Protective Effect of Siegesbeckia orientalis on Pancreatic ?-Cells under High Glucose-Induced Glucotoxicity
Abstract
:1. Introduction
2. Results
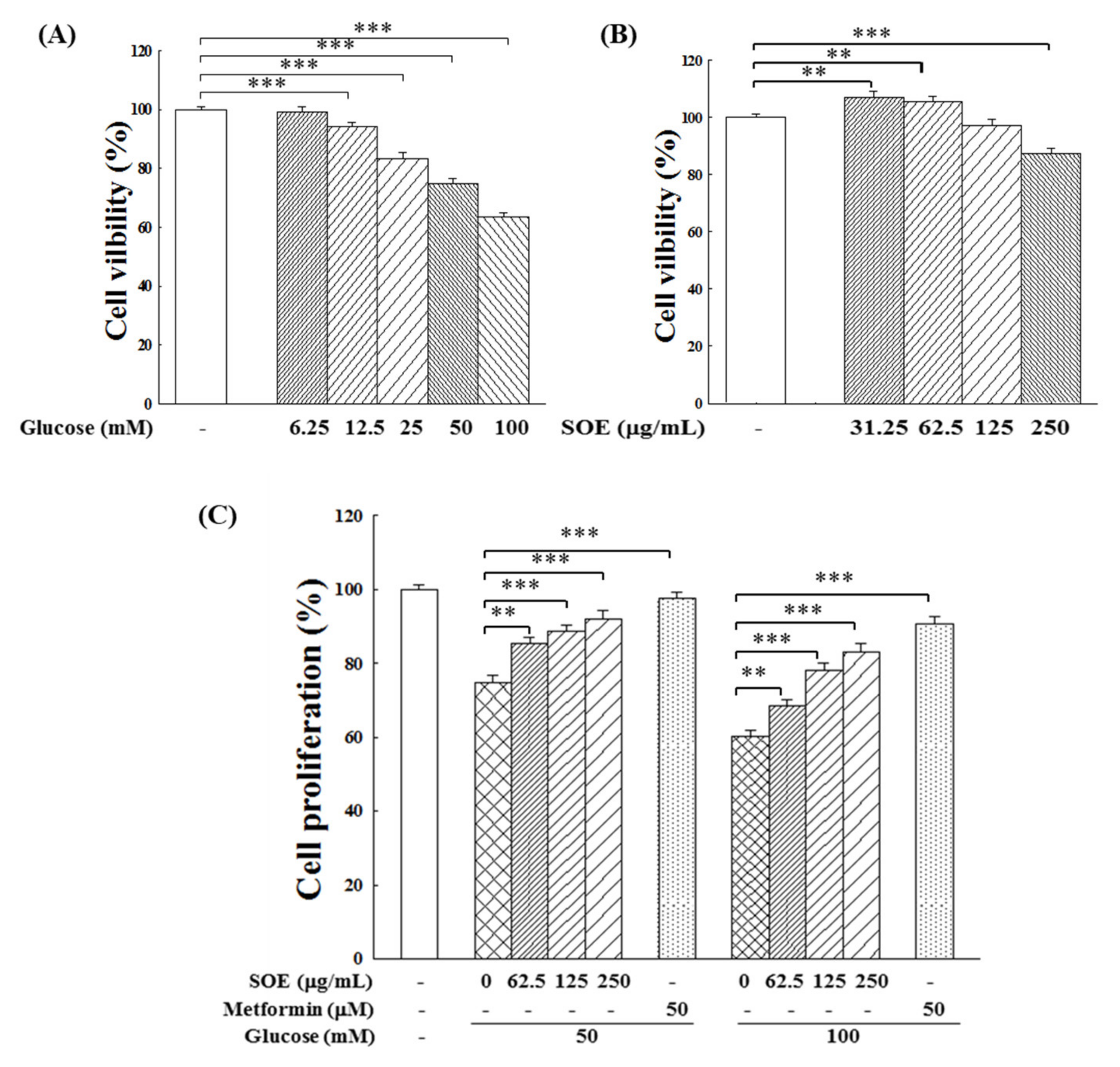
2.1. Effect on Proliferation of HG-Stimulated β-Cells
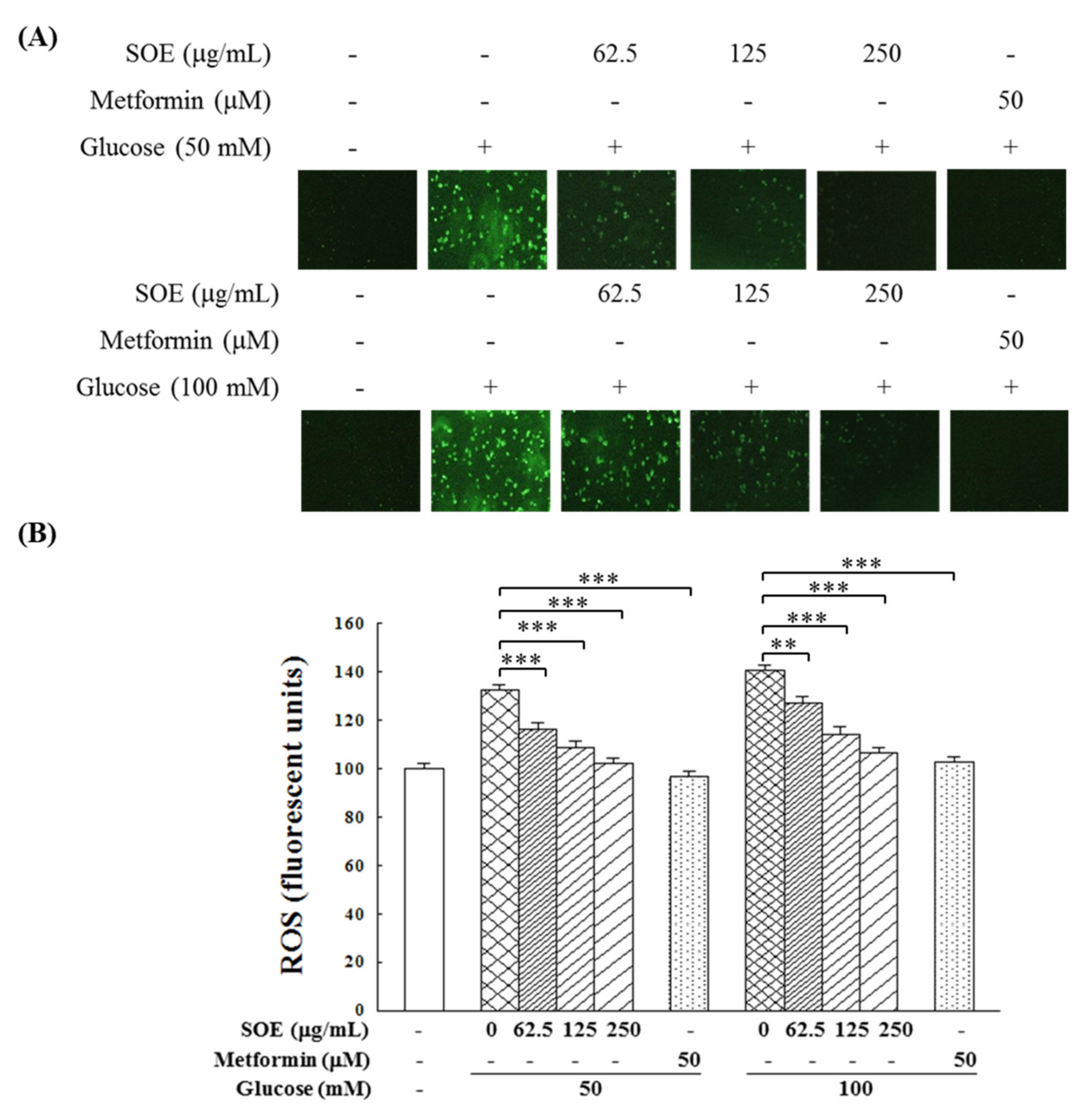
2.2. Effect on ROS Generation in HG-Stimulated β-Cells
2.3. Effect on Antioxidant Enzymes in HG-Stimulated β-Cells
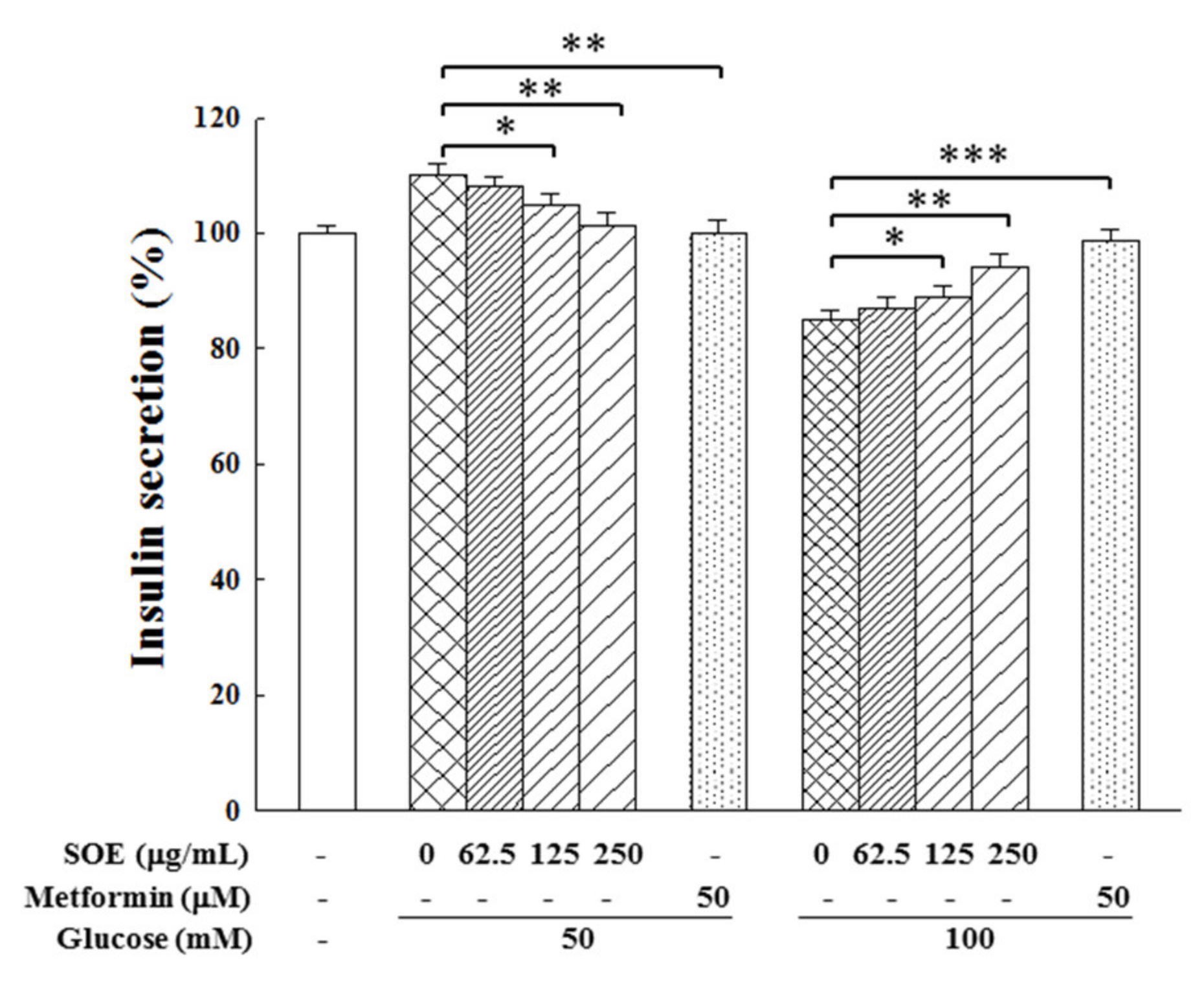
2.4. Effect on Insulin Secretion in HG-Stimulated β-Cells
2.5. Chemical Composition of SOE
3. Discussion
4. Materials and Methods
4.1. Preparation of SOE
4.2. Cell Culture and Viability Analysis
4.3. Assay of Intracellular ROS Level
4.4. Assay of Antioxidant Enzymes Activity and Reduced Glutathione
4.5. Western Blot Assay
4.6. Analysis of Insulin Secretion
4.7. Analysis of Chemical Composition
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bensellam, M.; Laybutt, D.R.; Jonas, J.C. The molecular mecha nisms of pancreatic β-cell glucotoxicity: Recent findings and future research directions. Mol. Cell Endocrinol. 2012, 364, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Imamura, F.; Mukamal, K.J.; Meigs, J.B.; Luchsinger, J.A.; Ix, J.H.; Siscovick, D.S.; Mozaffarian, D. Risk factors for type 2 diabetes mellitus preceded by β-cell dysfunction, insulin resistance, or both in older adults. Am. J. Epidemiol. 2013, 177, 1418–1429. [Google Scholar] [CrossRef] [Green Version]
- Moens, C.; Bensellam, M.; Himpe, E.; Muller, C.J.F.; Jonas, J.C.; Bouwens, L. Aspalathin protects insulin-producing β cells against glucotoxicity and oxidative stress-induced cell death. Mol. Nutr. Food Res. 2020, 64, e1901009. [Google Scholar] [CrossRef] [PubMed]
- Ajith, T.A.; Vinodkumar, P. Advanced glycation end products: Association with the pathogenesis of diseases and the current therapeutic advances. Curr. Clin. Pharmacol. 2016, 11, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Yuan, W.; Roan, H.Y.; Chang, J.L.; Huang, H.C.; Lee, Y.C.; Tsay, H.J.; Liu, H.K. The ethyl acetate fraction of corn silk exhibits dual antioxidant and anti-glycation activities and protects insulin-secreting cells from glucotoxicity. BMC Complement. Altern. Med. 2016, 16, 432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rani, N.; Bharti, S.; Bhatia, J.; Nag, T.C.; Ray, R.; Arya, D.S. Chrysin, a PPAR-γ agonist improves myocardial injury in diabetic rats through inhibiting AGE-RAGE mediated oxidative stress and inflammation. Chem. Biol. Interact. 2016, 250, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, M.; Park, L.; Shin, G.; Hong, H.; Kang, I.; Park, Y. Induction of apoptosis of β cells of the pancreas by advanced glycation end-products, important mediators of chronic complications of diabetes mellitus. Ann. N. Y. Acad. Sci. 2008, 1150, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.A.; Lee, S.; Tak, E.; Lee, J.; Choi, T.G.; Lee, J.W.; Kim, J.B.; Youn, J.H.; Kang, I.; Ha, J.; et al. Carbonyl reductase 1 protects pancreatic β-cells against oxidative stress-induced apoptosis in glucotoxicity and glucolipotoxicity. Free Radic. Biol. Med. 2010, 49, 1522–1533. [Google Scholar] [CrossRef] [PubMed]
- Brun, T.; Maechler, P. Beta-cell mitochondrial carriers and the diabetogenic stress response. Biochim. Biophys. Acta 2016, 1863, 2540–2549. [Google Scholar] [CrossRef]
- Stefano, G.B.; Challenger, S.; Kream, R.M. Hyperglycemia-associated alterations in cellular signaling and dysregulated mitochondrial bioenergetics in human metabolic disorders. Eur. J. Nutr. 2016, 55, 2339–2345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharroubi, I.; Ladriere, L.; Cardozo, A.K.; Dogusan, Z.; Cnop, M.; Eizirik, D.L. Free fatty acids and cytokines induce pancreatic beta-cell apoptosis by different mechanisms: Role of nuclear factor-κB and endoplasmic reticulum stress. Endocrinology 2004, 145, 5087–5096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harmon, J.S.; Stein, R.; Robertson, R.P. Oxidative stress-mediated, posttranslational loss of MafA protein as a contributing mechanism to loss of insulin gene expression in glucotoxic β cells. J. Biol. Chem. 2005, 280, 11107–11113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, E.A.; Yam, M.F.; Ang, L.F.; Mohamed, A.J.; Asmawi, M.Z. Antidiabetic properties and mechanism of action of Orthosiphon stamineus Benth bioactive sub-fraction in streptozotocin-induced diabetic rats. J. Acupunct. Meridian Stud. 2013, 6, 31–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shivanna, N.; Naika, M.; Khanum, F.; Kaul, V.K. Antioxidant, anti-diabetic and renal protective properties of Stevia rebaudiana. J. Diabetes Complicat. 2013, 27, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Han, J.S. Padina arborescens extract protects high glucose-induced apoptosis in pancreatic β cells by reducing oxidative stress. Nutr. Res. Pract. 2014, 8, 494–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Ren, C.J.; Lu, G.B.; Mu, Z.M.; Cui, W.Z.; Gao, H.J.; Wang, Y.W. Anti-diabetic effect of mulberry leaf polysaccharide by inhibiting pancreatic islet cell apoptosis and ameliorating insulin secretory capacity in diabetic rats. Int. Immunopharmacol. 2014, 22, 248–257. [Google Scholar] [CrossRef]
- Park, J.E.; Seo, Y.; Han, J.S. HM-Chromanone isolated from Portulaca oleracea L. protects INS-1 pancreatic β cells against glucotoxicity-induced apoptosis. Nutrients 2019, 11, 404. [Google Scholar] [CrossRef] [Green Version]
- Hong, Y.H.; Weng, L.W.; Chang, C.C.; Hsu, H.F.; Wang, C.P.; Wang, S.W.; Houng, J.Y. Anti-inflammatory effects of Siegesbeckia orientalis ethanol extract in in vitro and in vivo models. BioMed Res. Int. 2014, 2014, 329712. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Thuong, P.T.; Hwang, I.H.; Hoang, T.K.; Nguyen, M.K.; Nguyen, H.A.; Na, M. Anti-hyperuricemic, anti-inflammatory and analgesic effects of Siegesbeckia orientalis L. resulting from the fraction with high phenolic content. BMC Complement. Altern. Med. 2017, 17, 191. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.X.; Wang, H. Immunosuppressive activity of the ethanol extract of Siegesbeckia orientalis on the immune responses to ovalbumin in mice. Chem. Biodivers. 2006, 3, 754–761. [Google Scholar] [CrossRef]
- Wang, J.P.; Luo, Q.; Ruan, J.L.; Chen, Y.A.; Chen, M.X. Effect of Siegesbeckia orientalis L. on cervical cancer HeLa cell in vitro. Her. Med. 2009, 28, 45–46. [Google Scholar]
- Chang, C.C.; Hsu, H.F.; Huang, K.H.; Wu, J.M.; Kuo, S.M.; Ling, X.H.; Houng, J.Y. Anti-proliferative effects of Siegesbeckia orientalis ethanol extract on human endometrial RL-95 cancer cells. Molecules 2014, 19, 19980–19994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.C.; Ling, X.H.; Hsu, H.F.; Wu, J.M.; Wang, C.P.; Yang, J.F.; Fang, L.W.; Houng, J.Y. Siegesbeckia orientalis extract inhibits TGFβ1-induced migration and invasion of endometrial cancer cells. Molecules 2016, 21, 1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, W.C.; Ling, X.H.; Chang, C.C.; Hsu, H.F.; Wang, S.W.; Lee, Y.C.; Luo, C.; Lee, Y.T.; Houng, J.Y. Inhibitory effects of Siegesbeckia orientalis extracts on advanced glycation end products formation and key enzymes related to metabolic syndrome. Molecules 2017, 22, 1785. [Google Scholar] [CrossRef] [Green Version]
- Piro, S.; Rabuazzo, A.M.; Renis, M.; Purrello, F. Effects of metformin on oxidative stress, adenine nucleotides balance, and glucose-induced insulin release impaired by chronic free fatty acids exposure in rat pancreatic islets. J. Endocrinol. Investig. 2012, 35, 504–510. [Google Scholar]
- Adeshara, K.; Tupe, R. Antiglycation and cell protective actions of metformin and glipizide in erythrocytes and monocytes. Mol. Biol. Rep. 2016, 43, 195–205. [Google Scholar] [CrossRef]
- Wu, C.H.; Hsieh, H.T.; Lin, J.A.; Yen, G.C. Alternanthera paronychioides protects pancreatic β-cells from glucotoxicity by its antioxidant, antiapoptotic and insulin secretagogue actions. Food Chem. 2013, 139, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.P. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet β cells in diabetes. J. Biol. Chem. 2004, 279, 42351–42354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, R.P.; Harmon, J.S.; Tran, P.O.; Poitout, V. β-Cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes 2004, 53 (Suppl. 1), S119–S124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenzen, S. Oxidative stress: The vulnerable β-cell. Biochem. Soc. Trans. 2008, 36, 343–347. [Google Scholar] [CrossRef]
- Lowell, B.B.; Shulman, G.I. Mitochondrial dysfunction and type 2 diabetes. Science 2005, 307, 384–387. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.; Rashid, M.A.; Jang, M.; Kim, Y.; Won, H.; Lee, J.; Woo, J.T.; Kim, Y.S.; Murphy, M.P.; Ali, L.; et al. Mitochondria-targeted antioxidants protect pancreatic β-cells against oxidative stress and improve insulin secretion in glucotoxicity and glucolipotoxicity. Cell. Physiol. Biochem. 2011, 28, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.; Gilon, P. Can tea extracts exert a protective effect against diabetes by reducing oxidative stress and decreasing glucotoxicity in pancreatic β-cells? Diabetes Metab. J. 2015, 39, 27–30. [Google Scholar] [CrossRef] [Green Version]
- Robertson, R.P.; Zhou, H.; Zhang, T.; Harmon, J.S. Chronic oxidative stress as a mechanism for glucose toxicity of the β cell in type 2 diabetes. Cell Biochem. Biophys. 2007, 48, 139–146. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Yu, X.; He, S.; Wu, X.; Wang, Y. Mulberry leaf flavonoids protect against glucotoxicity-induced INS-1 cell apoptosis. J. Tradit. Chin. Med. 2019, 39, 153–159. [Google Scholar]
- Masuda, Y.; Vaziri, N.D.; Li, S.; Le, A.; Hajighasemi-Ossareh, M.; Robles, L.; Foster, C.E.; Stamos, M.J.; Al-Abodullah, I.; Ricordi, C.; et al. The effect of Nrf2 pathway activation on human pancreatic islet cells. PLoS ONE 2015, 10, e0131012. [Google Scholar]
- Tiedge, M.; Lortz, S.; Drinkgern, J.; Lenzen, S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes 1997, 46, 1733–1742. [Google Scholar] [CrossRef]
- Grankvist, K.; Marklund, S.; Täljedal, I.B. Superoxide dismutase is a prophylactic against alloxan diabetes. Nature 1981, 294, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.P.; Harmon, J.S. Pancreatic islet β-cell and oxidative stress: The importance of glutathione peroxidase. FEBS Lett. 2007, 581, 3743–3748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krause, M.S.; McClenaghan, N.H.; Flatt, P.R.; de Bittencourt, P.I.; Murphy, C.; Newsholme, P. L-Arginine is essential for pancreatic β-cell functional integrity, metabolism and defense from inflammatory challenge. J. Endocrinol. 2011, 211, 87–97. [Google Scholar] [CrossRef]
- Zhang, J.; An, H.; Ni, K.; Chen, B.; Li, H.; Li, Y.; Sheng, G.; Zhou, C.; Xie, M.; Chen, S.; et al. Glutathione prevents chronic oscillating glucose intake-induced β-cell dedifferentiation and failure. Cell Death Dis. 2019, 10, 321. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.F.; Chen, Z.H.; Chang, S.F.; Wang, C.P.; Chiou, S.J.; Yen, J.H.; Chang, C.C.; Tsai, Y.D.; Fang, L.W.; Houng, J.Y. Evaluating the anti-metastatic potential of Anisomeles indica extract by using human oral squamous carcinoma FaDu cells. Afr. J. Pharm. Pharmacol. 2012, 6, 1782–1791. [Google Scholar]


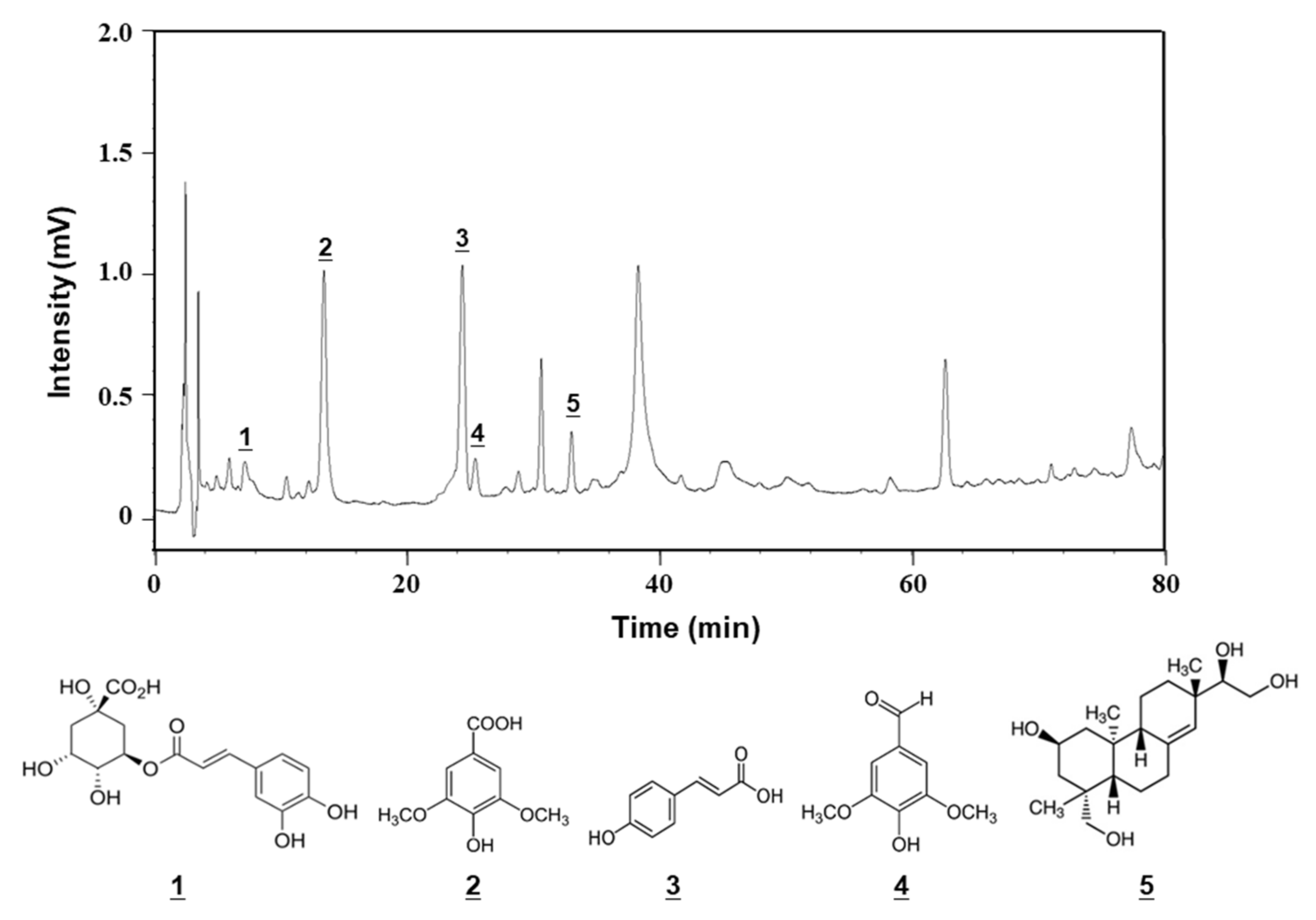
| No. | Component | Retention Time (min) | Content (mg/g Dry Extract) |
|---|---|---|---|
| 1 | Chlorogenic acid | 7.2 | 2.04 ± 0.04 |
| 2 | Syringic acid | 13.4 | 10.76 ± 0.11 |
| 3 | p-Coumaric acid | 24.4 | 0.70 ± 0.03 |
| 4 | Syringaldehyde | 25.4 | 0.39 ± 0.02 |
| 5 | Kirenol | 37.3 | 7.88 ± 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-C.; Houng, J.-Y.; Wang, S.-W.; Hsuan, C.-F.; Lu, Y.-C.; Chang, T.-H.; Chen, Y.-L. Protective Effect of Siegesbeckia orientalis on Pancreatic ?-Cells under High Glucose-Induced Glucotoxicity. Appl. Sci. 2021, 11, 10963. https://doi.org/10.3390/app112210963
Chang C-C, Houng J-Y, Wang S-W, Hsuan C-F, Lu Y-C, Chang T-H, Chen Y-L. Protective Effect of Siegesbeckia orientalis on Pancreatic ?-Cells under High Glucose-Induced Glucotoxicity. Applied Sciences. 2021; 11(22):10963. https://doi.org/10.3390/app112210963
Chicago/Turabian StyleChang, Chi-Chang, Jer-Yiing Houng, Shih-Wei Wang, Chin-Feng Hsuan, Yung-Chuan Lu, Tzu-Hsien Chang, and Ya-Ling Chen. 2021. "Protective Effect of Siegesbeckia orientalis on Pancreatic ?-Cells under High Glucose-Induced Glucotoxicity" Applied Sciences 11, no. 22: 10963. https://doi.org/10.3390/app112210963
APA StyleChang, C.-C., Houng, J.-Y., Wang, S.-W., Hsuan, C.-F., Lu, Y.-C., Chang, T.-H., & Chen, Y.-L. (2021). Protective Effect of Siegesbeckia orientalis on Pancreatic ?-Cells under High Glucose-Induced Glucotoxicity. Applied Sciences, 11(22), 10963. https://doi.org/10.3390/app112210963







