Developments in Smart Multi-Function Gait Assistive Devices for the Prevention and Treatment of Knee Osteoarthritis—A Literature Review
Abstract
:Featured Application
Abstract
1. Introduction
2. Biomechanical Factors for Knee Osteoarthritis
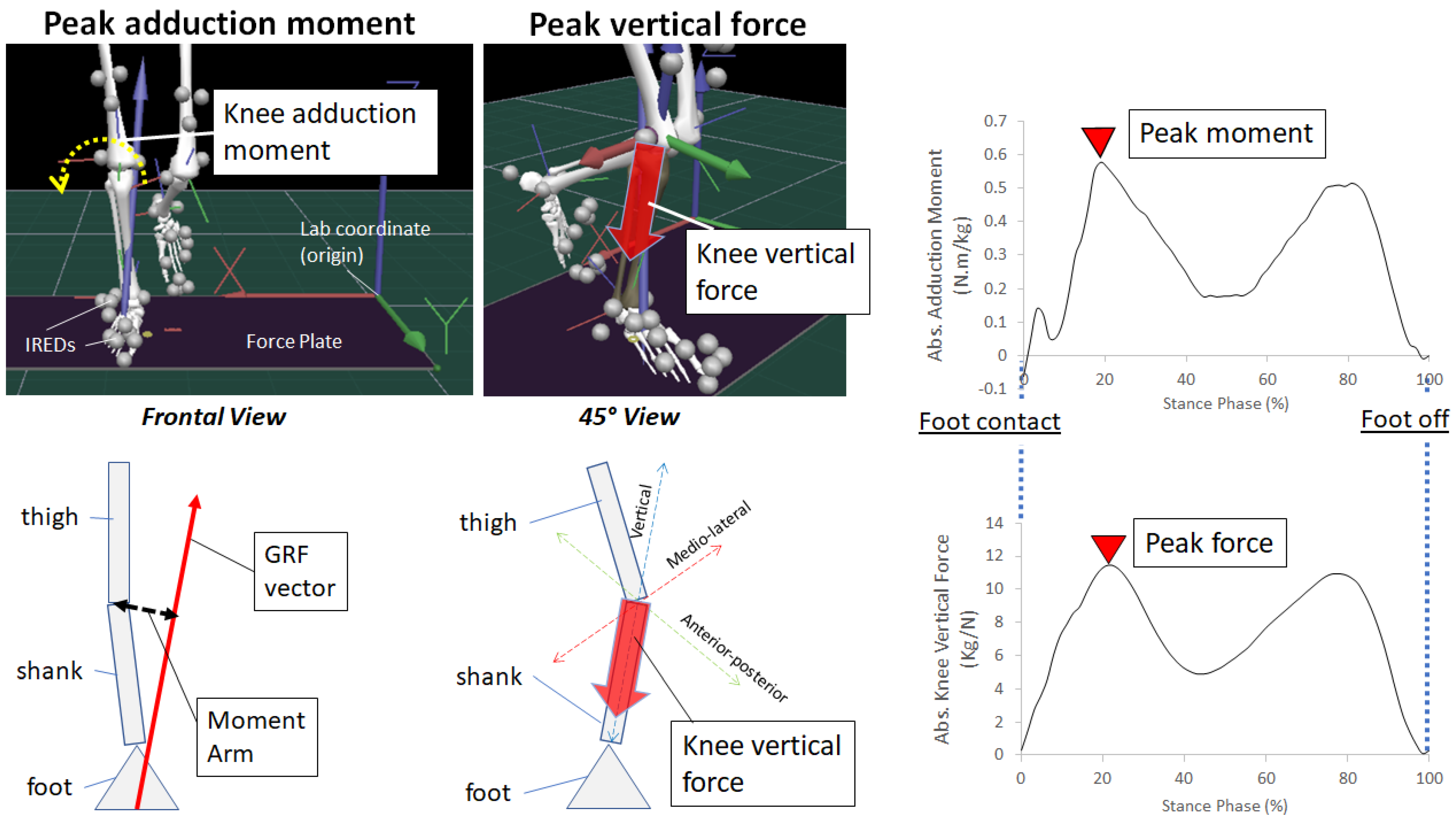
2.1. Knee Adduction Moment
2.2. Knee Compression Forces
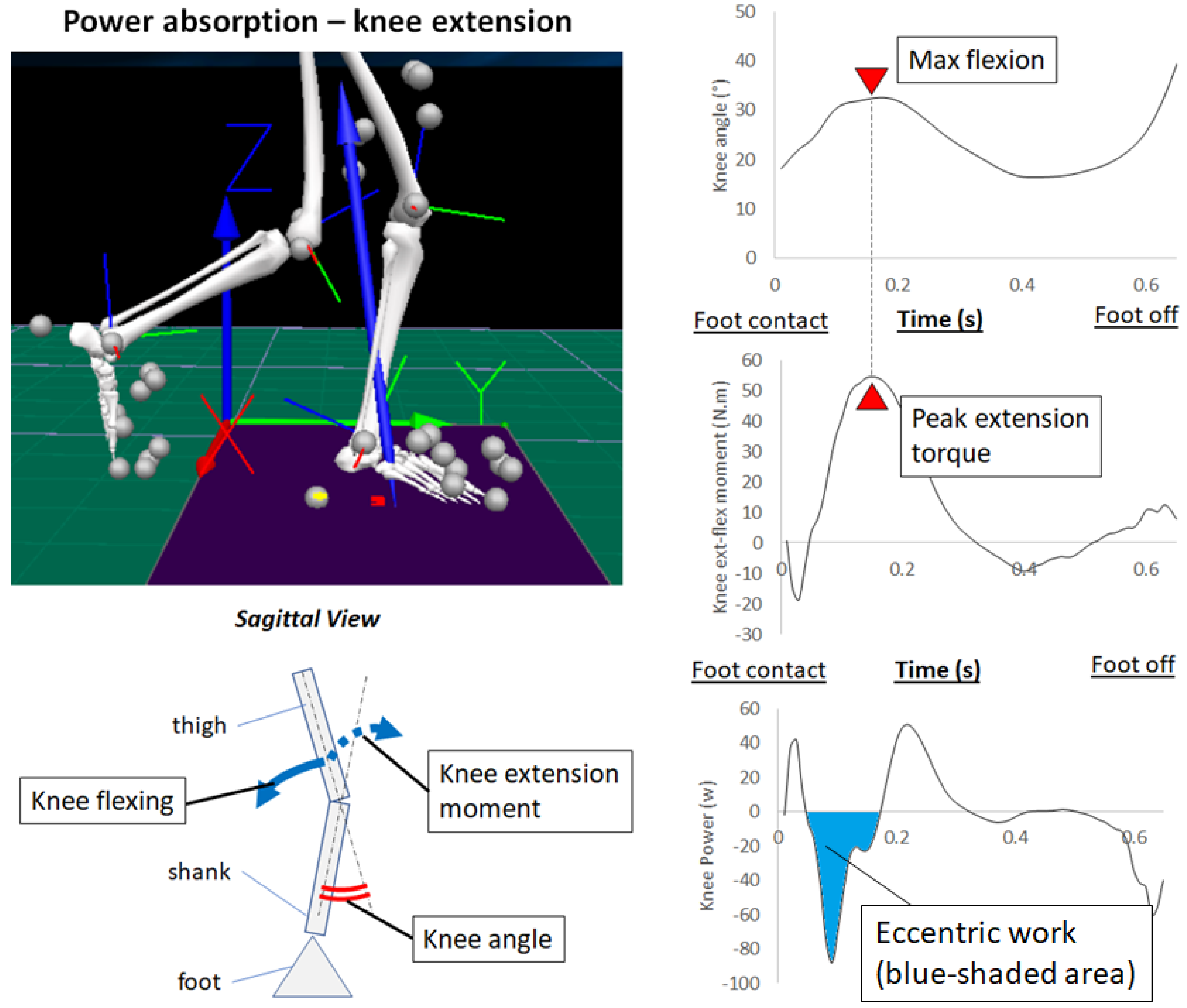
2.3. Power Absorption
2.4. Foot Contact Impact
3. Passive Mechanics of Wearable Devices for Knee OA Prevention
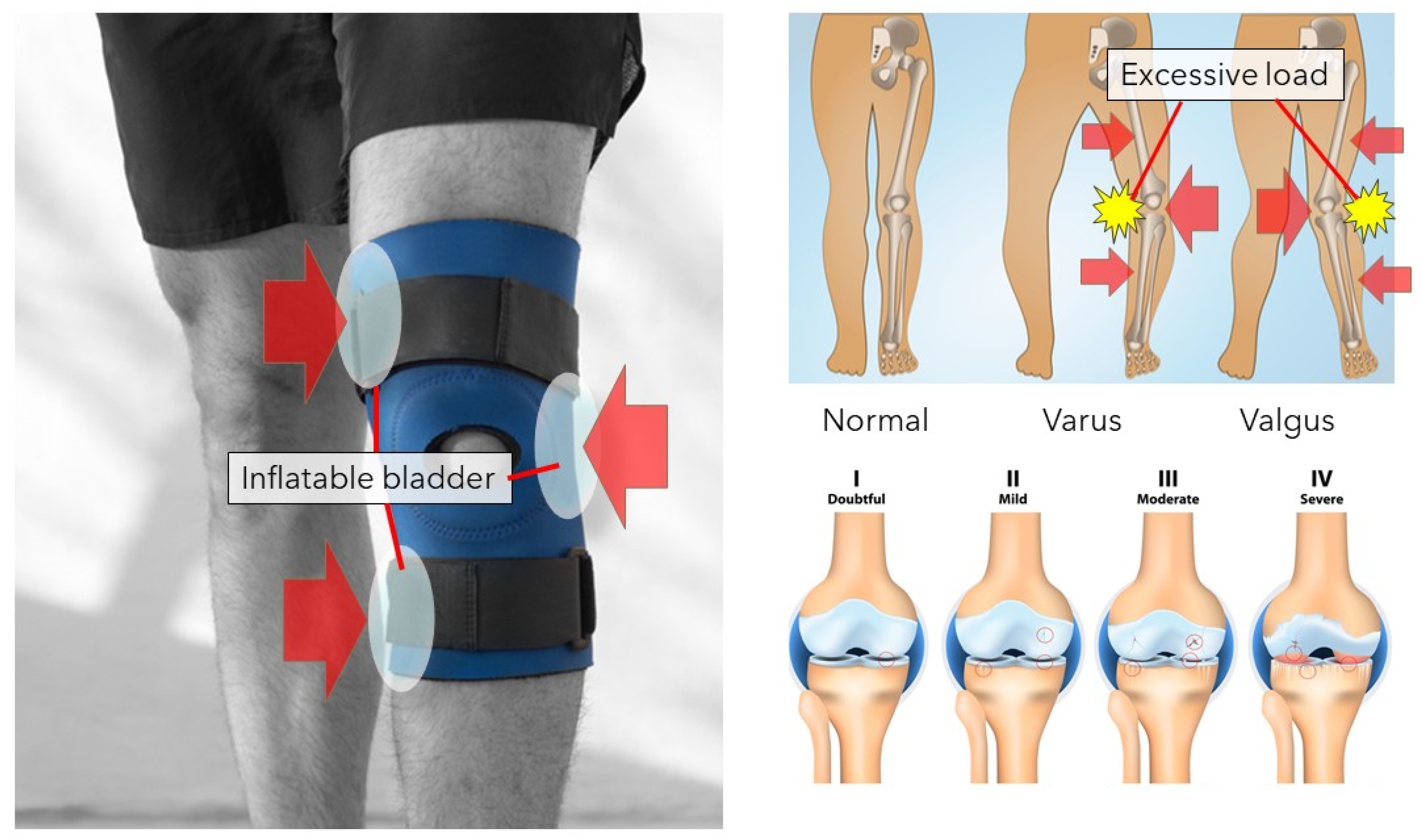
3.1. Knee Orthosis
3.2. Shoe Insole
4. Incorporation of Wearable Sensors to Measure OA Risks
4.1. Laboratory-Based 3D Motion Capture Systems
4.2. Inertial Measurement Units (IMUs)
4.3. Force Transducer
4.4. Electromyography (EMG)
4.5. Foot Pressure Measurement Insole
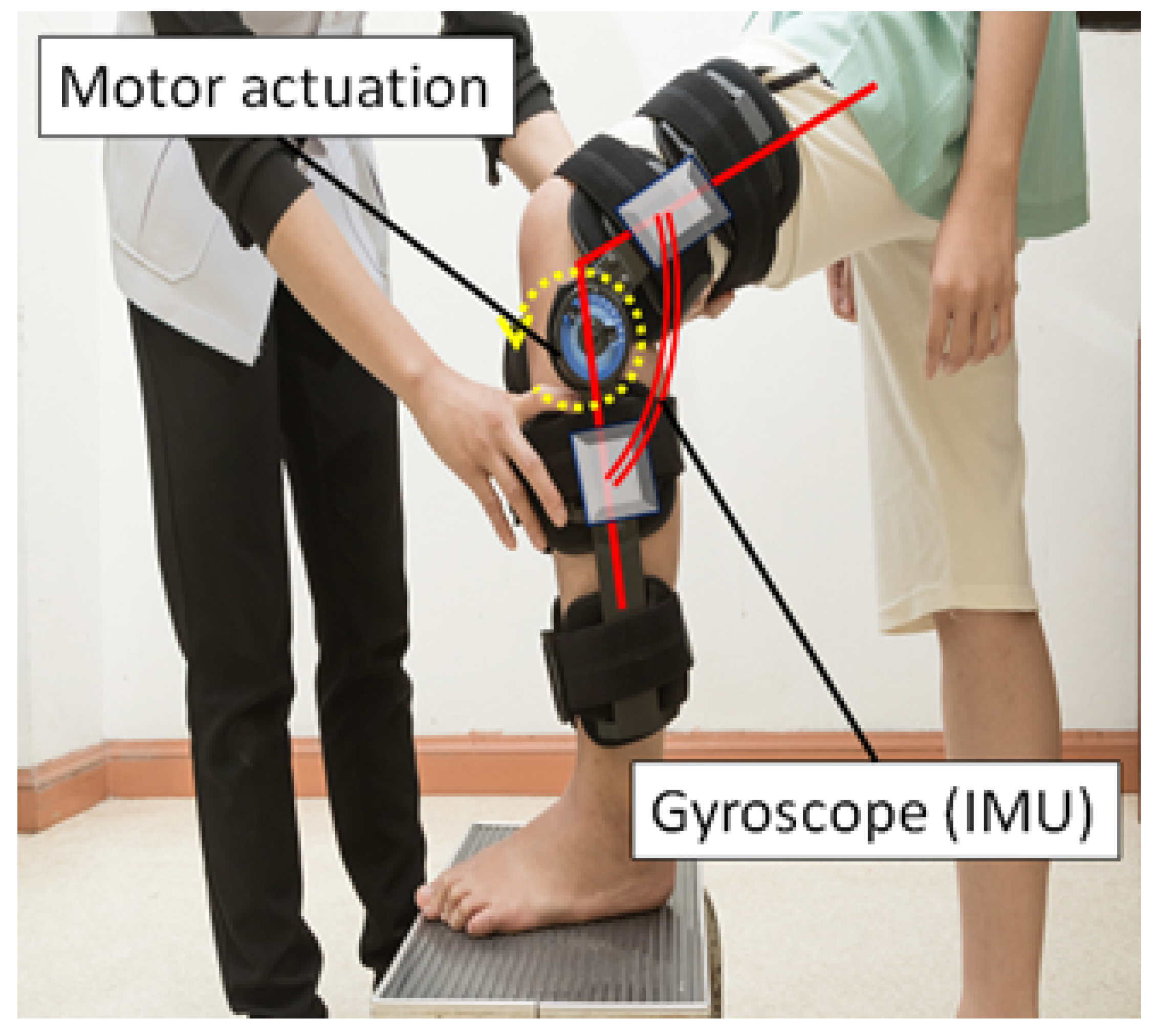
5. Exoskeletons to Minimise Risk Factors
6. Remedial Aspects of a Knee Orthosis
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, regional prevalence, incidence and risk factors of knee osteoar-thritis in population-based studies. EClinicalMedicine 2020, 29, 100587. [Google Scholar] [CrossRef] [PubMed]
- Cicuttini, F.M.; Wluka, A.E.; Stuckey, S.L. Tibial and femoral cartilage changes in knee osteoarthritis. Ann. Rheum. Dis. 2001, 60, 977–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quatman, C.E.; Harris, J.D.; Hewett, T.E. Biomechanical outcomes of cartilage repair of the knee. J. Knee Surg. 2012, 25, 197–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagano, H.; Tatsumi, I.; Sarashina, E.; Sparrow, W.; Begg, R.K. Modelling knee flexion effects on joint power absorption and adduction moment. Knee 2015, 22, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Nagano, H.; Levinger, P.; Downie, C.; Hayes, A.; Begg, R. Contribution of lower limb eccentric work and different step re-sponses to balance recovery among older adults. Gait Posture 2015, 42, 257–262. [Google Scholar] [CrossRef]
- McGibbon, C.; Sexton, A.; Jayaraman, A.; Deems-Dluhy, S.; Fabara, E.; Adans-Dester, C.; Bonato, P.; Marquis, F.; Turmel, S.; Belzile, E. Evaluation of a lower-extremity robotic exoskeleton for people with knee osteoarthritis. Assist. Technol. 2021, 1–14. [Google Scholar] [CrossRef] [PubMed]
- McGibbon, C.A.; Brandon, S.; Bishop, E.L.; Cowper-Smith, C.; Biden, E.N. Biomechanical Study of a Tricompartmental Unloader Brace for Patellofemoral or Multicompartment Knee Osteoarthritis. Front. Bioeng. Biotechnol. 2021, 8, 1528. [Google Scholar] [CrossRef] [PubMed]
- Nagano, H.; Begg, R. A shoe-insole to improve ankle joint mechanics for injury prevention among older adults. Ergonomics 2021, 64, 1271–1280. [Google Scholar] [CrossRef]
- Ezzibdeh, R.; Arora, P.; Amanatullah, D.F. Utilization of a pneumatic exoskeleton after total knee arthroplasty. Arthroplast. Today 2019, 5, 314–315. [Google Scholar] [CrossRef] [Green Version]
- Pamungkas, D.S.; Caesarendra, W.; Soebakti, H.; Analia, R.; Susanto, S. Overview: Types of Lower Limb Exoskeletons. Electronics 2019, 8, 1283. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wu, X.; Zhang, Y.; Chen, C.; Liu, S.; Liu, Y.; Peng, A.; Ma, Y. A Semi-active Exoskeleton Based on EMGs Reduces Muscle Fatigue When Squatting. Front. Neurorobot. 2021, 15, 625479. [Google Scholar] [CrossRef] [PubMed]
- Vantilt, J.; Tanghe, K.; Afschrift, M.; Bruijnes, A.K.; Junius, K.; Geeroms, J.; Aertbeliën, E.; De Groote, F.; Lefeber, D.; Jonkers, I.; et al. Model-based control for exoskeletons with series elastic actuators evaluated on sit-to-stand movements. J. Neuroeng. Rehabil. 2019, 16, 65. [Google Scholar] [CrossRef]
- Stetter, B.J.; Krafft, F.C.; Ringhof, S.; Stein, T.; Sell, S. A Machine Learning and Wearable Sensor Based Approach to Estimate External Knee Flexion and Adduction Moments During Various Locomotion Tasks. Front. Bioeng. Biotechnol. 2020, 8, 9. [Google Scholar] [CrossRef]
- Rönn, K.; Reischl, N.; Gautier, E.; Jacobi, M. Current Surgical Treatment of Knee Osteoarthritis. Arthritis 2011, 2011, 454873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aini, H.; Itaka, K.; Fujisawa, A.; Uchida, H.; Uchida, S.; Fukushima, S.; Kataoka, K.; Saito, T.; Chung, U.-I.; Ohba, S. Messenger RNA delivery of a cartilage-anabolic transcription factor as a disease-modifying strategy for osteoarthritis treatment. Sci. Rep. 2016, 6, 18743. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.-D.; Won, Y.H.; Park, S.-H.; Seo, J.-H.; Kim, D.-S.; Ko, M.-H.; Kim, G.-W. Efficacy and Safety of a Stimulator Using Low-Intensity Pulsed Ultrasound Combined with Transcutaneous Electrical Nerve Stimulation in Patients with Painful Knee Osteoarthritis. Pain Res. Manag. 2019, 2019, 7964897. [Google Scholar] [CrossRef] [PubMed]
- Heidari, B. Knee osteoarthritis diagnosis, treatment and associated factors of progression: Part II. Casp. J. Intern. Med. 2011, 2, 249–255. [Google Scholar]
- Kutzner, I.; Trepczynski, A.; Heller, M.O.; Bergmann, G. Knee adduction moment and medial contact force—Facts about their correlation during gait. PLoS ONE 2013, 8, e81036. [Google Scholar] [CrossRef]
- Nagano, H.; Begg, R. Ageing-related gait adaptations to knee joint kinetics: Implications for the development of knee oste-oarthritis. Appl. Sci. 2020, 10, 8881. [Google Scholar] [CrossRef]
- Robbins, S.M.K.; Birmingham, T.B.; Maly, M.R.; Chesworth, B.M.; Giffin, J.R. Comparative diagnostic accuracy of knee ad-duction moments in knee osteoarthritis: A case for not normalizing to body size. J. Biomech. 2011, 44, 968–971. [Google Scholar] [CrossRef]
- Beschorner, K.E.; Albert, D.L.; Redfern, M.S. Required coefficient of friction during level walking is predictive of slipping. Gait Posture 2016, 48, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Kram, R.; Griffin, T.M.; Donelan, J.M.; Chang, Y.H. Force treadmill for measuring vertical and horizontal ground reaction forces. J. Appl. Physiol. 1998, 85, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Purevsuren, T.; Kim, K.; Nha, K.W.; Kim, Y.H. Evaluation of compressive and shear joint forces on medial and lateral com-partments in knee joint during walking before and after medial open-wedge high tibial osteotomy. Int. J. Precis. Eng. Manuf. 2016, 17, 1365–1370. [Google Scholar] [CrossRef]
- Richards, R.; Andersen, M.; Harlaar, J.; Noort, J.V.D. Relationship between knee joint contact forces and external knee joint moments in patients with medial knee osteoarthritis: Effects of gait modifications. Osteoarthr. Cartil. 2018, 26, 1203–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmer, S.J.; Madigan, M.L.; LaStayo, P.C.; Martin, J.C. Joint-specific power absorption during eccentric cycling. Clin. Biomech. 2010, 25, 154–158. [Google Scholar] [CrossRef]
- Hernandez, H.J.; McIntosh, V.; Leland, A.; Harris-Love, M.O. Progressive Resistance Exercise with Eccentric Loading for the Management of Knee Osteoarthritis. Front. Med. 2015, 2, 45. [Google Scholar] [CrossRef] [Green Version]
- Kao, P.-C.; Ferris, D.P. Motor adaptation during dorsiflexion-assisted walking with a powered orthosis. Gait Posture 2009, 29, 230–236. [Google Scholar] [CrossRef] [Green Version]
- Meinders, M.; Gitter, A.; Czemiecki, J.M. The role of ankle plantar flexor muscle work during walking. Scand. J. Rehabil. Med. 1998, 30, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Ahn, A.; Brayton, C.; Bhatia, T.; Martin, P. Muscle activity and kinematics of forefoot and rearfoot strike runners. J. Sport Health Sci. 2014, 3, 102–112. [Google Scholar] [CrossRef]
- Beerse, M.; Lelko, M.; Wu, J. Acute effect of whole-body vibration on acceleration transmission and jumping performance in children. Clin. Biomech. 2020, 81, 105235. [Google Scholar] [CrossRef] [PubMed]
- Chadefaux, D.; Gueguen, N.; Thouze, A.; Rao, G. 3D propagation of the shock-induced vibrations through the whole low-er-limb during running. J. Biomech. 2019, 96, 109343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruber, A.H.; Boyer, K.A.; Derrick, T.; Hamill, J. Impact shock frequency components and attenuation in rearfoot and forefoot running. J. Sport Health Sci. 2014, 3, 113–121. [Google Scholar] [CrossRef]
- Vahdatpour, B.; Taheri, P.; Asl, M.M.; Ramezanian, H. Effects of Taping on Pain and Functional Outcome of Patients with Knee Osteoarthritis: A Pilot Randomized Single-blind Clinical Trial. Adv. Biomed. Res. 2017, 6, 139. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, L.C.; Rudolph, K.S. Muscle stabilization strategies in people with medial knee osteoarthritis: The effect of instability. J. Orthop. Res. 2008, 26, 1180–1185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khosravi, M.; Arazpour, M.; Saeedi, H.; Rezaei, M. Design evaluation in novel orthoses for patients with medial knee oste-oarthritis. J. Biomed. Phys. Eng. 2019, 9, 719–732. [Google Scholar]
- Ramsey, D.K.; Russell, M.E. Unloader braces for medial compartment knee osteoarthritis: Implications on mediating progression. Sports Health 2009, 1, 416–425. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, V.; Simões, R.; Gonçalves, R.S.; Machado, L.; Roriz, P. The optimal degree of lateral wedge insoles for reducing knee joint load: A systematic review and meta-analysis. Arch. Physiother. 2019, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Hinman, R.S.; Bowles, K.-A.; Metcalf, B.; Wrigley, T.; Bennell, K. Lateral wedge insoles for medial knee osteoarthritis: Effects on lower limb frontal plane biomechanics. Clin. Biomech. 2012, 27, 27–33. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.; Zhang, C. Ineffectiveness of lateral-wedge insoles on the improvement of pain and function for medial knee osteoarthritis: A meta-analysis of controlled randomized trials. Arch. Orthop. Trauma Surg. 2018, 138, 1453–1462. [Google Scholar] [CrossRef] [Green Version]
- Parkes, M.J.; Maricar, N.; Lunt, M.; Lavalley, M.P.; Jones, R.K.; Segal, N.A.; Takahashi-Narita, K.; Felson, D.T. Lateral Wedge Insoles as a Conservative Treatment for Pain in Patients with Medial Knee Osteoarthritis: A Meta-analysis. JAMA 2013, 310, 722–730. [Google Scholar] [CrossRef] [Green Version]
- Chiu, H.-T.; Shiang, T.-Y. Effects of Insoles and Additional Shock Absorption Foam on the Cushioning Properties of Sport Shoes. J. Appl. Biomech. 2007, 23, 119–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulmala, J.-P.; Kosonen, J.; Nurminen, J.; Avela, J. Running in highly cushioned shoes increases leg stiffness and amplifies impact loading. Sci. Rep. 2018, 8, 17496. [Google Scholar] [CrossRef] [Green Version]
- Maropoulos, S.; Korakidis, G.; Fasnakis, D.; Papanikolaou, S.; Papagiannaki, M.; Arabazti, F. The effect of cushioning system on impact attenuation of athletic footwear. MATEC Web Conf. 2017, 112, 8018. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.W.; Rudins, A. Foot Biomechanics During Walking and Running. Mayo Clin. Proc. 1994, 69, 448–461. [Google Scholar] [CrossRef] [Green Version]
- Bolgla, L.A.; Malone, T.R. Plantar fasciitis and the windlass mechanism: A biomechanical link to clinical practice. J. Athl. Train. 2004, 39, 77–82. [Google Scholar] [PubMed]
- Adesida, Y.; Papi, E.; McGregor, A.H. Exploring the Role of Wearable Technology in Sport Kinematics and Kinetics: A Systematic Review. Sensors 2019, 19, 1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lizzio, V.A.; Cross, A.G.; Guo, E.W.; Makhni, E.C. Using Wearable Technology to Evaluate the Kinetics and Kinematics of the Overhead Throwing Motion in Baseball Players. Arthrosc. Tech. 2020, 9, e1429–e1431. [Google Scholar] [CrossRef]
- De Brabandere, A.; Emmerzaal, J.; Timmermans, A.; Jonkers, I.; Vanwanseele, B.; Davis, J. A Machine Learning Approach to Estimate Hip and Knee Joint Loading Using a Mobile Phone-Embedded IMU. Front. Bioeng. Biotechnol. 2020, 8, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhlár, Á.; Ambrus, M.; Kékesi, M.; Fodor, E.; Grand, L.; Szathmáry, G.; Rácz, K.; Lacza, Z. Kinect Azure-based accurate measurement of dynamic valgus of osteoarthritis. Appl. Sci. 2021, 11, 5536. [Google Scholar] [CrossRef]
- Faber, H.; Van Soest, A.J.; Kistemaker, D.A. Inverse dynamics of mechanical multibody systems: An improved algorithm that ensures consistency between kinematics and external forces. PLoS ONE 2018, 13, e0204575. [Google Scholar] [CrossRef] [PubMed]
- Riley, P.O.; Paolini, G.; Della Croce, U.; Paylo, K.W.; Kerrigan, D.C. A kinematic and kinetic comparison of overground and treadmill walking in healthy subjects. Gait Posture 2007, 26, 17–24. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, J.; Zhang, Y.; Zhu, R. A wearable motion capture device able to detect dynamic motion of human limbs. Nat. Commun. 2020, 11, 5615. [Google Scholar] [CrossRef] [PubMed]
- Bonroy, B.; Meijer, K.; Dunias, P.; Cuppens, K.; Gransier, R.; Vanrumste, B. Ambulatory Monitoring of Physical Activity Based on Knee Flexion/Extension Measured by Inductive Sensor Technology. ISRN Biomed. Eng. 2013, 2013, 908452. [Google Scholar] [CrossRef] [Green Version]
- Eitzen, I.; Renberg, J.; Færevik, H. The Use of Wearable Sensor Technology to Detect Shock Impacts in Sports and Occupational Settings: A Scoping Review. Sensors 2021, 21, 4962. [Google Scholar] [CrossRef] [PubMed]
- Iwama, Y.; Harato, K.; Kobayashi, S.; Niki, Y.; Ogihara, N.; Matsumoto, M.; Nakamura, M.; Nagura, T. Estimation of the ex-ternal knee adduction moment during gait using an inertial measurement unit in patients with knee osteoarthritis. Sensors 2021, 21, 1418. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Ha, D.; Kang, Y.-S.; Park, H.-S. Biomechanical Analysis of the Effects of Bilateral Hinged Knee Bracing. Front. Bioeng. Biotechnol. 2016, 4, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reaz, M.B.I.; Hussain, M.S.; Mohd-Yasin, F. Techniques of EMG signal analysis: Detection, processing, classification and applications. Biol. Proced. Online 2006, 8, 11–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kastalskiy, I.; Mironov, V.; Lobov, S.; Krilova, N.; Pimashkin, A.; Kazantsev, V. A Neuromuscular Interface for Robotic Devices Control. Comput. Math. Methods Med. 2018, 2018, 8948145. [Google Scholar] [CrossRef]
- Lodish, H.; Berk, A.; Zipursky, S.L.; Matsudaira, P.; Baltimore, D.; Darnell, J. 2. The action potential and conduction of electric impulses. In Molecular Cell Biology, 4th ed.; W.H. Freeman: New York, NY, USA, 2000; p. 21. [Google Scholar]
- Mills, K.R. The basics of electromyography. J. Neurol. Neurosurg. Psychiatry 2005, 76, ii32–ii35. [Google Scholar] [CrossRef] [Green Version]
- Vigotsky, A.D.; Halperin, I.; Lehman, G.J.; Trajano, G.S.; Vieira, T.M. Interpreting signal amplitudes in surface electromyography studies in sport and rehabilitation sciences. Front. Physiol. 2018, 8, 985. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Liu, J.; Gong, Z.; Lei, Y.; Ouyang, X.; Chan, C.C.; Ruan, S. Wearable Physiological Monitoring System Based on Electrocardiography and Electromyography for Upper Limb Rehabilitation Training. Sensors 2020, 20, 4861. [Google Scholar] [CrossRef] [PubMed]
- Giminiani, R.D.; Cardinale, M.; Ferrari, M.; Quaresima, V. Validation of fabric-based thigh-wearable EMG sensors and oxi-metry for monitoring quadriceps activity during strength and endurance exercises. Sensors 2020, 20, 4664. [Google Scholar] [CrossRef] [PubMed]
- Hanatsu, N.H.; Begg, R.K. Shoe-Insole Technology for Injury Prevention in Walking. Sensors 2018, 18, 1468. [Google Scholar] [CrossRef] [Green Version]
- Lin, F.; Wang, A.; Zhuang, Y.; Tomita, M.R.; Xu, W. Smart insole: A wearable sensor device for unobstrusive gait monitoring in daily life. IEEE Trans. Ind. Inf. 2016, 12, 2281–2291. [Google Scholar] [CrossRef]
- Zhao, W.; Song, A. Active Motion Control of a Knee Exoskeleton Driven by Antagonistic Pneumatic Muscle Actuators. Actuators 2020, 9, 134. [Google Scholar] [CrossRef]
- Baron, R. Normative data for muscle strength in relation to age, knee angle and velocity. Wien. Med. Wochenschr. 1995, 145, 600–606. [Google Scholar]
- Jandacka, D.; Plesek, J.; Skypala, J.; Uchytil, J.; Silvernail, J.F.; Hamill, J. Knee Joint Kinematics and Kinetics During Walking and Running After Surgical Achilles Tendon Repair. Orthop. J. Sports Med. 2018, 6, 2325967118779862. [Google Scholar] [CrossRef] [Green Version]
- Bennett, H.J.; Fleenor, K.; Weinhandl, J.T. A normative database of hip and knee joint biomechanics during dynamic tasks using anatomical regression prediction methods. J. Biomech. 2018, 81, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Wannop, J.; Worobets, J.T.; Stefanyshyn, D. Normalization of Ground Reaction Forces, Joint Moments, and Free Moments in Human Locomotion. J. Appl. Biomech. 2012, 28, 665–676. [Google Scholar] [CrossRef]
- Meinders, E.; Booij, M.J.; van den Noort, J.C.; Harlaar, J. How to compare knee kinetics at different walking speeds? Gait Posture 2021, 88, 225–230. [Google Scholar] [CrossRef]
- Norcross, M.F.; Johnson, S.T.; Pollard, C.D.; Chang, E.W.; Hoffman, M.A. Normalization influences knee adduction moment results: Could it influence ACL-injury research, too? J. Sci. Med. Sport 2017, 20, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Lietman, S.A. Induced pluripotent stem cells in cartilage repair. World J. Orthop. 2016, 7, 149–155. [Google Scholar] [CrossRef]
- Hiemer, B.; Krogull, M.; Bender, T.; Ziebart, J.; Krueger, S.; Bader, R.; Jonitz-Heincke, A. Effect of electric stimulation on human chondrocytes and mesenchymal stem cells under normoxia and hypoxia. Mol. Med. Rep. 2018, 18, 2133–2141. [Google Scholar] [CrossRef] [Green Version]
- Veronesi, F.; Fini, M.; Giavaresi, G.; Ongaro, A.; De Mattei, M.; Pellati, A.; Setti, S.; Tschon, M. Experimentally induced cartilage degeneration treated by pulsed electromagnetic field stimulation; an in vitro study on bovine cartilage. BMC Musculoskelet. Disord. 2015, 16, 308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, H.J.; Kim, S.B.; Somaiya, D.; Noh, M.J.; Choi, K.; Lim, C.; Lee, H.; Lee, Y.; Yi, Y.; Lee, K.H. Type II collagen and gly-cosaminoglycan expression induction in primary human chondrocyte by TGE-β1. BMC Musculoskelet. Disord. 2015, 16, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavénis, K.; Andereya, S.; Schmidt-Rohlfing, B.; Mueller-Rath, R.; Silny, J.; Schneider, U. Millicurrent stimulation of human articular chondrocytes cultivated in a collagen type-I gel and of human osteochondral explants. BMC Complement. Altern. Med. 2010, 10, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakata, S.; Kunimatsu, R.; Tsuka, Y.; Nakatani, A.; Hiraki, T.; Gunji, H.; Hirose, N.; Yanoshita, M.; Putranti, N.A.R.; Tanimoto, K. High-frequency near-infrared diode laser irraditation attenuates IL-1β-induced expression of inflammatory cytokines and matrix metalloproteinases in human primary chondrocytes. J. Clin. Med. 2020, 9, 881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Factor | Problem | How to Manage by Active Exoskeletons | |
|---|---|---|---|
| 1 | Knee adduction moment | Excessive | Provide counteracting moment |
| 2 | Knee extension moment | Insufficient | Provide additional moment |
| 3 | Knee power | Insufficient | Same as 2 |
| 4 | Knee eccentric work | Insufficient | Provide knee power for a prolonged time |
| 5 | Maximum knee flexion | Insufficient | Same as 4 |
| 6 | Knee compression force | Excessive | Provide counteracting force, absorb foot contact impact |
| 7 | Knee abduction angle | Excessive | Regulate excessive joint motion |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagano, H.; Sparrow, W.; Begg, R. Developments in Smart Multi-Function Gait Assistive Devices for the Prevention and Treatment of Knee Osteoarthritis—A Literature Review. Appl. Sci. 2021, 11, 10947. https://doi.org/10.3390/app112210947
Nagano H, Sparrow W, Begg R. Developments in Smart Multi-Function Gait Assistive Devices for the Prevention and Treatment of Knee Osteoarthritis—A Literature Review. Applied Sciences. 2021; 11(22):10947. https://doi.org/10.3390/app112210947
Chicago/Turabian StyleNagano, Hanatsu, William Sparrow, and Rezaul Begg. 2021. "Developments in Smart Multi-Function Gait Assistive Devices for the Prevention and Treatment of Knee Osteoarthritis—A Literature Review" Applied Sciences 11, no. 22: 10947. https://doi.org/10.3390/app112210947
APA StyleNagano, H., Sparrow, W., & Begg, R. (2021). Developments in Smart Multi-Function Gait Assistive Devices for the Prevention and Treatment of Knee Osteoarthritis—A Literature Review. Applied Sciences, 11(22), 10947. https://doi.org/10.3390/app112210947








