1. Introduction
In 2020, about 2.3 million women were diagnosed with breast cancer, of which 685,000 were observed globally [
1]. The National Cancer Institute recorded about 3,676,262 breast cancer women in US in 2018, with an estimate of 281,550 new cases in 2021, of which around 43,600 will be estimated deaths [
2].
Breast cancer is a group of diseases possessing phenotypic and genotypic heterogeneity. Each type of breast cancer possesses distinct characteristics which differentiate it from the other type/subtype and determine the type of effective treatment regime. Overexpression of human epidermal growth factor receptor 2 (HER2) is an important marker of an aggressive form of tumors [
3], while as a type of basal breast cancer, triple negative breast cancer (TNBC) where expression of all the three hormone receptors (estrogen receptor-ER, progesterone receptor-PR, and HER2) is negative represents one of the most aggressive forms which possess distant metastasis patterns and lack targeted therapies [
4,
5]. Histologically, breast canceris categorized into ductal or lobular, with 80% of invasive carcinomas being ductal in origin. Accounting to one fourth of breast cancers, ductal carcinoma in situ (DCIS) consists of heterogeneous lesions with varied clinical features, morphology, and biological functioning [
3].
The underlying mechanism of invasiveness and progression of cancer is yet to be fully understood. However, altercations in cell–cell adhesion, increase in motility, production of metalloproteases (MMPs), invasion of basement membrane, degradation of extracellular membrane, intravastation into blood and lymphatic vessels, extravasation, proliferation, and colonization at distant sites are some of the essential and critical steps involved [
6,
7,
8]. During the primary and secondary cancer development several cell molecules and receptors which are vital for normal cellular development, proliferation, growth, and survival can malfunction. This results in altered gene expression, uncontrolled proliferation, and cellular growth. One such essential category of the molecules is CAMs. CAMs such as integrins mediate interactions between tumor cells and surrounding environment to contribute to cancer progression and metastasis. These molecules associate with receptors and other cell adhesion molecules, triggering cascades of pathways such as ERK/MAPK, PI3K-AKT, wingless/integrated (WNT), c-Jun N-terminal kinase (JNK), and transforming growth factor beta (TGF-β) to initiate, progress, and metastasize the tumors [
9,
10,
11]. Based on the stage of cancer, the upregulation and downregulation of CAMs at transcriptional and translational level cause changes in protein expression, activation of pathways, and modifications in cell adhesion patterns. Altered expression of adhesion complex proteins which help in maintaining the breast epithelia contributes towards tissue disorganization, leading to development of breast cancer [
12]. In breast cancer, depending upon type of breast carcinoma and its extent metastasis, these adhesion molecules have been found either under expressed or overexpressed [
12,
13,
14,
15].
Integrins, family of glycoproteins, serve as main extracellular matrix (ECM) receptors, linking ECM and cytoskeleton via ligands. Structurally, integrin receptors consisting of 18 α and 8 β-subunits are linked non-covalently, forming at least 24 heterodimer pairs. While α subunit is larger than β subunit, a distinct similarity has been found within both. Localized at plasma membrane, each integrin binds to various proteins on the intracellular side, and interacts with specific ligands such as fibronectin, fibrinogen, vitronectin, laminin, and collagen on extracellular domain, accounting for bidirectional signaling [
16,
17,
18,
19]. Integrin serves as a main ECM receptor of mammary epithelium, where signaling pathways help modulate the cellular responses and control the breast biology. This includes mammary gland functioning, ductal morphogenesis, mammary alveoli, and lumen formation. While, in normal breasts, the histochemical assays show presence of integrins in myoepitheial layer of basement membrane, their concentration and expression pattern are different in breast cancer [
12,
20,
21,
22]. Abnormal integrin expression contributes to the migratory properties of tumor cells. This permits them to pass through blood vessels and collagen matrix, invade surrounding cells and tissues, and eventually colonize tumor cells at secondary tissues [
20,
21]. Various in vitro and in vivo studies show compromised expression of integrin receptors and their associated signaling pathways. Integrins such as αvβ5, αvβ6, α2β1, α6β1, and α6β4 play critical roles in breast cancer growth, invasion, and metastasis [
22]. Integrin αv (ITGαv) which forms heterodimer with six β integrin sub is a receptor for various proteins [
23]. Each heterodimer of αv-β plays an important part in the regulation of breast cancer and metastasis. For example, integrin αvβ3 causes bone metastasis in breast cancer through enhanced tumor adhesion. While the expression of this integrin is found in both primary tumors and metastatic cells, it is more active in metastatic cells [
24,
25]. Apart from ITGαv, integrin α6 (ITGα6) also has an important role in invasion, adhesion, migration, and progression of breast cancer cells. Overexpressed in breast cancer cells and tissues, it is associated with poor prognosis and reduced survival rates. Furthermore, studies show that in various breast cancer cells its overexpression alters the processes such as apoptosis, cell cycle arrest, and DNA damage and double-strand break repair mechanism [
26]. This integrin subunit can form heterodimers with β1 or β4 subunits, forming α6β1 and α6β4 integrins, with each integrin unit forming receptor for laminins. By participating via different mechanisms, both α6β1 and α6β4 contribute to the progression and survival of breast cancer [
27].
While there are 8 subunits of β integrin which play important roles in cellular processes, integrins such as β1, β3, β4, β5, β6, and β8 have vital role in breast cancer. Each type of β subunits plays a distinct role in breast cancer where they are essentially involved in pro-tumorigenic processes such as epithelial mesenchymal transition (EMT), tumor development, progression, survival, angiogenesis, neo-angiogenesis, inflammation, and metastasis [
16,
28,
29,
30,
31,
32]. For example, the role of β1 integrin based on the type of breast cancer varies, whereby its decreased and increased expression plays essential roles in tumorigenesis. Regular expression of this integrin helps in development of epithelial tissues via cellular proliferation and differentiation, but when overexpressed it can participate in angiogenesis, progression, and metastasis of breast cancer in invasive ductal carcinoma (IDC) [
33]. Like α subunit, β subunit also associates with various receptor molecules, modulating different signaling pathways in breast cancer. For example, ITGβ5, which is present in primary and metastatic breast cancer cells, activates TGF-β and ERK signaling pathways via focal adhesion molecules such as focal adhesion kinase (FAK) and paxilin, and epidermal growth factor receptor (EGFR) signaling via proto-oncogene tyrosine-protein kinase (Src), causing tumor cell migration, invasion proliferation, and metastasis [
28].
The overexpression of these integrins provides resistance to chemotherapy and radiations, resulting in a decrease in the survival rate of breast cancer patients. Hence, modulating their expressions can help in limiting the growth and invasion of tumor cells, thereby increasing the survival rates. This could be achieved by silencing the gene expression of overregulated genes using small interfering RNAs (siRNAs). siRNAs are short double stranded RNA sequences that impede the expression of over expressed genes by degrading the mRNA sequences in cell cytoplasm. In addition to curbing the upregulated gene expression, siRNA gene silencing can also help in chemosensitizing the tumor cells for various drugs, resulting in increased chemotherapy efficacy [
34,
35,
36]. However, targeted delivery of siRNAs to the cancer cells is a major challenge because of inability to pass the plasma membrane, and sensitiveness to nuclease-based breakdown [
34,
36]. Therefore, it is necessary to use a delivery vehicle to carry nucleic acids and/or chemotherapy drugs to the target site. Currently nanoparticles serve as one of the efficient carrier vehicles. While several nanoparticles such as lipid based carriers, polymers, carbon nanotubes, and inorganic nanoparticles are being used at various pre-clinical and clinical stages, pH receptive inorganic CA is also gaining science based evidence for a carrier vehicle [
37,
38,
39]. With the help of cation (Ca
2+)-rich domains, CA is capable of electrostatically binding a nucleic acid sequence possessing an anionic phosphate backbone. The presence of carbonate as a core constituent of CA notably helps in preventing particle aggregation and thus maintaining the size within few hundred nanometers—a size range potentially beneficial not only in increasing loading of nucleic acids, but also in accelerating passive tumor accumulation by enhanced permeability and retention (EPR) effect. While CA is very stable at physiological pH, it is highly sensitive to dissolution at endosomal acidic pH—a fascinating property that facilitates fast release of payload (DNA or siRNA) after the particles are endocytosed by the target cells. The particle dissolution might also assist in endosomal destabilization through development of osmotic pressure across the endosomal membrane due to accumulation of Ca
2+, phosphate, and carbonate ions—the core constituents of CA, thus enabling release of bound DNA or siRNA from both the carrier and cellular endosomes to cytosol. This in turn results in targeted therapy whereby expression of a desirable protein or specific cleavage of a mRNA transcript is achieved, without causing any toxicity to healthy cells [
40,
41,
42,
43]. Therefore, by targeting altered integrin genes via CA-mediated intracellular delivery of specific siRNAs, the reduction in proliferation and survival of breast cancer cells could be achievable. Apart from the regular treatments, focus on the cell adhesion biomarkers could enhance the survival rate in breast cancer patients with poor prognosis. In this study we delivered individual siRNAs targeting integrin αv, α6, β1, β3, β4, β5, and β6 via CA nanoparticles in breast cancer cell lines as well as murine metastatic breast cancer model to evaluate the effect on tumor cell proliferation and growth.
5. Discussion
Integrins maintain cellular architecture, survival, proliferation, migration, and overall tissue integrity by binding to ECM, cytoskeleton, and connecting via various cell adaptor proteins and ligands [
16,
18]. Integrin αv, α6, β1, β3, β4, β5, and β6 subunits are the essential targets responsible for tumor proliferation, progression, and growth. High level of integrins or high activity of the signaling components associated with them synergize with oncogenes, growth factors, and other cell adhesion proteins, leading to the activation of signaling pathways and affecting both upstream and downstream molecules [
6,
21,
28,
31,
44,
45,
46,
47,
48]. Therefore, they serve an essential targeting molecule to control tumor development. Dysregulation in the integrin expressions which participate in breast cancer development using siRNA-CA nano-formulation may lead to reduced association of the integrins with other cell adhesion molecules and proteins, thus hampering the cancer proliferation and survival.
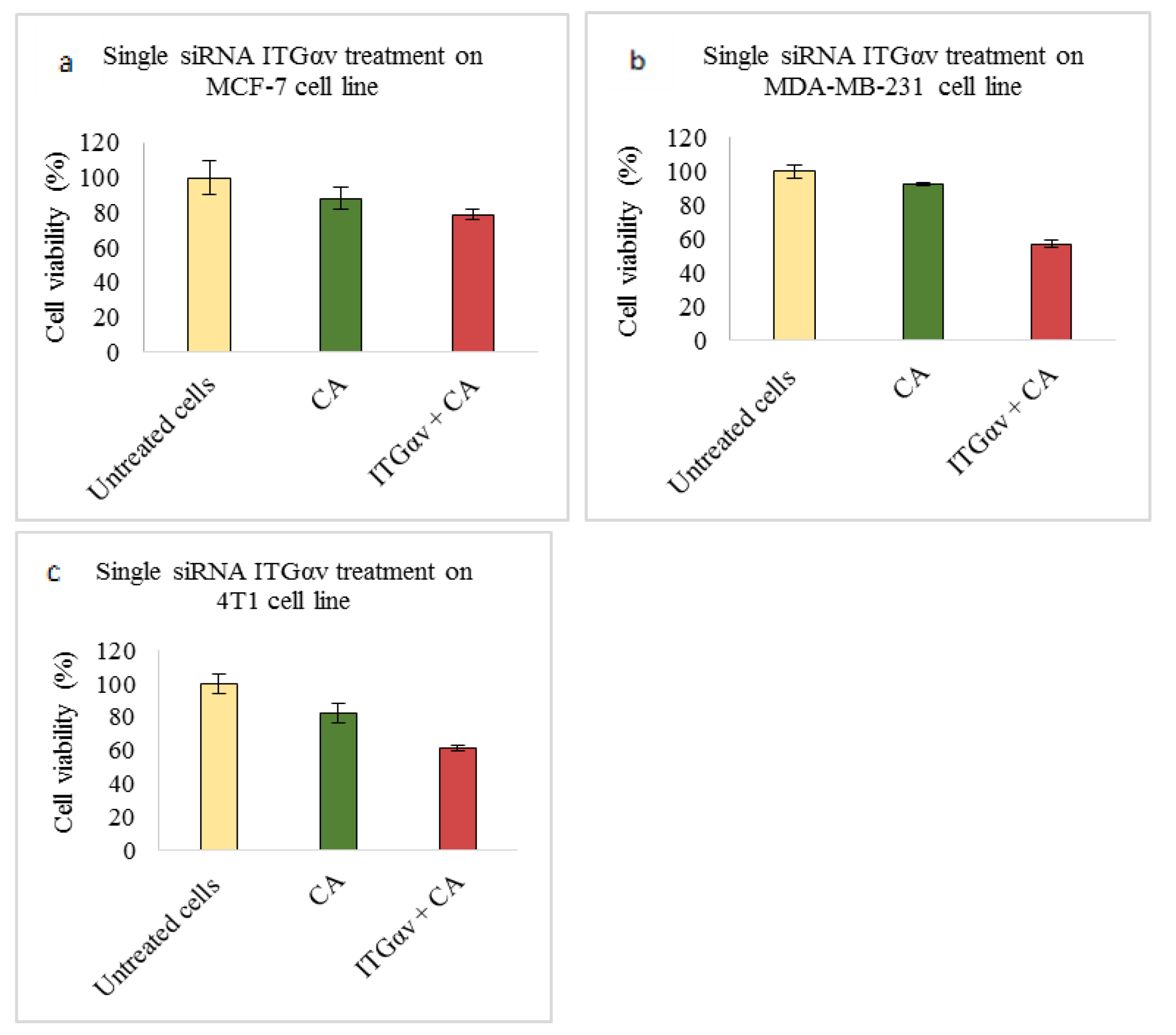
In our integrin siRNA transfection experiments MCF-7 cells showed no visible decrease in cell viability when they were transfected with CA loaded with ITGαv siRNA (
Figure 1a), while a significant reduction in viability of ~57% was observed in MDA-MB-231 (
Figure 1b), causing about 35% cytotoxicity (
Table 3) and viability of ~61% in 4T1 cells (
Figure 1c) which caused about 21% cytotoxicity (
Table 4). Research shows MDA-MB-231 cells contain high expression of ITGαv causing different signaling [
25]. Targeting this sub-unit in MDA-MB-231 cells might have reduced the viability by mainly targeting AKT and MAPK pathways, as from Western blot analysis we could see reduced band intensities after siRNA-CA transfection (
Figure 8b). On the other hand, in MCF-7 cells phosphorylated AKT and total MAPK showed no decrease in band intensity while total AKT and phosphorylated MAPK showed significant reduction compared to CA (
Figure 8a). Breast cancer is a heterogeneous disease, and each cell line represents a different model, with different characteristics and association with different protein molecules and signaling pathways. The expression of integrin αv can vary among different metastatic and non-metastatic cells [
25]. The upregulation of certain pathways in the cells upon delivery of αv integrin might be due to cross talks with other pathways. One such pathway which could interfere is TGF-β pathway. TGF-β signaling mutually influence each other and its cross-talks with other pathways result in successful induction of cancer progression [
48].
Upregulated in invasive and distant metastasis, studies show ITGαv associates with various β sub-units and contributes towards breast cancer metastasis [
25,
30]. When 4T1 cells were induced in the in vivo model of female Balb/c mice for tumor, after first dose of treatment of CA complexed with ITGαv siRNA, reduced tumor volume trend was observed compared to CA treated and untreated groups (
Figure 10) with highest fold decrease of 2.3 on Day 11 (
Table 5). The tumor volume remained smaller compared to the control groups (
Figure 12a). Statistical analysis performed between CA nanoparticle treated and siRNA-CA treated groups showed significant difference with
p < 0.05 on selected time points (
Figure 10). The CA loaded delivery of the integrin siRNA molecule increases its delivery efficiency to the target site. This is achieved by its ability to cause instability in the endosome, and release prior to its loss due to endosomal degradation, thus causing a sufficient decrease in the tumor volume [
39]. The body weight remaining the same for both control and the treatment group indicates that there is no effect on the overall physiology of animals. One of the essential roles of ITGαv is during angiogenesis and progression in breast cancer. Besides frequently being overexpressed in neoplastic cell surfaces and involved in invasion of cancer cells and metastatic dissemination, ITGαv via its ligand association interacts with various cell proteases such as MMP2 which helps in enhancing the activity of tumor cells to degrade ECM and eventually migrate within the environment [
49]. Several pathways are responsible for the migration of tumor cells. As we could see, transfection of this integrin siRNA caused reduction in protein expressions of phosphorylated AKT and MAPK as well as total MAPK (
Figure 8c). This indicates that these pathways have a critical role in modulation of ITGαv expression. However, apart from AKT and MAPK, the TGF-β pathway also plays an essential role in integrin signaling and these pathways have been found to be co-related to each other. In 4T1 cells, research shows that TGF-β can induce EMT, cell migration, and phosphorylation of mothers against decapentaplegic homolog (Smad)-2 protein and transcriptional responses are dependent on the PI3K-AKT pathway [
50]. Further, elevated endogenous levels of various MAPK proteins by this sub-unit have also been found responsible in increasing the tumor invasiveness [
51]. Therefore, inhibiting these pathways with the help of antagonist such as ITGαv siRNA could modulate the morphogenic, transcriptional, and migratory activities of activated signaling pathways such as TGF-β, AKT, and MAPK in 4T1 models (in vitro and in vivo). While αv integrin can form heterodimers with other β integrin units as well as connect with additional cell cytoskeletal proteins [
52], disrupting its function could possibly decrease the association with these molecules, which could further reduce the invasion of the tumor cells. Therefore, its knockdown could be vital in reducing the metastatic dissemination of the cancer in breasts and eventually reducing the cell invasion.
Integrin α6 (ITGα6), widely upregulated in tumors, participates in migration and invasion in cancer cells and as compared to normal cells, has a high level of protein and mRNA expressions [
26,
53], causing poor prognosis and survival. Transfection of CA-ITGα6 siRNA slightly decreased cell viability (~72%) in MCF-7 cells (
Figure 2a) with cytotoxicity of ~11% (
Table 2). ITGα6 siRNA transfection also showed the decrease expression of phosphorylated and total AKT and MAPK proteins in MCF-7 cells (
Figure 8a). A study performed by Cariati et al. (2008) showed ITGα6 was necessary for MCF-7 tumorigenicity and overexpression of α6 has been found to provide growth and survival of tumor cells. Further, within this cell line, the sub population of MSS-derived cells attribute the stemness, which is stem cell characteristics and ITGα6 is one of its markers for identification. Therefore, by reducing the expression of this integrin via these proteins the invasive and migration properties of these cells could be reduced [
54]. On the other hand, in MDA-MB-231 and 4T1 cells (
Figure 2b,c respectively) no visible decrease in the viability was observed. As the level of α6 integrin is very high in TNBC and in metastatic breast cancers, the transfection at smaller dose may not be sufficient to carry out the reduction in cell viability in the respective MDA-MB-231 and 4T1 cell lines. Additionally, ITGα6is found in two transcript variants [
26,
53,
54]. Hence, with one of the variants more expressed than the other, it may be possible that this pre-designed siRNA is not sufficient to reduce the activity of both the variants in the cells, leading to unaffected cell cytotoxicity. Reduction in levels of phosphorylated and total AKT and MAPK in MDA-MB-231 and in 4T1 cells (
Figure 8b,c respectively) suggest MAPK and AKT pathways are critical in overregulated α6. They play an essential role in causing chemoresistance in breast cancer via α6 [
26], and by controlling their expression by down regulating molecules such as ITGα6 they could prove beneficial for chemotherapeutics. However, at the same time targeting these pathways may not be enough to reduce the cell viability in certain high proliferative and metastatic in vitro cells. Cross talks might affect more downstream molecules of AKT and MAPK pathway, leading to increased viability, and involvement of other pathways and molecules such as growth factors, TGF-β, hedgehog, cell adhesion, and p63 might play critical role [
55,
56]. Studies show upon knockdown of ITGα6, reduction in the cell migration, invasion, and pulmonary metastasis can be observed. This can be seen in our in vivo model as well, where clear reduction in the tumor volume was observed post second dose of siRNA-CA treatment (Day 11 onwards) with maximum fold reduction of 2.65 on Day 17 (
Table 5) and small tumors were observed at the end of the treatment (
Figure 12b). As we can see from
Figure 10, the trend for outgrowth of tumor over the time for ITGα6 siRNA complexed with CA nanoparticle was smaller compared to CA nanoparticle and untreated group. Statistical analysis performed between nanoparticle treated and siRNA-CA nanoparticle treated groups showed significant difference (*) with
p < 0.05 on selected time points. Research shows apart from targeting main cell proliferation pathways such as PI3K/AKT or ERK/MAPK one of the ways the reduction of ITGα6 expression could take place is also by inhibiting cells to enter S phase. This is done by up-regulating p27, a cyclin protein, which results in down-regulation of cyclin E/CDK2 complexes [
57].
Integrin β1 (ITGβ1), the largest subgroup of integrin family, plays a vital role in breast cancer. Responsible for tumor proliferation, causing the switch of tumor cells from the dormant stage to metastatic stage, and providing resistance to tumor cells against adjuvant therapy and ionization radiation [
58], targeting this component of integrin could be a promising strategy in reducing the tumor metastasis and increasing the efficiency in therapy. Based on our MTT results significant reduction in the cell viability after 48 h of transfection with this siRNA in all three cell lines was seen. While MCF-7 showed cell viability ~63% (
Figure 3a) and cytotoxicity of ~32% (
Table 2), MDA-MB-231 showed cell viability of ~86% (
Figure 3b) and cytotoxicity ~10% (
Table 3) and 4T1 showed cell viability of ~81% and cytotoxicity of ~12.5% (
Table 4). The reduced expression of phosphorylated and total MAPK and AKT levels was observed in MCF-7, MDA-MB-231, and 4T1 cells (
Figure 8a–c respectively). In animal model, tumor regression analysis also showed decrease in the tumor volume upon delivery of this integrin siRNA sub-unit along with CA nanoparticles (
Figure 11). The significant difference was observed post first dose of treatment till the mice were sacrificed with highest fold decrease of 6.3 in tumor volume (
Table 6) and smaller tumor compared to CA group (
Figure 12c). From these results the essential role of this integrin subunit in various types and stages of breast cancer and its significant correlation with AKT and MAPK pathways can be inferred. Previous studies have shown knockdown of β1 in MDA-MB-231 cell line decreased the expression levels of β1-associated major α subunits and decreased the phosphorylation levels of FAK, thereby disrupting the focal adhesion and inhibiting mobility [
59]. ITGβ1 is involved in bidirectional signaling where the domain interacts with cytoskeleton as well as the intracellular proteins in the cell. This helps ITGβ1 to contribute towards several tumor generating steps such as initiation, its survival, progression, reversion, and eventually metastasis. Various experimental studies, in vitro and in vivo, have shown, by targeting this sub-unit, its downstream signaling mediator is able to prevent the transition of the dormant tumor cells to function into metastatic cells and attenuate cell proliferation [
58,
60]. Further, by inhibiting its ability to cease apoptosis caused by various drugs such as paclitaxel could aid in reducing the chemoresistance via deactivation of AKT and MAPK pathways and effector molecules such as B-cell lymphoma-2 (Bcl-2) [
45]. In
Figure 13 we have elucidated how this integrin sub-unit could involve in signaling pathways, and introduction of an antagonistic could help in hampering the pathways at various sites.
Integrin β3 (ITGβ3) a critical sub-unit which plays role in tumor invasion, neoangiogenesis, and inflammation, makes it one of the promising cancer targets. Tumor cells which show high expression of ITGβ3 exhibit enhancement in their proliferation and metastasis, especially to bones [
61,
62]. The role of this integrin in breast cancer has been studied in various experiments, in vitro as well as in vivo, suggesting its knockdown or deletion can play significant role in decreasing the metastasis. In our MTT assay, delivery of β3 siRNA loaded CA reduced the cell viability to ~60% in MCF-7 (
Figure 4a), with cytotoxicity of ~14% (
Table 2), while in MDA-MB-231 and 4T1 cell lines no reduction in cell viability was seen (
Figure 4b,c respectively). However, in vivo model showed contrary results compared to cell line. Significant tumor reduction was observed from Day 10 (
Figure 11), with highest of 4.9-fold decrease on Day 12 compared to CA control (
Table 6) and reduced tumor size at the end of tumor excision (
Figure 12d). Protein expression study showed the transfection of CA loaded β3 integrin siRNA caused reduction of total and phosphorylated MAPK and AKT proteins in MCF-7, MDA-MB-23, and 4T1 cells (
Figure 8a–c respectively). One reason for high cell viability despite reduced AKT and MAPK pathways may be due to cross talks with signaling molecules such as that of signal transducer and activator of transcription (STAT)-6/STAT1. These cross talks could have resulted in controlling the balance between anti-tumor and pro-tumor immune cells [
61] in the in vitro model. In several biological contexts, the locus of
ITGB3 is regulated epigenetically, which in turn is responsible for regulating its function and expression. This regulation is seen to control tumor senescence as well. For example, increased levels of endogenous β3 via transcriptional regulation of polycomb protein chromobox 7 (CBX7) is observed during oncogene-induced senescence (OIS) with concomitant increase in β3 mRNA expression [
62]. Therefore, by hampering expression of this integrin along with other transcriptional proteins via an efficient vehicle may provide improved efficacy in tumor regulation.
Integrin β4 (ITGβ4) overexpression, chemoresistance to drugs such as tamoxifien, and its related aggressiveness has been found especially in basal-like breast cancers. Cell migration and invasion takes place with the aid of actin binding proteins, which help in stabilization of actin protrusions [
31]. Targeting this subtype of integrin has shown reduction in tumors in aggressive cancers such as TNBC. Based on our results, ITGβ4 siRNA-CA delivery did not caused visible reduction in cell viability in MCF-7 and 4T1 cells (
Figure 5a,c respectively), while in MDA-MB-231, cell viability of ~88% (
Figure 5b), with cytotoxicity of ~11% (
Table 3) was observed. Presence of integrin β4 has been observed in TNBC patients. Inter and intra tumoral heterogeneity and presence of cancer stem like cells (CSC) being critical feature of this breast cancer [
63]. Therefore, reducing its expression may help in reduction of these stem-like cells and result in lesser tumor heterogeneity. ITGβ4 binds directly to laminin protein which in turn activates PI3K/AKT pathways and promotes proliferation, invasion, and survival of cells [
64]. Protein expression study by Western blot technique showed total and phosphorylated AKT and MAPK expression was reduced upon β4 integrin siRNA transfection, in all the three cell lines (
Figure 8a–c). Reduction in the expression of this integrin reportedly reduces the lung metastasis in aggressive breast cancers [
64]. Reduction in protein expression without decrease in cell viability indicates that, in cells such as MCF-7 and 4T1, other pathways are actively involved in causing the upregulation of this gene. For example, ERα signaling plays an essential role in upregulating ITGβ4 signaling. Research done by Yi Ho et al. (2016) showed estrogen enhances the viability of breast cancer cells by activating ERα-∆Np63-integrin β4 signaling. Additionally, cross talk with various growth factors, and activation of Ras homolog family member A (RhoA)/Rho-associated protein kinase 2 (ROCK-2) signaling cascades could enhance the cell motility and viability in vitro [
64]. Even though no reduction in cell viability was observed in 4T1 cells, the tumor regression model showed significant decrease (
p < 0.05) in the tumor volume compared to CA control (
Figure 11) and smaller tumor at the end of the treatment (
Figure 12e). This indicates targeting this integrin and reduced signaling of vital cell proliferation pathways such as AKT and MAPK pathways play vital role in regulating β4 expression and its tumor induced modelling. While various cross talks are involved between the signaling molecules and several upstream and downstream molecules are targeted, one of the ways by which integrins cooperate with AKT and ERK is by suppressing cyclin-dependent kinase inhibitor p21/cyclin dependent kinase inhibitor p27 jointly, which causes shift in the balance of forkhead box O3 (Foxo3a), an essential transcriptional activator for apoptosis, from the nucleus to cytosol [
65]. Similarly, it plays role in hampering of pathways involved with cell cycle, thereby causing a significant reduction in tumors.
Integrin β5 (ITGβ5), when highly expressed, participates in tumor growth and angiogenesis. After delivering ITGβ5 siRNA along with CA nanoparticles and incubating the cells for 48 h, MTT assay revealed a decrease in cell viability of ~63% in MCF-7 (
Figure 6a) with ~21% cytotoxicity (
Table 2), ~84% in MDA-MB-231 (
Figure 6b) with slight cytotoxicity of ~8% (
Table 3), and cell viability of ~84% in 4T1 cells (
Figure 6c) with cytotoxicity of ~7% (
Table 4). Protein expression was reduced for both phosphorylated and total AKT and MAPK in MCF-7 and 4T1 cells (
Figure 8a,c respectively), while in MDA-MB-231 no knockdown was seen (
Figure 8b). Variation in the protein expressions may be due to different phenotypic and genotypic characteristics of cells, and while PI3K-ERK serves as one of the pathways which contribute to signaling via this integrin, several other pathways such as Src-FAK, MEK may be involved in regulating the cell viability in MDA-MB-231 cells [
28]. At the end of the study, tumor regression showed significant (
p < 0.05) reduction in tumor volume compared to CA control (
Figure 11), with smaller tumor in comparison with CA control (
Figure 12f), indicating successful delivery of target antagonist via CA delivery vehicle. Recent studies have shown integrin β5 contributes to TGF-β-induced EMT and resistance to radiotherapy and chemotherapy in various stages of breast cancer [
28]. Therefore, by sequestering this molecule, besides targeting AKT and MAPK oncogenic proliferation pathways, vital EMT pathways could also be affected. Hence, this integrin sub-unit might serve as an efficient therapeutic target in the breast cancers which are resistant to most of the therapies.
Integrin β6 (ITGβ6) gene shows its expression in several carcinoma cell lines, including breast cancer [
66,
67]. All three cell lines showed reduced cell viability of ~67% (
Figure 7a), ~63% (
Figure 7b), and ~60% (
Figure 7c). High cytotoxicity of ~16% in MCF-7 (
Table 2), ~29% in MDA-MB-231 (
Table 3) and ~33% in 4T1 cells (
Table 4), upon transfection with β6 siRNA loaded CA nanoparticles was observed. At protein level, expression of phosphorylated and total AKT and MAPK was reduced in MCF-7 (
Figure 8a). In MDA-MB-231 cells, total MAPK showed no reduction (
Figure 8b) compared to CA treated control, while in 4T1 cell line undetectable reduction was seen in p-AKT (
Figure 8c). In vivo model showed reduction in tumor volume with significant difference (
p < 0.05) at certain time points post treatment doses (
Figure 11) and smaller tumor post excision compared to CA control (
Figure 12g). With only 50-fold more siRNA (roughly equivalent to 3.325 μg/kg in a 20 g mouse) compared to that used in each well of a 24 well-plate, the anti-tumor effect was remarkably high, which could be due to the efficient tumor targeting ability of siRNA-loaded CA nanoparticles and, finally, intracellular release of the therapeutic siRNA following endocytosis and pH-responsive dissolution of the particles. The expression of p-AKT may have contributed towards significant reduction only at certain days. Mechanism of this integrin is mainly regulated at transcription level. Studies report various transcription factors such as ETS Proto-Oncogene 1 (Ets-1), Signal transducer, and activator of transcription 3 (STAT3) (involved in basal epithelial cells), and Smad3 mediate the initiation of ITGβ6 expression. While the exact mechanism underlying integrin β6 regulation is unknown, it serves as a prognostic indicator in various invasive breast cancers, especially HER2+ subtype [
67,
68].
The contrasting in vitro and in vivo results could be due to varied microenvironments in both systems and different sensitivity to siRNAs in cells compared to animal model. While in vivo cells are biologically embedded in the ECM, which in itself is a complex three-dimensional gel, providing mechanical support, directing cellular behavior, and mimicking the physiology and pathology of human systems, in vitro support system provides a two-dimensional environment which not only provides a less suitable complex system for cell-substrate interaction but also results in certain limitations such as dissimilarities in cellular adhesion, migration, and cytoskeletal organization [
69]. Further, slight, or undetectable change in the expression of proteins in certain studies could indicate that the proteins were already expressed before the knockdown by siRNA treatment. In addition, integrin signaling is complex and, besides MAPK and AKT, there are several pathways and molecules, which play distinct roles in each type of integrin regulation. Hence, in treatments where knockdown in these pathways is observed, without cell viability being affected, it may be the other pathways which enhance the proliferation.