citral and related compounds in culture. J. Agric. Food Chem. 2003, 51, 2637–2640. [Google Scholar] [CrossRef]
- Wannissorn, B.; Jarikasem, S.; Soontorntanasart, T. Antifungal activity of lemon grass oil and lemon grass oil cream. Phytother. Res. 1996, 10, 551–554. [Google Scholar] [CrossRef]
- Onawunmi, G.O. Evaluation of the antimicrobial activity of citral. Lett. Appl. Microbiol. 1989, 9, 105–108. [Google Scholar] [CrossRef]
- Silva, C.d.B.d.; Guterres, S.S.; Weisheimer, V.; Schapoval, E.E. Antifungal activity of the lemongrass oil and citral against Candida spp. Braz. J. Infect. Dis. 2008, 12, 63–66. [Google Scholar] [CrossRef] [Green Version]
- Marques, A.M.; Lima, C.H.; Alviano, D.S.; Esteves, R.L.; Kaplan, M.A.C. Traditional use, chemical composition and antimicrobial activity of Pectis brevipedunculata essential oil: A correlated lemongrass species in Brazil. Emir. J. Food Agric. 2013, 25, 798–808. [Google Scholar] [CrossRef] [Green Version]
- McCallion, R.F.; Cole, A.; Walker, J.; Blunt, J.; Munro, M. Antibiotic substances from New Zealand plants. Planta Med. 1982, 44, 134–138. [Google Scholar] [CrossRef]
- Lim, T.K. Tasmannia lanceolata. In Edible Medicinal and Non-Medicinal Plants: Fruits; Springer: Dordrecht, The Netherlands, 2013; Volume 6, pp. 493–499. [Google Scholar]
- Kubo, I.; Fujita, K.I. Naturally occurring anti-Salmonella agents. J. Agric. Food Chem. 2001, 49, 5750–5754. [Google Scholar] [CrossRef]
- Banihashemi, Z.; Abivardi, C. Evaluation of fungicidal and fungistatic activity of plant essential oils towards plant pathogenic and saprophytic fungi. Phytopathol. Mediterr. 2011, 50, 245–256. [Google Scholar]
- Taniguchi, M.; Yano, Y.; Tada, E.; Ikenishi, K.; Oi, S.; Haraguchi, H.; Hashimoto, K.; Kubo, I. Mode of action of polygodial, an antifungal sesquiterpene dialdehyde. Agric. Biol. Chem. 1988, 52, 1409–1414. [Google Scholar]
- Abe, F.; Hiraki, T. Mechanistic role of ergosterol in membrane rigidity and cycloheximide resistance in Saccharomyces cerevisiae. Biochim. Biophys. Acta (BBA) Biomembr. 2009, 1788, 743–752. [Google Scholar] [CrossRef] [Green Version]
- de Lira Mota, K.S.; de Oliveira Pereira, F.; de Oliveira, W.A.; Lima, I.O.; de Oliveira Lima, E. Antifungal activity of Thymus vulgaris L. essential oil and its constituent phytochemicals against Rhizopus oryzae: Interaction with ergosterol. Molecules 2012, 17, 14418–14433. [Google Scholar] [CrossRef] [Green Version]
- Miron, D.; Battisti, F.; Silva, F.K.; Lana, A.D.; Pippi, B.; Casanova, B.; Gnoatto, S.; Fuentefria, A.; Mayorga, P.; Schapoval, E.E. Antifungal activity and mechanism of action of monoterpenes against dermatophytes and yeasts. Rev. Bras. Farmacogn. 2014, 24, 660–667. [Google Scholar] [CrossRef]
- de Almeida Freires, I.; Murata, R.M.; Furletti, V.F.; Sartoratto, A.; de Alencar, S.M.; Figueira, G.M.; de Oliveira Rodrigues, J.A.; Duarte, M.C.T.; Rosalen, P.L. Coriandrum sativum L.(coriander) essential oil: Antifungal activity and mode of action on Candida spp., and molecular targets affected in human whole-genome expression. PLoS ONE 2014, 9, e99086. [Google Scholar]
- de Castro, R.D.; de Souza, T.M.P.A.; Bezerra, L.M.D.; Ferreira, G.L.S.; de Brito Costa, E.M.M.; Cavalcanti, A.L. Antifungal activity and mode of action of thymol and its synergism with nystatin against Candida species involved with infections in the oral cavity: An in vitro study. BMC Complement. Altern. Med. 2015, 15, 417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajkowska, K.; Nowak, A.; Kunicka-Styczyńska, A.; Siadura, A. Biological effects of various chemically characterized essential oils: Investigation of the mode of action against Candida albicans and HeLa cells. RSC Adv. 2016, 6, 97199–97207. [Google Scholar] [CrossRef] [Green Version]
- Leite, M.C.; de Brito Bezerra, A.P.; de Sousa, J.P.; de Oliveira Lima, E. Investigating the antifungal activity and mechanism(s) of geraniol against Candida albicans strains. Med. Mycol. 2015, 53, 275–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Microorganisms | TPL EO | LM EO | Amphotericin B | Chloramphenicol | ||||
|---|---|---|---|---|---|---|---|---|
| MIC (mg/mL) | MBC/MFC (mg/mL) | MIC (mg/mL) | MBC/MFC (mg/mL) | MIC (µg/mL) | MFC (µg/mL) | MIC (µg/mL) | MBC (µg/mL) | |
| E. coli | 0.42 ± 0.02 a | 0.42 ± 0.02 | 1.67 ± 0.07 a | 3.33 ± 0.14 | NT | NT | 26.03 ± 1.81 b | 52.08 ± 3.61 |
| S. aureus | 0.22 ± 0.01 a | 0.30 ± 0.02 | 0.83 ± 0.04 a | 0.83 ± 0.04 | NT | NT | 20.82 ± 1.81 b | 41.67 ± 3.61 |
| Z. rouxii | 0.05 ± 0.002 a | 0.08 ± 0.01 | 0.31 ± 0.001 a | 0.52 ± 0.02 | 5.20 ± 0.45 b | 7.80 ± 0.001 | NT | NT |
| Z. bailii | 0.07 ± 0.002 a | 0.09 ± 0.01 | 0.26 ± 0.01 a | 0.31 ± 0.001 | 13.02 ± 0.90 b | 13.02 ± 0.90 | NT | NT |
| C. albicans | 0.03 ± 0.001 a | 0.03 ± 0.001 | 0.26 ± 0.01 a | 0.26 ± 0.01 | 41.67 ± 3.61 b | 52.08 ± 3.61 | NT | NT |
| S. cerevisiae | 0.05 ± 0.003 a | 0.05 ± 0.002 | 0.21 ± 0.01 a | 0.26 ± 0.01 | 3.25 ± 0.23 b | 7.80 ± 0.001 | NT | NT |
| R. mucilaginosa | 0.05 ± 0.003 a | 0.05 ± 0.003 | 0.21 ± 0.01 a | 0.42 ± 0.02 | 3.25 ± 0.23 b | 6.50 ± 0.45 | NT | NT |
| C. krusei | 0.03 ± 0.001 a | 0.04 ± 0.001 | 0.21 ± 0.01 a | 0.26 ± 0.01 | 3.25 ± 0.23 b | 3.90 ± 0.45 | NT | NT |
| R. glutinis | 0.03 ± 0.001 a | 0.03 ± 0.001 | 0.21 ± 0.01 a | 0.37 ± 0.02 | 2.60 ± 0.23 b | 5.21 ± 0.45 | NT | NT |
| S. pombe | 0.07 ± 0.008 a | 0.07 ± 0.008 | 0.16 ± 0.001 a | 0.21 ± 0.01 | 2.60 ± 0.23 b | 6.50 ± 0.45 | NT | NT |
| D. anomala | 0.03 ± 0.001 a | 0.03 ± 0.001 | 0.11 ± 0.01 a | 0.13 ± 0.005 | 2.93 ± 0.34 b | 3.90 ± 0.001 | NT | NT |
| D. bruxellensis | 0.03 ± 0.001 a | 0.03 ± 0.001 | 0.07 ± 0.002 a | 0.11 ± 0.004 | 13.02 ± 0.90 b | 13.02 ± 0.90 | NT | NT |
| S. pombe | C. albicans | Z. rouxii | Z. bailii | C. Krusei | S. cerevisiae | D. anomala | D. bruxellensis | ||
|---|---|---|---|---|---|---|---|---|---|
| TPL EO and ergosterol | R2 | 0.986 ** | 0.771 ns | 0.861 ns | 0.771 ns | 0.910 * | 0.938 * | 0.771 ns | 0.910 * |
| LM EO and ergosterol | R2 | 0.986 ** | 0.916 * | 0.940 * | 0.918 * | 0.933 * | 0.996 ** | 0.771 ns | 0.914 * |
| Microorganisms | Combinations | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TPL EO (A) + LM EO (B) | TPL EO (A) + AM EO (B) | LM EO (A) + AM EO (B) | ||||||||||
| MIC (v/v%) | FIC (v/v%) | FICI | Action | MIC (v/v%) | FIC (v/v%) | FICI | Action | MIC (v/v%) | FIC (v/v%) | FICI | Action | |
| S. pombe | 0.016 (A) | 0.004 | 0.38 | Synergy | 0.016 (A) | 0.008 | 0.5 | Synergy | 0.016 (A) | 0.016 | 1 | Additive |
| 0.016 (B) | 0.002 | >2.0 (B) | 0.008 | >2.0 (B) | 0.008 | |||||||
| S. cerevisiae | 0.031 (A) | 0.008 | 0.28 | Synergy | 0.031 (A) | 0.016 | 0.5 | Synergy | 0.031 (A) | 0.031 | 1 | Additive |
| 0.031 (B) | 0.001 | >2.0 (B) | 0.008 | >2.0 (B) | 0.008 | |||||||
| D. anomala | 0.002 (A) | 0.002 | 1.95 | Indifferent | 0.002 (A) | 0.004 | 1.95 | Indifferent | 0.008 (A) | 0.016 | 1.95 | Indifferent |
| 0.008 (B) | 0.008 | >2.0 (B) | 0.0625 | >2.0 (B) | 0.125 | |||||||
| C. albicans | 0.008 (A) | 0.002 | 0.49 | Synergy | 0.008 (A) | 0.004 | 0.49 | Synergy | 0.063 (A) | -- | Antagonistic | |
| 0.063 (B) | 0.016 | >2.0 (B) | 0.063 | >2.0 (B) | -- | |||||||
| Z. bailii | 0.031 (A) | 0.008 | 0.31 | Synergy | 0.031 (A) | 0.008 | 0.25 | Synergy | 0.031 (A) | 0.031 | 0.99 | Additive |
| 0.031 (B) | 0.002 | >2.0 (B) | 0.063 | >2.0 (B) | 0.0625 | |||||||
| Z. rouxii | 0.004 (A) | 0.002 | 0.5 | Synergy | 0.004 (A) | 0.004 | 0.98 | Additive | 0.016 (A) | 0.031 | 1 | Additive |
| 0.031 (B) | 0.0005 | >2.0 (B) | 0.008 | >2.0 (B) | 0.008 | |||||||
| R. mucilaginosa | 0.002 (A) | 0.001 | 0.74 | Additive | 0.002 (A) | 0.0005 | 0.24 | Synergy | 0.016 (A) | 0.0005 | 0.03 | Synergy |
| 0.016 (B) | 0.004 | >2.0 (B) | 0.031 | >2.0 (B) | 0.125 | |||||||
| R. glutinis | 0.008 (A) | 0.002 | 0.28 | Synergy | 0.008 (A) | 0.004 | 0.49 | Synergy | 0.016 (A) | 0.016 | 1 | Additive |
| 0.016 (B) | 0.0005 | >2.0 (B) | 0.008 | >2.0 (B) | 0.063 | |||||||
| D. bruxellensis | 0.002 (A) | 0.0005 | 0.31 | Synergy | 0.002 (A) | 0.002 | 0.98 | Additive | 0.008 (A) | 0.016 | 1.95 | Indifferent |
| 0.008 (B) | 0.0005 | >2.0 (B) | 0.008 | >2.0 (B) | 0.063 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alderees, F.; Mereddy, R.; Were, S.; Netzel, M.E.; Sultanbawa, Y. Anti-Yeast Synergistic Effects and Mode of Action of Australian Native Plant Essential Oils. Appl. Sci. 2021, 11, 10670. https://doi.org/10.3390/app112210670
Alderees F, Mereddy R, Were S, Netzel ME, Sultanbawa Y. Anti-Yeast Synergistic Effects and Mode of Action of Australian Native Plant Essential Oils. Applied Sciences. 2021; 11(22):10670. https://doi.org/10.3390/app112210670
Chicago/Turabian StyleAlderees, Fahad, Ram Mereddy, Stephen Were, Michael E. Netzel, and Yasmina Sultanbawa. 2021. "Anti-Yeast Synergistic Effects and Mode of Action of Australian Native Plant Essential Oils" Applied Sciences 11, no. 22: 10670. https://doi.org/10.3390/app112210670
APA StyleAlderees, F., Mereddy, R., Were, S., Netzel, M. E., & Sultanbawa, Y. (2021). Anti-Yeast Synergistic Effects and Mode of Action of Australian Native Plant Essential Oils. Applied Sciences, 11(22), 10670. https://doi.org/10.3390/app112210670








