Fenton Reaction for Enhancing Polishing Rate and Protonated Amine Functional Group Polymer for Inhibiting Corrosion in Ge1Sb4Te5 Film Surface Chemical-Mechanical-Planarization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemical-Mechanical Planarization
2.3. Measurement Equipment
3. Results and Discussion
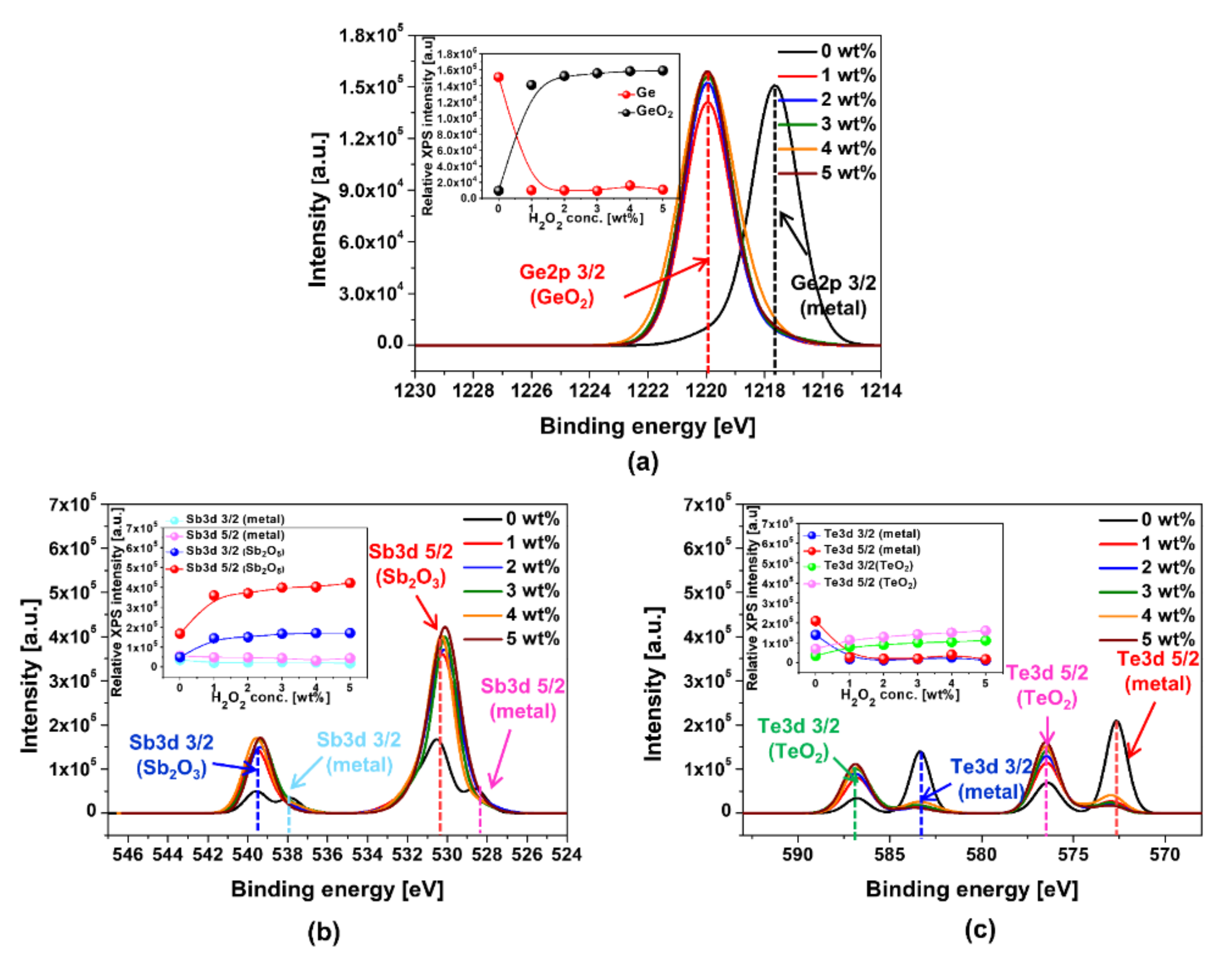
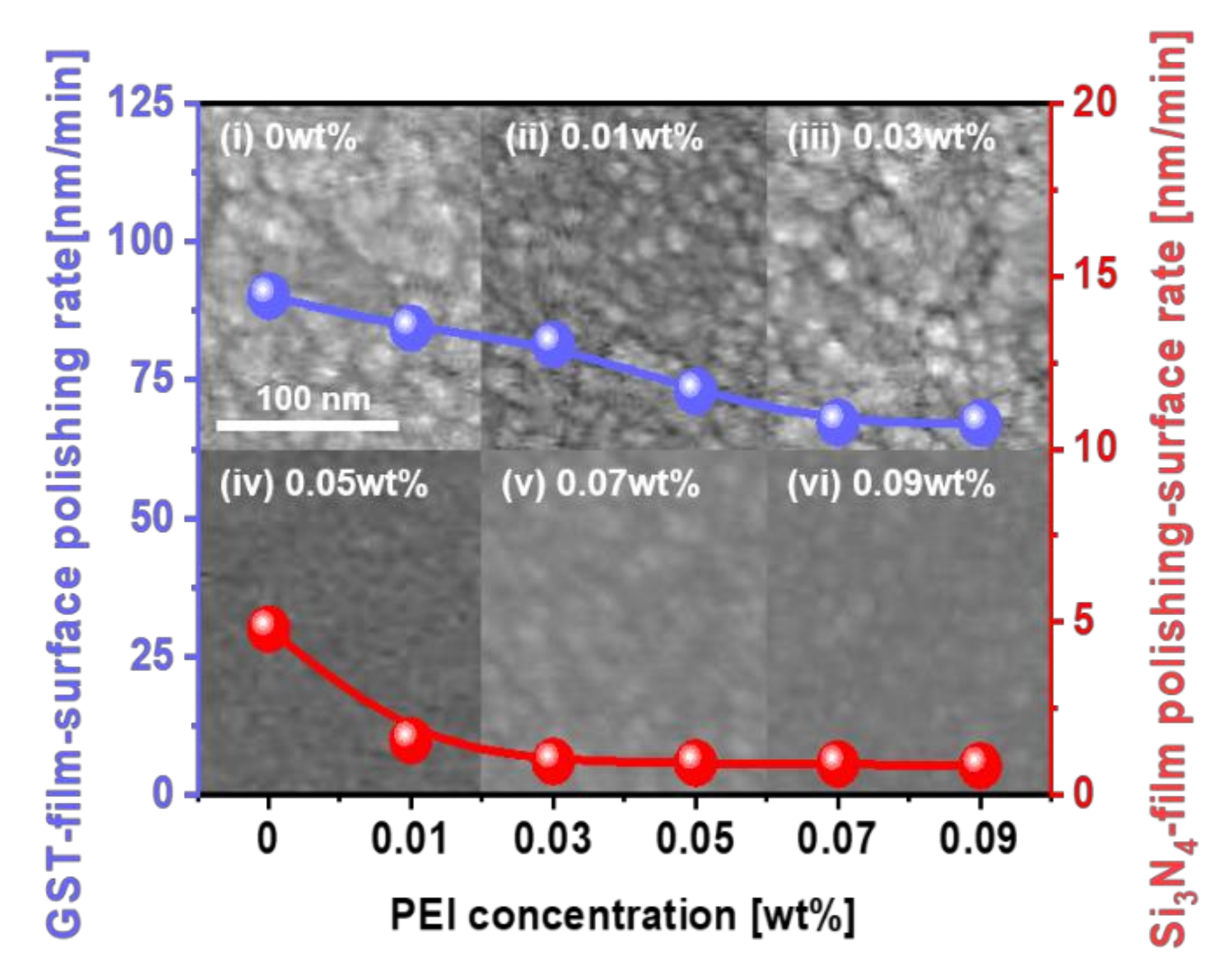
3.1. Effect of Fenton Reaction on Enhancement in Ge1Sb4Te5 Film Polishing Rate and Corrosion Suppression during CMP
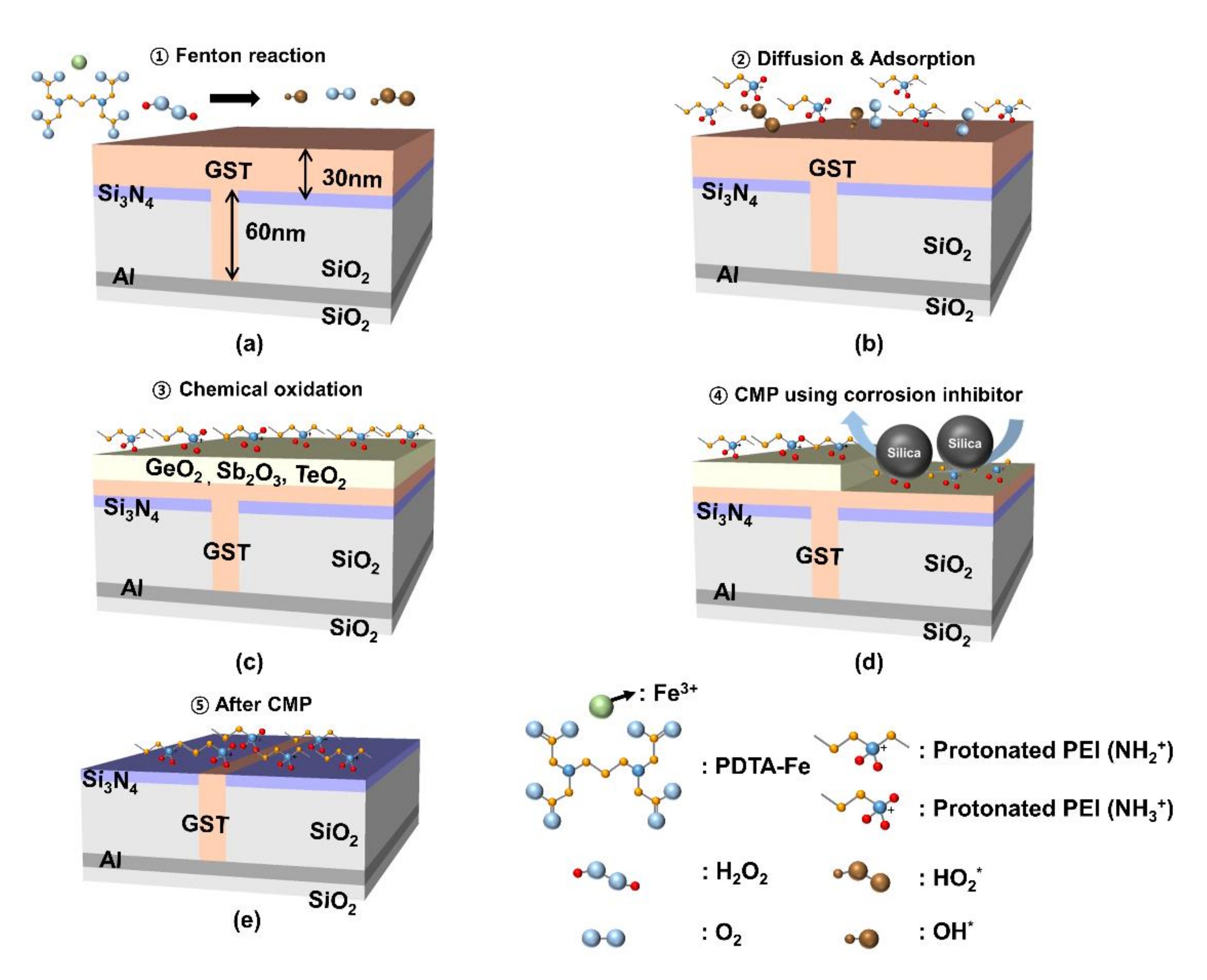
3.2. Fenton Reaction Mechanism for Enhancing Polishing Rate and Suppressing Corrosion on the Ge1Sb4Te5 Film Surface during CMP
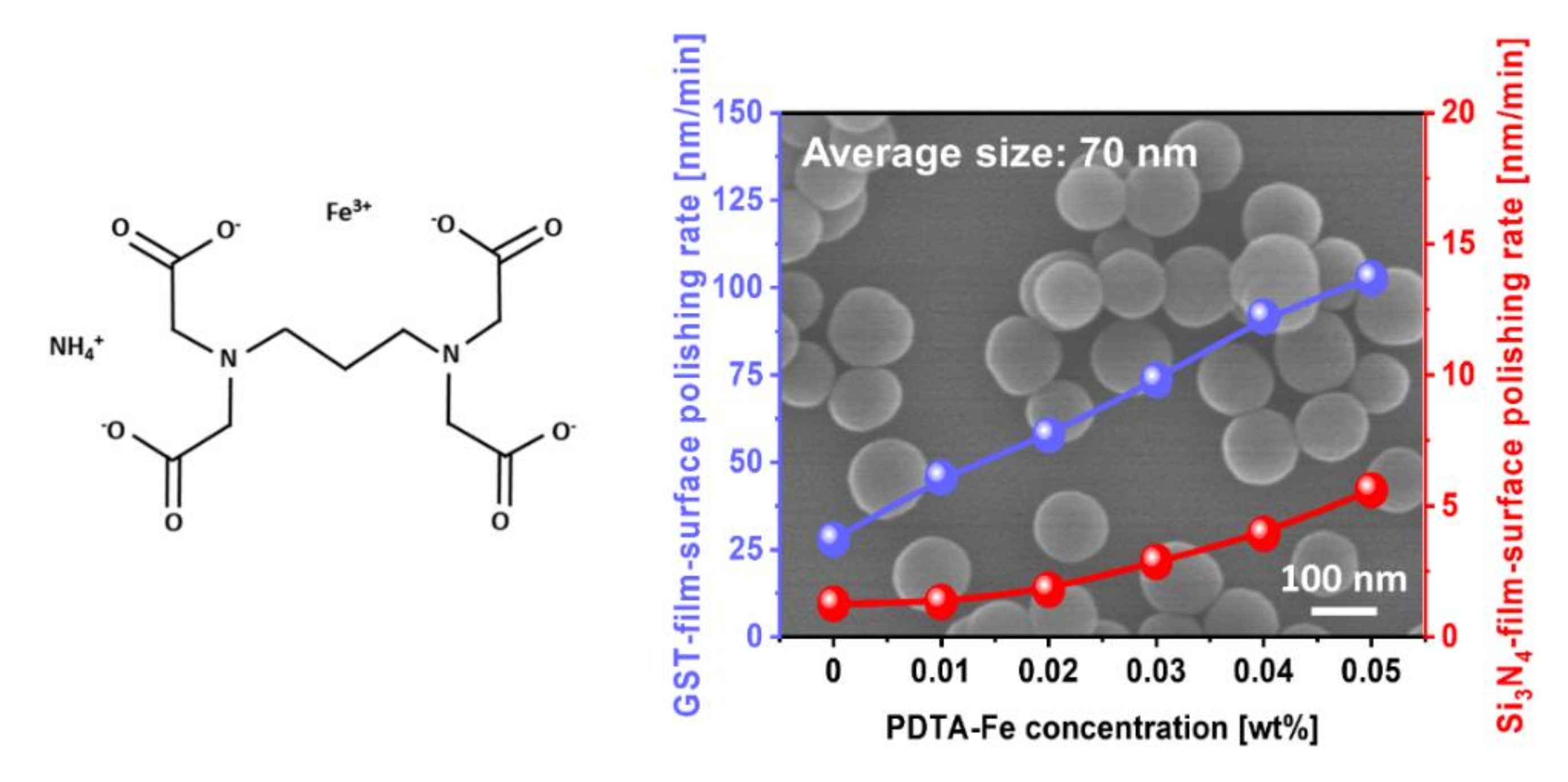
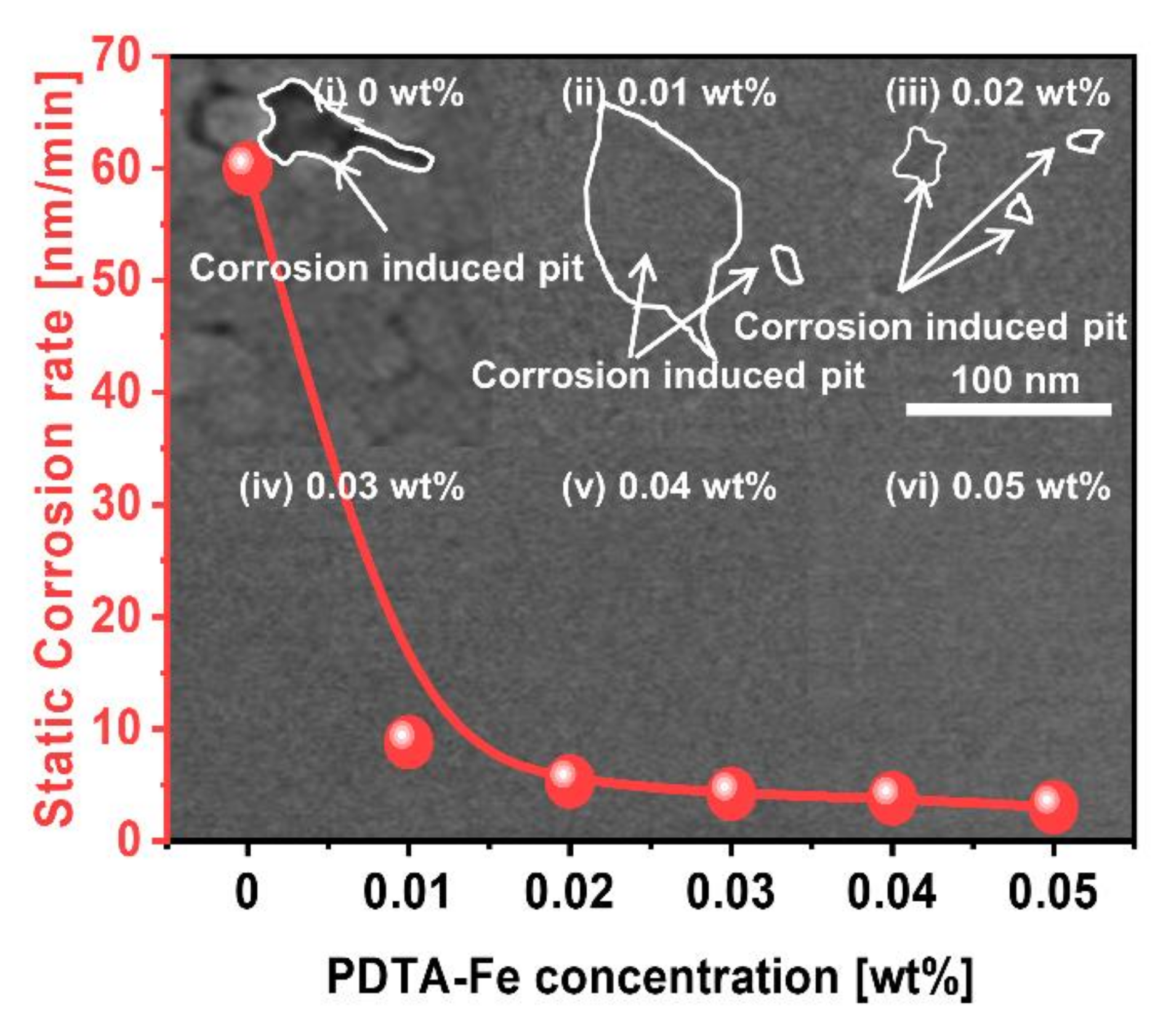
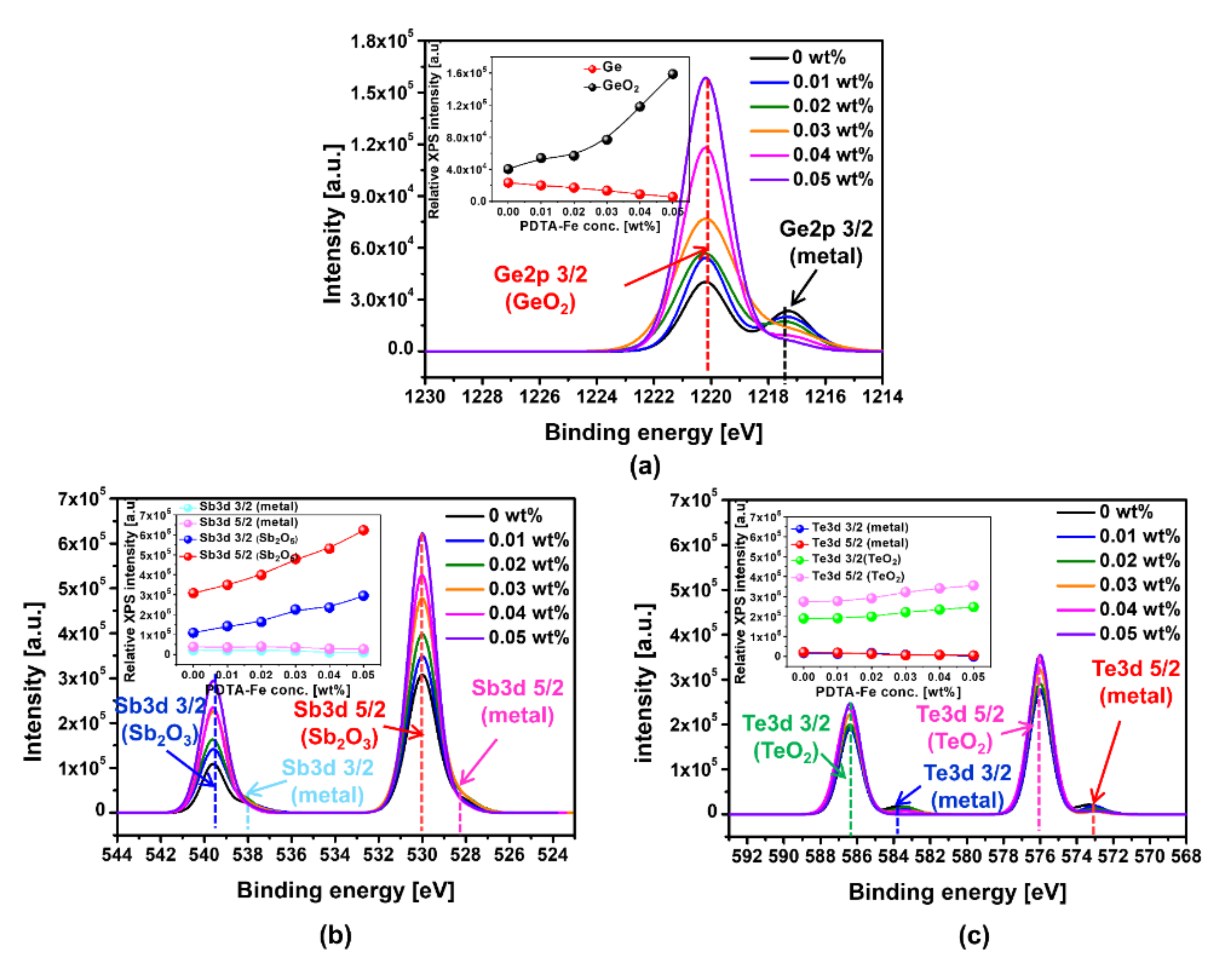
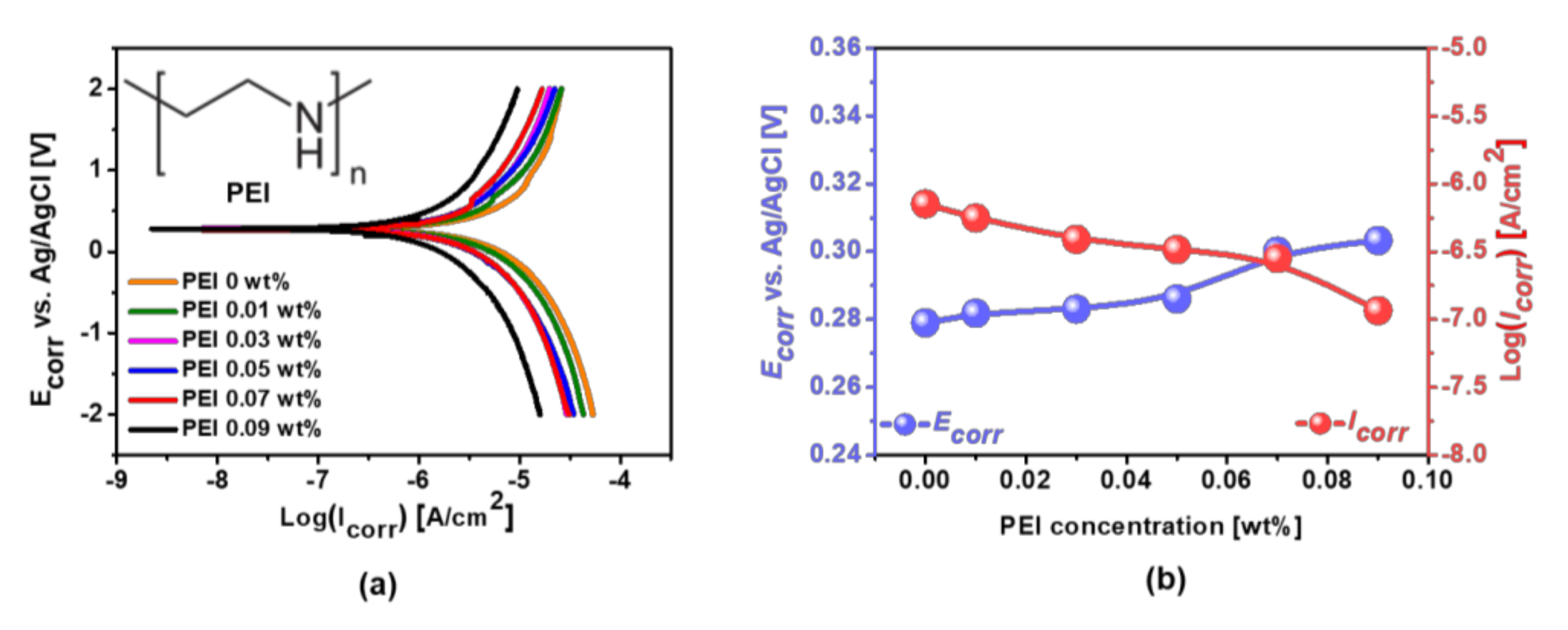
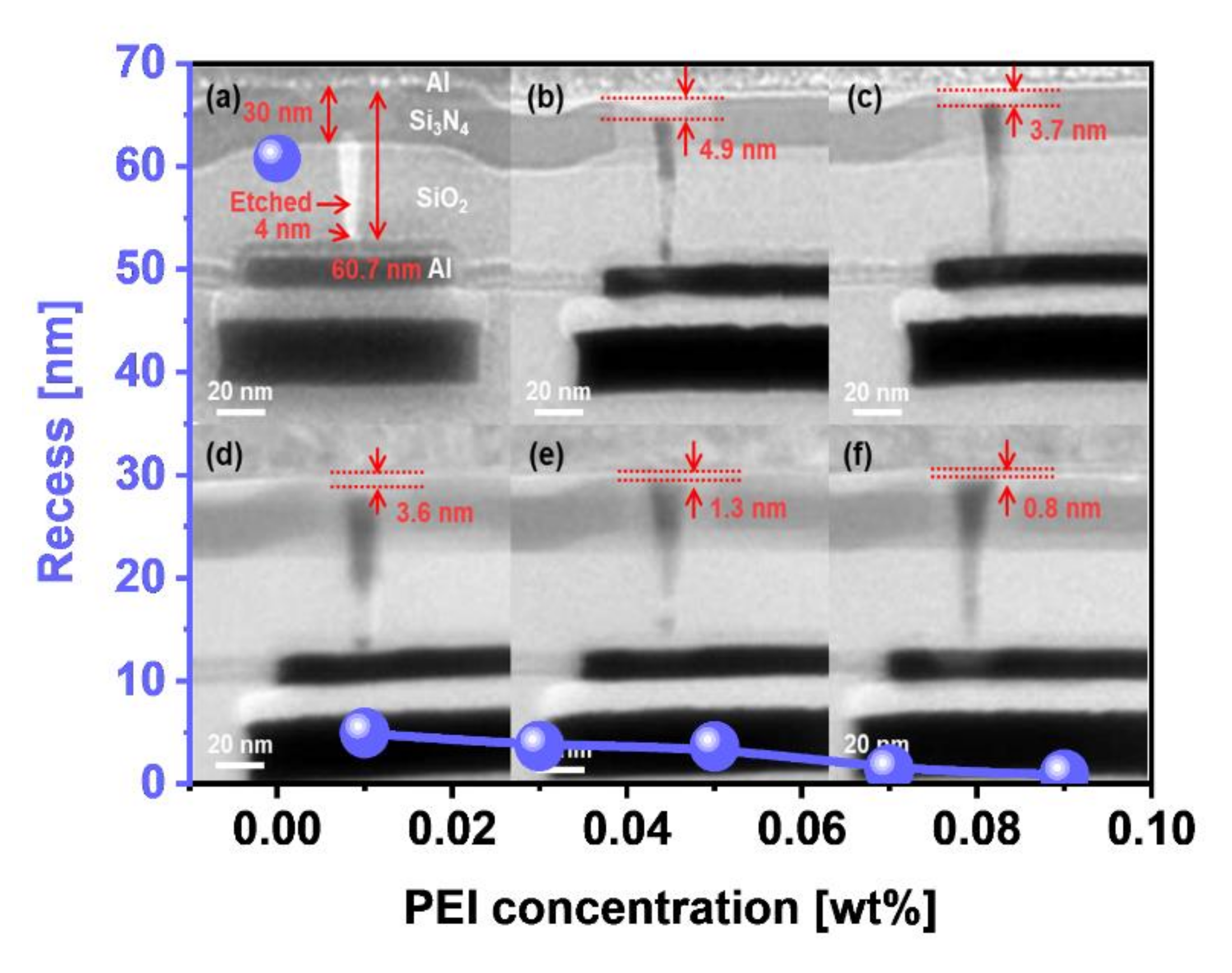
3.3. Effect of Corrosion Inhibitor with Protonated Amine Groups on Corrosion Suppression and Corrosion Inhibition Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Makarov, A.; Sverdlov, V.; Selberherr, S. Emerging Memory Technologies: Trends, Challenges, and Modeling Methods. Microelectron. Reliab. 2012, 52, 628–634. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, I.J.; Lee, J.S. CMOS-Compatible Ferroelectric NAND Flash Memory for High-Density, Low-Power, and High-Speed Three-Dimensional Memory. Sci. Adv. 2021, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Mohan, J.; Summerfelt, S.R.; Kim, J. Ferroelectric Hf0.5Zr0.5O2 Thin Films: A Review of Recent Advances. JOM 2019, 71, 246–255. [Google Scholar] [CrossRef]
- Raoux, S.; Burr, G.W.; Breitwisch, M.J.; Rettner, C.T.; Chen, Y.-C.; Shelby, R.M.; Salinga, M.; Krebs, D.; Chen, S.-H.; Lung, H.-L.; et al. Phase-Change Random Access Memory: A Scalable Technology. IBM J. Res. Dev. 2008, 52, 465–479. [Google Scholar] [CrossRef]
- Yamada, N.; Ohno, E.; Nishiuchi, K.; Akahira, N.; Takao, M. Rapid-Phase Transitions of GeTe-Sb2Te3 Pseudobinary Amorphous Thin Films for an Optical Disk Memory. J. Appl. Phys. 1991, 69, 2849–2856. [Google Scholar] [CrossRef]
- Wang, W.J.; Shi, L.P.; Zhao, R.; Lim, K.G.; Lee, H.K.; Chong, T.C.; Wu, Y.H. Fast Phase Transitions Induced by Picosecond Electrical Pulses on Phase Change Memory Cells. Appl. Phys. Lett. 2008, 93, 3–6. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Jeong, J.-H.; Cheong, B.-K. Overview of the Current Status of Technical Development for a Highly Scalable, High-Speed, Non-Volatile Phase-Change Memory. J. Semicond. Technol. Sci. 2008, 8, 1–10. [Google Scholar] [CrossRef]
- Xiong, F.; Yalon, E.; Behnam, A.; Neumann, C.M.; Grosse, K.L.; Deshmukh, S.; Pop, E. Towards Ultimate Scaling Limits of Phase-Change Memory. In Proceedings of the 2016 IEEE International Electron Devices Meeting (IEDM), San Francisco, CA, USA, 3–7 December 2016; pp. 4.1.1–4.1.4. [Google Scholar] [CrossRef]
- Kim, S.B.; Sosa, N.; Brightsky, M.; Mori, D.; Kim, W.; Zhu, Y.; Suu, K.; Lam, C. A Phase Change Memory Cell with Metal Nitride Liner as a Resistance Stabilizer to Reduce Read Current Noise for MLC Optimization. IEEE Trans. Electron Devices 2016, 63, 3922–3927. [Google Scholar] [CrossRef]
- Cho, S.L.; Yi, J.H.; Ha, Y.H.; Kuh, B.J.; Lee, C.M.; Park, J.H.; Nam, S.D.; Horii, H.; Cho, B.O.; Ryoo, K.C.; et al. Highly Scalable On-Axis Confined Cell Structure for High Density PRAM beyond 256Mb. In Proceedings of the 2005 Symposium on VLSI Technology, Kyoto, Japan, 14–16 June 2005; Volume 2005, pp. 96–97. [Google Scholar] [CrossRef]
- Feldmann, J.; Youngblood, N.; Li, X.; Wright, C.D.; Bhaskaran, H.; Pernice, W.H.P. Integrated 256 Cell Photonic Phase-Change Memory with 512-Bit Capacity. IEEE J. Select. Top. Quantum Electron. 2020, 26, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Fong, S.W.; Neumann, C.M.; Wong, H.-S.P. Phase-Change Memory—Towards a Storage-Class Memory. IEEE Trans. Electron Devices 2017, 64, 4374–4385. [Google Scholar] [CrossRef]
- Tang, Q.; He, T.; Yu, K.; Cheng, Y.; Qi, R.; Huang, R.; Zhao, J.; Song, W.; Song, Z. The Effect of Thickness on Texture of Ge2Sb2Te5 Phase-Change Films. J. Mater. Sci. Mater. Electron. 2020, 31, 5848–5853. [Google Scholar] [CrossRef]
- Song, Z.; Wang, R.; Xue, Y.; Song, S. The “Gene” of Reversible Phase Transformation of Phase Change Materials: Octahedral Motif. Nano Res. 2021, 12-20. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Zhang, H. The Study of Deep Trench Etch Process for PCRAM. In Proceedings of the 2017 China Semiconductor Technology International Conference (CSTIC), Shanghai, China, 12–13 March 2017. [Google Scholar] [CrossRef]
- Song, Y.J.; Ryoo, K.C.; Hwang, Y.N.; Jeong, C.W.; Lim, D.W.; Park, S.S.; Kim, J.I.; Kim, J.H.; Lee, S.Y.; Kong, J.H.; et al. Highly Reliable 256Mb PRAM with Advanced Ring Contact Technology and Novel Encapsulating Technology. In Proceedings of the 2006 Symposium on VLSI Technology, Honolulu, HI, USA, 13–15 June 2006; pp. 118–119. [Google Scholar] [CrossRef]
- Kim, W.; Brightsky, M.; Masuda, T.; Sosa, N.; Kim, S.; Bruce, R.; Carta, F.; Fraczak, G.; Cheng, H.Y.; Ray, A.; et al. ALD-Based Confined PCM with a Metallic Liner Toward Unlimited Endurance. In Proceedings of the 2016 IEEE International Electron Devices Meeting (IEDM), San Francisco, CA, USA, 3–7 December 2016; pp. 4.2.1–4.2.4. [Google Scholar] [CrossRef]
- Burr, G.W.; Shelby, R.M.; Sidler, S.; Di Nolfo, C.; Jang, J.; Boybat, I.; Shenoy, R.S.; Narayanan, P.; Virwani, K.; Giacometti, E.U.; et al. Experimental Demonstration and Tolerancing of a Large-Scale Neural Network (165 000 Synapses) Using Phase-Change Memory as the Synaptic Weight Element. IEEE Trans. Electron Devices 2015, 62, 3498–3507. [Google Scholar] [CrossRef]
- Sebastian, A.; Le Gallo, M.; Burr, G.W.; Kim, S.; Brightsky, M.; Eleftheriou, E. Tutorial: Brain-Inspired Computing Using Phase-Change Memory Devices. J. Appl. Phys. 2018, 124, 111101. [Google Scholar] [CrossRef] [Green Version]
- Sarwat, S.G. Materials Science and Engineering of Phase Change Random Access Memory. Mater. Sci. Technol. 2017, 33, 1890–1906. [Google Scholar] [CrossRef]
- Kim, S.; Ishii, M.; Lewis, S.; Perri, T.; Brightsky, M.; Kim, W.; Jordan, R.; Burr, G.W.; Sosa, N.; Ray, A.; et al. NVM Neuromorphic Core with 64k-Cell (256-by-256) Phase Change Memory Synaptic Array with On-Chip Neuron Circuits for Continuous In-Situ Learning. In Proceedings of the 2015 IEEE International Electron Devices Meeting (IEDM), Washington, DC, USA, 7–9 December 2015; pp. 17.1.1–17.1.4. [Google Scholar] [CrossRef]
- Kim, S.-B.; Cui, H.; Cho, J.-Y.; Seo, E.-B.; Yun, S.-S.; Son, Y.-H.; Jeong, G.-P.; Bae, J.-Y.; Park, J.-H.; Kang, S.-G.; et al. Surface-Tensile-Stress Induced Polishing-Voids Suppression via H2O2 Oxidizer Effect in Cross-Point Phase-Change-Memory-Cells. ECS J. Solid State Sci. Technol. 2019, 8, P667–P672. [Google Scholar] [CrossRef]
- Kim, S.; Cui, H.; Cho, J.; Seo, E.; Yoon, S.; Son, Y.; Jeong, G.; Bae, J.; Park, J.; Park, J. Surface-Tensile-Stress Induced Polishing-Voids in Cross-Point Phase-Change-Memory Cells: Corrosion Mechanism and Solution. Semicond. Sci. Technol. 2019, 34, 65002. [Google Scholar] [CrossRef]
- Feng, D.; Liu, W.; Liu, Y.; Song, Z.; Wang, W.; Wei, Z.; Zhao, G. Chemical Mechanical Planarization of Carbon-Doped Amorphous Ge2Sb2Te5 Film with Hydrogen Peroxide as Oxidizer in Alkaline Slurry. ECS J. Solid State Sci. Technol. 2020, 9, 024007. [Google Scholar] [CrossRef]
- Song, Z.; Liu, W.; Wang, L. Chemical mechanical polishing slurry for amorphous Ge2Sb2Te5. Procedia Eng. 2015, 102, 582–589. [Google Scholar] [CrossRef] [Green Version]
- Yun, S.S.; Son, Y.H.; Jeong, G.P.; Lee, J.H.; Jeong, J.H.; Bae, J.Y.; Kim, S.I.; Park, J.H.; Park, J.G. Dishing-Free Chemical Mechanical Planarization for Copper Films. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126143. [Google Scholar] [CrossRef]
- Seo, E.-B.; Bae, J.-Y.; Kim, S.-I.; Choi, H.-E.; Son, Y.-H.; Yun, S.-S.; Park, J.-H.; Park, J.-G. Interfacial Chemical and Mechanical Reactions between Tungsten-Film and Nano-Scale Colloidal Zirconia Abrasives for Chemical-Mechanical-Planarization. ECS J. Solid State Sci. Technol. 2020, 9, 054001. [Google Scholar] [CrossRef]
- Seo, E.-B.; Bae, J.-Y.; Kim, S.-I.; Choi, H.-E.; Kim, P.; Lee, J.-C.; Son, Y.-H.; Yun, S.-S.; Park, J.-H.; Park, J.-G. Influence of Scavenger on Abrasive Stability Enhancement and Chemical and Mechanical Properties for Tungsten-Film Chemical-Mechanical-Planarization. ECS J. Solid State Sci. Technol. 2020, 9, 065001. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, G.-P.; Son, Y.-H.; Park, J.-S.; Kim, P.-S.; Han, M.-H.; Hong, S.-W.; Park, J.-H.; Cui, H.; Yoon, B.-U.; Park, J.-G. Fenton Reaction for Enhancing Polishing Rate and Protonated Amine Functional Group Polymer for Inhibiting Corrosion in Ge1Sb4Te5 Film Surface Chemical-Mechanical-Planarization. Appl. Sci. 2021, 11, 10872. https://doi.org/10.3390/app112210872
Jeong G-P, Son Y-H, Park J-S, Kim P-S, Han M-H, Hong S-W, Park J-H, Cui H, Yoon B-U, Park J-G. Fenton Reaction for Enhancing Polishing Rate and Protonated Amine Functional Group Polymer for Inhibiting Corrosion in Ge1Sb4Te5 Film Surface Chemical-Mechanical-Planarization. Applied Sciences. 2021; 11(22):10872. https://doi.org/10.3390/app112210872
Chicago/Turabian StyleJeong, Gi-Ppeum, Young-Hye Son, Jun-Seong Park, Pil-Su Kim, Man-Hyup Han, Seong-Wan Hong, Jin-Hyung Park, Hao Cui, Bo-Un Yoon, and Jea-Gun Park. 2021. "Fenton Reaction for Enhancing Polishing Rate and Protonated Amine Functional Group Polymer for Inhibiting Corrosion in Ge1Sb4Te5 Film Surface Chemical-Mechanical-Planarization" Applied Sciences 11, no. 22: 10872. https://doi.org/10.3390/app112210872





