Adverse Events Following COVID-19 Vaccine in Patients Previously Injected with Facial Filler: Scoping Review and Case Report
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
2.4. Article and Data Selection Process
2.5. Data Items
2.6. Case Report
3. Results
Study Selection and Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AE | Adverse events |
| ASAPS | American Society of Aesthetic Plastic Surgery |
| ASIA | Autoimmune/inflammatory Syndrome Induced by Adjuvants |
| BDDE | 1,4-butanediol diglycidyl ether |
| COVID-19 | Coronavirus Disease 19 |
| CSF | Cosmetic Surgery Forum |
| FDA | Food and Drugs Administration |
| HA | Hyaluronic Acid |
| HMW | High Molecular Weight |
| ICU | Intensive Care Unit |
| LMW | Low Molecular Weight |
| PEG | Polyethylene glycol |
| WHO | World Health Organization |
References
- Ghebreyesus, T.A. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19 11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-11-march-2020 (accessed on 27 September 2021).
- Kaye, K.; Paprottka, F.; Escudero, R.; Casabona, G.; Montes, J.; Fakin, R.; Moke, L.; Stasch, T.; Richter, D.; Benito-Ruiz, J. Elective, Non-urgent Procedures and Aesthetic Surgery in the Wake of SARS-COVID-19: Considerations Regarding Safety, Feasibility and Impact on Clinical Management. Aesthetic. Plast Surg. 2020, 44, 1014–1042. [Google Scholar] [CrossRef] [PubMed]
- Soreide, K.; Hallet, J.; Matthews, J.B.; Schnitzbauer, A.A.; Line, P.D.; Lai, P.B.S.; Otero, J.; Callegaro, D.; Warner, S.G.; Baxter, N.N.; et al. Immediate and long-term impact of the COVID-19 pandemic on delivery of surgical services. Br. J. Surg. 2020, 107, 1250–1261. [Google Scholar] [CrossRef]
- Rauso, R.; Chirico, F.; Federico, F.; Francesco Nicoletti, G.; Colella, G.; Fragola, R.; Pafundi, P.C.; Tartaro, G. Maxillo-facial reconstruction following cancer ablation during COVID-19 pandemic in southern Italy. Oral Oncol. 2021, 115, 105114. [Google Scholar] [CrossRef] [PubMed]
- Koscielecka, K.E.; Kuc, A.J.; Kubik, D.M.; Mecik-Kronenberg, T.; Ceglarz, D. Impact of the Covid-19 Pandemic on the Availability of Medical Care among Oncological Patients. Wiad. Lek. 2021, 74, 1542–1551. [Google Scholar] [CrossRef] [PubMed]
- Shanbehzadeh, M.; Kazemi-Arpanahi, H.; Kalkhajeh, S.G.; Basati, G. Systematic review on telemedicine platforms in lockdown periods: Lessons learned from the COVID-19 pandemic. J. Educ. Health Promot. 2021, 10, 211. [Google Scholar] [CrossRef] [PubMed]
- Adejumo, O.A.; Adejumo, O.A. Prospects of telemedicine during and post COVID-19: Highlighting the environmental health implications. Malawi Med. J. 2020, 32, 235–238. [Google Scholar] [CrossRef]
- Kim, H.; Lee, H.; Park, C.S.; Kim, S.; Cho, S.A.; Yoo, S.M.; Kim, J.A.; Lee, J.Y. Preliminary Results of Teleconsultations Temporarily Allowed during the COVID-19 Pandemic. Yonsei Med. J. 2021, 62, 850–857. [Google Scholar] [CrossRef]
- Lo Giudice, G.; Caterino, M.; Rauso, R.; Colella, G. The Multidisciplinary Approach in Head and Neck Oncology during COVID-19 Pandemic. J. Craniofac Surg 2021, 32, e835. [Google Scholar] [CrossRef]
- Iqbal, M. Zoom Revenue and Usage Statistics (2021). Available online: https://www.businessofapps.com/data/zoom-statistics/ (accessed on 27 September 2021).
- The Aesthetic Society. Aesthetic Plastic Surgery National Databank Statistics 2019. Available online: https://www.surgery.org/sites/default/files/Aesthetic-Society_Stats2019Book_FINAL.pdf (accessed on 27 September 2021).
- The Aesthetic Society. Aesthetic Plastic Surgery National Databank Statistics 2020. Available online: https://cdn.theaestheticsociety.org/media/statistics/aestheticplasticsurgerynationaldatabank-2020stats.pdf (accessed on 27 September 2021).
- Pfund, G.N.; Hill, P.L.; Harriger, J. Video chatting and appearance satisfaction during COVID-19: Appearance comparisons and self-objectification as moderators. Int. J. Eat. Disord. 2020, 53, 2038–2043. [Google Scholar] [CrossRef]
- Chen, J.; Chow, A.; Fadavi, D.; Long, C.; Sun, A.H.; Cooney, C.M.; Broderick, K.P. The Zoom Boom: How Video Calling Impacts Attitudes towards Aesthetic Surgery in the COVID-19 Era. Aesthet Surg. J. 2021, 41, NP2086–NP2093. [Google Scholar] [CrossRef]
- Rauso, R.; Tartaro, G.; Chirico, F.; Zerbinati, N.; Albani, G.; Rugge, L. Rhinofilling with hyaluronic acid thought as a cartilage graft. J. Craniomaxillofac. Surg. 2020, 48, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Rauso, R.; Federico, F.; Zerbinati, N.; De Cicco, D.; Nicoletti, G.F.; Tartaro, G. Hyaluronic Acid Injections to Correct Lips Deformity Following Surgical Removal of Permanent Implant. J. Craniofac. Surg. 2020, 31, e604–e606. [Google Scholar] [CrossRef] [PubMed]
- Rauso, R.; Sesenna, E.; Fragola, R.; Zerbinati, N.; Nicoletti, G.F.; Tartaro, G. Skin Necrosis and Vision Loss or Impairment after Facial Filler Injection. J. Craniofac. Surg. 2020, 31, 2289–2293. [Google Scholar] [CrossRef] [PubMed]
- Rauso, R.; Zerbinati, N.; Fragola, R.; Nicoletti, G.F.; Tartaro, G. Transvascular Hydrolysis of Hyaluronic Acid Filler with Hyaluronidase: An Ex Vivo Study. Derm. Surg. 2021, 47, 370–372. [Google Scholar] [CrossRef]
- Rauso, R.; Zerbinati, N.; Franco, R.; Chirico, F.; Ronchi, A.; Sesenna, E.; Colella, G.; Tartaro, G. Cross-linked hyaluronic acid filler hydrolysis with hyaluronidase: Different settings to reproduce different clinical scenarios. Derm. Ther. 2020, 33, e13269. [Google Scholar] [CrossRef]
- Vaccines and Related Biological Products Advisory Committee Meeting 17 December 2020. Available online: https://www.fda.gov/media/144434/download (accessed on 27 September 2021).
- Cirillo, N. Reported orofacial adverse effects of COVID-19 vaccines: The knowns and the unknowns. J. Oral Pathol. Med. 2021, 50, 424–427. [Google Scholar] [CrossRef]
- Riad, A.; Pokorna, A.; Attia, S.; Klugarova, J.; Koscik, M.; Klugar, M. Prevalence of COVID-19 Vaccine Side Effects among Healthcare Workers in the Czech Republic. J. Clin. Med. 2021, 10, 1428. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Michon, A. Hyaluronic acid soft tissue filler delayed inflammatory reaction following COVID-19 vaccination-A case report. J. Cosmet. Derm. 2021, 20, 2684–2690. [Google Scholar] [CrossRef]
- McMahon, D.E.; Amerson, E.; Rosenbach, M.; Lipoff, J.B.; Moustafa, D.; Tyagi, A.; Desai, S.R.; French, L.E.; Lim, H.W.; Thiers, B.H.; et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases. J. Am. Acad. Derm. 2021, 85, 46–55. [Google Scholar] [CrossRef]
- Munavalli, G.G.; Guthridge, R.; Knutsen-Larson, S.; Brodsky, A.; Matthew, E.; Landau, M. COVID-19/SARS-CoV-2 virus spike protein-related delayed inflammatory reaction to hyaluronic acid dermal fillers: A challenging clinical conundrum in diagnosis and treatment. Arch. Derm. Res. 2021. [Google Scholar] [CrossRef]
- Munavalli, G.G.; Knutsen-Larson, S.; Lupo, M.P.; Geronemus, R.G. Oral angiotensin-converting enzyme inhibitors for treatment of delayed inflammatory reaction to dermal hyaluronic acid fillers following COVID-19 vaccination-a model for inhibition of angiotensin II-induced cutaneous inflammation. JAAD Case Rep. 2021, 10, 63–68. [Google Scholar] [CrossRef]
- Artzi, O.; Cohen, J.L.; Dover, J.S.; Suwanchinda, A.; Pavicic, T.; Landau, M.; Goodman, G.J.; Ghannam, S.; Al Niaimi, F.; van Loghem, J.A.J.; et al. Delayed Inflammatory Reactions to Hyaluronic Acid Fillers: A Literature Review and Proposed Treatment Algorithm. Clin. Cosmet. Investig. Derm. 2020, 13, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Shoenfeld, Y.; Agmon-Levin, N. ‘ASIA’-Autoimmune/inflammatory syndrome induced by adjuvants. J. Autoimmun. 2011, 36, 4–8. [Google Scholar] [CrossRef]
- Alijotas-Reig, J.; Fernandez-Figueras, M.T.; Puig, L. Late-onset inflammatory adverse reactions related to soft tissue filler injections. Clin. Rev. Allergy Immunol. 2013, 45, 97–108. [Google Scholar] [CrossRef]
- Vaccino COVID-19 Moderna & Filler-Position Statement Ufficiale del Collegio Italiano delle Società Scientifiche di Medicina Estetica. Available online: https://www.lamedicinaestetica.it/wp-content/uploads/2021/01/POSITION-STATEMENT-VACCINO-COVID19-MODERNA-E-HA.pdf (accessed on 27 September 2021).
- Freeman, E.E.; McMahon, D.E.; Lipoff, J.B.; Rosenbach, M.; Kovarik, C.; Desai, S.R.; Harp, J.; Takeshita, J.; French, L.E.; Lim, H.W.; et al. The spectrum of COVID-19-associated dermatologic manifestations: An international registry of 716 patients from 31 countries. J. Am. Acad. Derm. 2020, 83, 1118–1129. [Google Scholar] [CrossRef]
- Rice, S.M.; Ferree, S.D.; Kourosh, A.S. Coronavirus Vaccine Considerations for the Aesthetic Patient. Facial Plast. Surg. Aesthet. Med. 2021, 23, 75–76. [Google Scholar] [CrossRef]
- Garvey, L.H.; Nasser, S. Anaphylaxis to the first COVID-19 vaccine: Is polyethylene glycol (PEG) the culprit? Br. J. Anaesth. 2021, 126, e106–e108. [Google Scholar] [CrossRef]
- Chen, B.M.; Cheng, T.L.; Roffler, S.R. Polyethylene Glycol Immunogenicity: Theoretical, Clinical, and Practical Aspects of Anti-Polyethylene Glycol Antibodies. ACS Nano 2021, 15, 14022–14048. [Google Scholar] [CrossRef] [PubMed]
- Zerbinati, N.; Haddad, R.G.; Bader, A.; Rauso, R.; D’Este, E.; Cipolla, G.; Calligaro, A.; Gonzalez, P.; Salvatore, S.; Serafin, D. A new hyaluronic acid polymer in the augmentation and restoration of labia majora. J. Biol. Regul Homeost. Agents 2017, 31, 153–161. [Google Scholar]
- Zerbinati, N.; Lotti, T.; Monticelli, D.; Rauso, R.; Gonzalez-Isaza, P.; D’Este, E.; Calligaro, A.; Sommatis, S.; Maccario, C.; Mocchi, R.; et al. In Vitro Evaluation of the Biosafety of Hyaluronic Acid PEG Cross-Linked with Micromolecules of Calcium Hydroxyapatite in Low Concentration. Open Access Maced. J Med. Sci. 2018, 6, 15–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zerbinati, N.; D’Este, E.; Farina, A.; Rauso, R.; Cherubino, M.; Calligaro, A. Morphological evidences following pegylated filler treatment in human skin. J. Biol. Regul. Homeost. Agents 2017, 31, 79–85. [Google Scholar] [PubMed]
- Humphrey, S.; Jones, D.H.; Carruthers, J.D.; Carruthers, A.; Beleznay, K.; Wesley, N.; Black, J.M.; Vanderveen, S.; Minokadeh, A. Retrospective review of delayed adverse events secondary to treatment with a smooth, cohesive 20-mg/mL hyaluronic acid filler in 4500 patients. J. Am. Acad. Derm. 2020, 83, 86–95. [Google Scholar] [CrossRef]
- Kim, J.E.; Sykes, J.M. Hyaluronic acid fillers: History and overview. Facial. Plast. Surg. 2011, 27, 523–528. [Google Scholar] [CrossRef]
- Decates, T.; Kadouch, J.; Velthuis, P.; Rustemeyer, T. Immediate nor Delayed Type Hypersensitivity Plays a Role in Late Inflammatory Reactions After Hyaluronic Acid Filler Injections. Clin. Cosmet. Investig. Derm. 2021, 14, 581–589. [Google Scholar] [CrossRef]
- Sadeghpour, M.; Quatrano, N.A.; Bonati, L.M.; Arndt, K.A.; Dover, J.S.; Kaminer, M.S. Delayed-Onset Nodules to Differentially Crosslinked Hyaluronic Acids: Comparative Incidence and Risk Assessment. Derm. Surg. 2019, 45, 1085–1094. [Google Scholar] [CrossRef]
- Niforos, F.; Acquilla, R.; Ogilvie, P.; Safa, M.; Signorini, M.; Creutz, L.; Kerson, G.; Silberberg, M. A Prospective, Open-Label Study of Hyaluronic Acid-Based Filler with Lidocaine (VYC-15L) Treatment for the Correction of Infraorbital Skin Depressions. Derm. Surg. 2017, 43, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Beleznay, K.; Carruthers, J.D.; Carruthers, A.; Mummert, M.E.; Humphrey, S. Delayed-onset nodules secondary to a smooth cohesive 20 mg/mL hyaluronic acid filler: Cause and management. Derm. Surg. 2015, 41, 929–939. [Google Scholar] [CrossRef]
- Mikkilineni, R.; Wipf, A.; Farah, R.; Sadick, N. New Classification Schemata of Hypersensitivity Adverse Effects after Hyaluronic Acid Injections: Pathophysiology, Treatment Algorithm, and Prevention. Derm. Surg. 2020, 46, 1404–1409. [Google Scholar] [CrossRef]
- Rowland-Warmann, M.J. Hypersensitivity reaction to Hyaluronic Acid Dermal filler following novel Coronavirus infection-A case report. J. Cosmet Derm. 2021, 20, 1557–1562. [Google Scholar] [CrossRef]
- Artzi, O.; Loizides, C.; Verner, I.; Landau, M. Resistant and Recurrent Late Reaction to Hyaluronic Acid-Based Gel. Derm. Surg. 2016, 42, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Turkmani, M.G.; De Boulle, K.; Philipp-Dormston, W.G. Delayed hypersensitivity reaction to hyaluronic acid dermal filler following influenza-like illness. Clin. Cosmet. Investig. Derm. 2019, 12, 277–283. [Google Scholar] [CrossRef] [Green Version]
- Goodman, G.J.; Swift, A.; Remington, B.K. Current Concepts in the Use of Voluma, Volift, and Volbella. Plast. Reconstr. Surg. 2015, 136, 139S–148S. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan as an immune regulator in human diseases. Physiol. Rev. 2011, 91, 221–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheibner, K.A.; Lutz, M.A.; Boodoo, S.; Fenton, M.J.; Powell, J.D.; Horton, M.R. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J. Immunol. 2006, 177, 1272–1281. [Google Scholar] [CrossRef] [Green Version]
- Schlessinger, J. Update on COVID-19 Vaccines and Dermal Fillers. Available online: https://practicaldermatology.com/articles/2021-feb/update-on-covid-19-vaccines-and-dermal-fillers (accessed on 27 September 2021).

| Author | Patient Features (Gender; Age) | Medical Status | Filler Injected/Anatomical Site | Injection Date | Time-Lapse between COVID-19 Vaccine and Adverse Event | Adverse Event | Treatment Performed | Outcome |
|---|---|---|---|---|---|---|---|---|
| Michon A. [24] | F; 39 | Physically fit and healthy, and without any medical comorbidities nor allergies. | Juvéderm Volite (Allergan, Irvine CA)/tear trough | October 2020 | 2 days after the 1st dose of mRNA Pfizer-BioNTech COVID-19 vaccine (April 2021) | Tender, erythematous swelling at the left tear trough area | Watch-and-wait. | Resolution |
| Michon A. [24] | F; 61 | Physically healthy, without allergies or significant medical history, except for intermittent benign vertigo. | Juvéderm Voluma; Juvederm Volux; Juvéderm Volift; Juvederm Volbella (Allergan, Irvine CA)/Pan facial injections | June 2020 | A few days after the 1st dose of mRNA Pfizer-BioNTech COVID-19 vaccine (April 2021) | Intermittent facial swelling on either side of the face, localized primarily at the cheeks and undereye | Hyaluronidase injections | Resolution |
| McMahon et al. [25] | 3 patients (gender and age N.S.) | N.S. | N.S. | N.S. | After the 1st dose of mRNA Moderna COVID-19 vaccine | Swelling at the site of cosmetic filler injection | N.S. | N.S. |
| McMahon et al. [25] | 5 patients (gender and N.S.) | N.S. | N.S. | N.S. | After the 2st dose of mRNA Moderna COVID-19 vaccine | Swelling at the site of cosmetic filler injection | N.S. | N.S. |
| McMahon et al. [25] | 1 patient (gender and N.S.) | N.S. | N.S. | N.S. | After the 2st dose of mRNA Pfizer-BioNTech COVID-19 vaccine | Swelling at the site of cosmetic filler injection | N.S. | N.S. |
| Munavalli et al. [26] | F; 36 | Healthy | Juvederm. Voluma (Allergan, Irvine CA)/tear troughs. Juvederm. Ultra (Allergan, Irvine CA)/upper and lower lip | November 2019 | 24/48 h following the 1st dose of mRNA Moderna COVID-19 vaccine (5 January 2020) | Unilateral infraorbital edema and perioral edema | Early certirizine administration (twice); due to worsening Lisinopril was administrated. | Resolution |
| Munavalli et al. [26] | F; 43 | Healthy | Juvederm Voluma (Allergan, Irvine CA)/lateral parts of her cheeks. | 2.5 years before | 24 h following the 2nd dose of mRNA Pfizer-BioNTech COVID-19 vaccine (7 January 2021) | Swelling in periorbital area | Medrol administration; linisopril administration reported on the second paper (JAAD Case Reports 2021;10:63-8.) | Resolution |
| Munavalli et al. [27] | F; 76 | Healthy | Juvederm Ultra (Allergan, Irvine CA)/cheeks | 2019 | Ten days after the first dose of mRNA Pfizer-BioNTech COVID-19 Vaccine (date not reported) | Panfacial and periorbital swelling | Linisopril administration | Resolution |
| Munavalli et al. [27] | F; 31 | No known drug allergies and no history of angioedema | Juvederm Volbella (Allergan, Irvine CA)/Lips. Juvederm Voluma (Allergan, Irvine CA)/Earlobes. Juvederm Ultra (Allergan, Irvine CA)/Nasolabial folds. Juvederm Voluma (Allergan, Irvine CA)/malar cheeks | Fall 2018, October 2018, June 2019 and June 2020 | Twenty-four hours after the second dose of mRNA Moderna COVID-19 vaccine (January/February 2021) | Swelling of the upper mucosal lip, which progressed to involve the left earlobe and bilateral zygomas | Linisopril administration | Resolution |
| Moderna Clinical Trial [20] | F; 46 | Healthy | Juvederm XC (Allergan, Irvine CA)/Cheeks | March 2020 | Within 7 days from the 2nd dose of mRNA Moderna COVID-19 vaccine (29 September 2020) | Significant bilateral cheek swelling | Diphenhydramine and methylprednisolone administration | Resolution |
| Moderna Clinical Trial [20] | F; 51 | Healthy | Juvederm XC (Allergan, Irvine CA)/Cheeks | October 2020 | Within 7 days from the 2nd dose of mRNA Moderna COVID-19 vaccine (15 October 2020) | Bilateral facial swelling | Prednisolone administration | Resolution |
| Moderna Clinical Trial [20] | F; 29 | The patient reported the same lip angioedema after receipt of an influenza vaccine in the past. | N.S./lips | unknown how long prior to vaccination | 2 days after vaccine injection (date not reported) | Lip angioedema | N.S. | It is assumed to be solved |
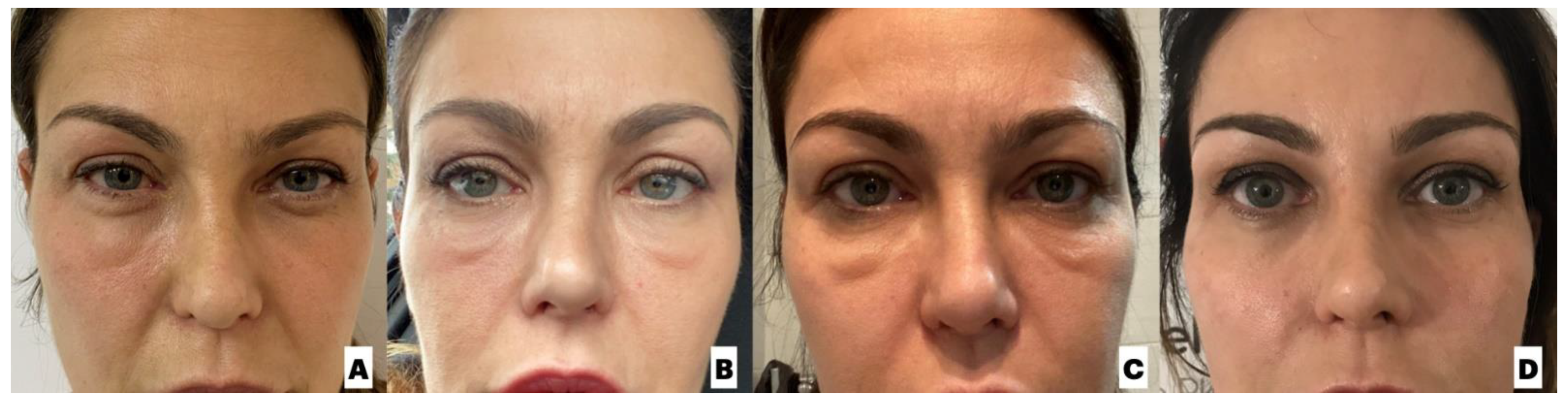
| Rauso et al. (unpublished data) | F; 44 | Healthy. Tardive lower eyelid swelling already experienced following Juv ederm Volbella (Allergan), solved following 3 session of hyaluronidase injections | Redensity II (Teoxane; Geneve, Swiss)/tear trough | January 2020 | Seven days after the second dose of mRNA Pfizer-BioNTech COVID-19 vaccine (8 June 2021) | Right lower eyelid swelling and ipsilateral conjunctivitis | Watch-and-wait | Resolution |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rauso, R.; Lo Giudice, G.; Zerbinati, N.; Nicoletti, G.F.; Fragola, R.; Tartaro, G. Adverse Events Following COVID-19 Vaccine in Patients Previously Injected with Facial Filler: Scoping Review and Case Report. Appl. Sci. 2021, 11, 10888. https://doi.org/10.3390/app112210888
Rauso R, Lo Giudice G, Zerbinati N, Nicoletti GF, Fragola R, Tartaro G. Adverse Events Following COVID-19 Vaccine in Patients Previously Injected with Facial Filler: Scoping Review and Case Report. Applied Sciences. 2021; 11(22):10888. https://doi.org/10.3390/app112210888
Chicago/Turabian StyleRauso, Raffaele, Giorgio Lo Giudice, Nicola Zerbinati, Giovanni Francesco Nicoletti, Romolo Fragola, and Gianpaolo Tartaro. 2021. "Adverse Events Following COVID-19 Vaccine in Patients Previously Injected with Facial Filler: Scoping Review and Case Report" Applied Sciences 11, no. 22: 10888. https://doi.org/10.3390/app112210888
APA StyleRauso, R., Lo Giudice, G., Zerbinati, N., Nicoletti, G. F., Fragola, R., & Tartaro, G. (2021). Adverse Events Following COVID-19 Vaccine in Patients Previously Injected with Facial Filler: Scoping Review and Case Report. Applied Sciences, 11(22), 10888. https://doi.org/10.3390/app112210888









