Modeling the Process of Thawing of Tailings Dam Base Soils by Technological Waters
Abstract
:1. Introduction
2. Materials and Methods
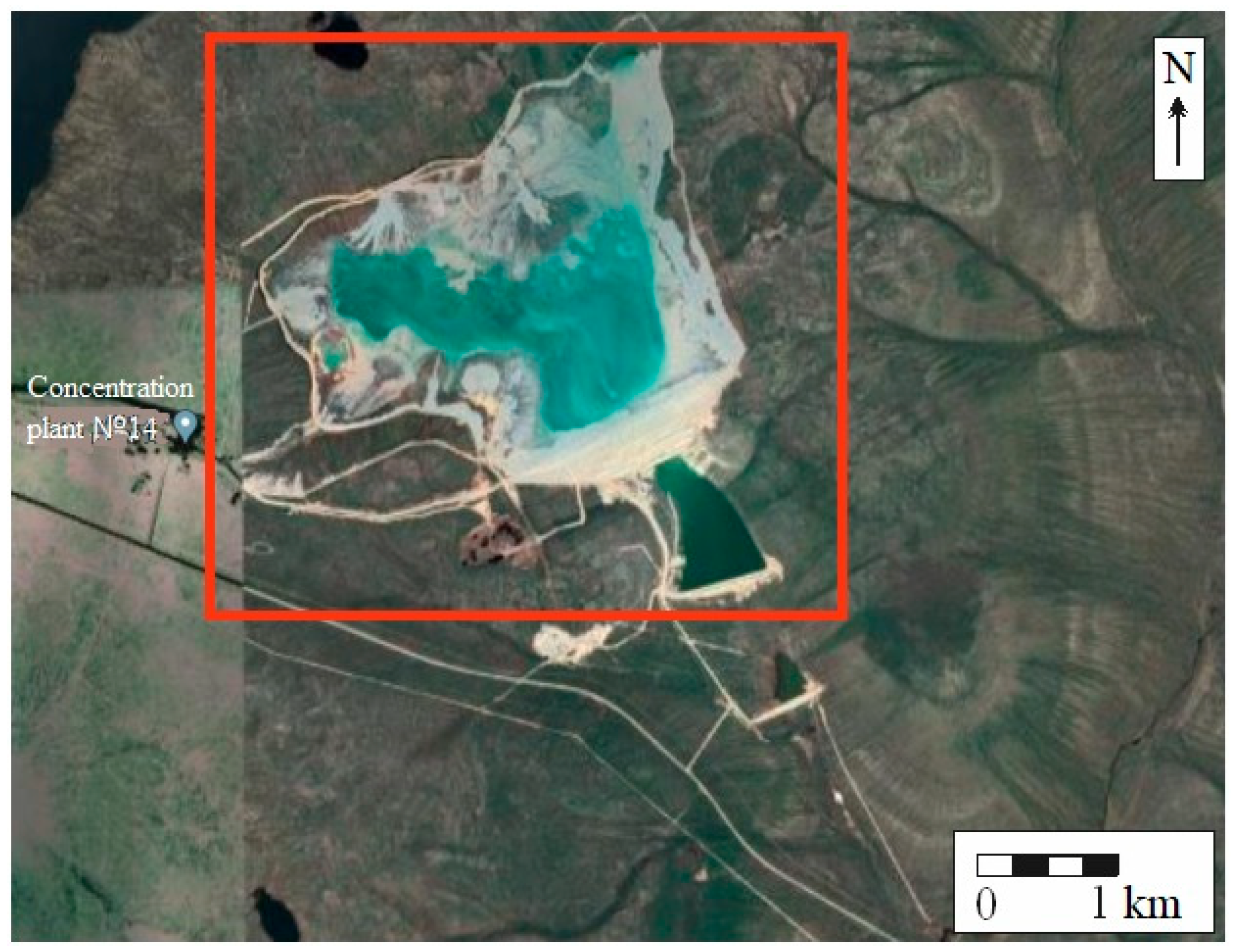
2.1. Study Object
2.2. Sampling and Sample Preparation
2.3. Analytical Work
2.4. Thermophysical Modeling
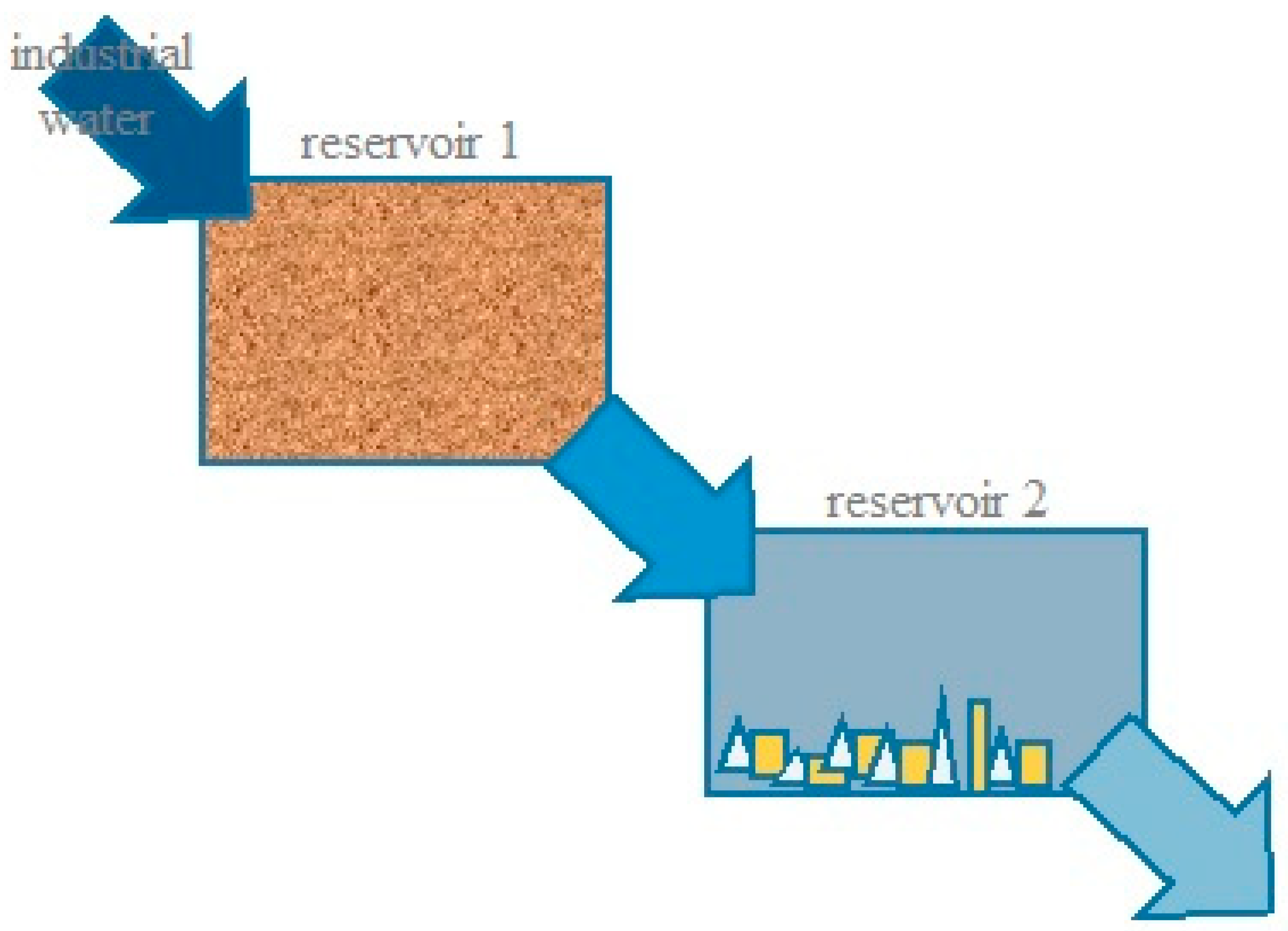
Model Description
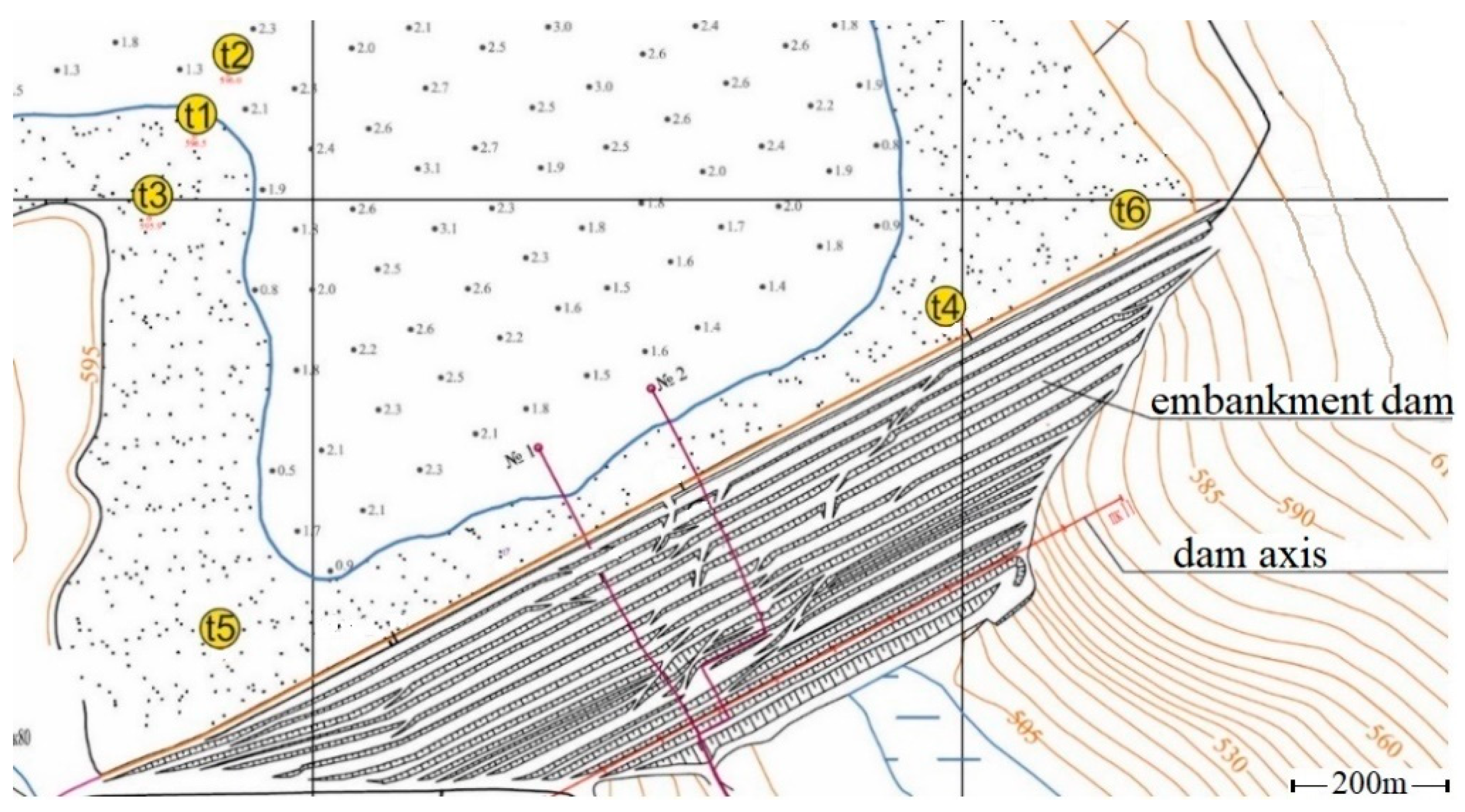
- Well t1: total depth 75 m. Structure according to Figure 5: 0–17 m—technogenic deposits with known properties (t 1/1, Table 1); 17–66 m—fractured limestones (t 1/2, Table 1); 66–75 m—weakly fractured marl (impermeable, not modeled). The initial temperature of the well t1 rocks and the temperature at large distances from the filtration channel are assumed to be constant and time-invariant (Ti = 4 °C, Table 1).
- Well t3: total depth 70 m. Structure according to Figure 5: 0–8 m—technogenic deposits with known properties (t 3/1, Table 1); 8–68 m—fractured limestones (t 3/2, Table 1); 68 m and below—marl (impermeable, not modeled). The initial temperature of the well t3 rocks (units 3/1 and 3/2) and the temperature at large distances from the filtration channel are assumed to be constant (Ti = −4 °C, Table 1).
2.5. Thermodynamic Modeling
2.5.1. Model Description
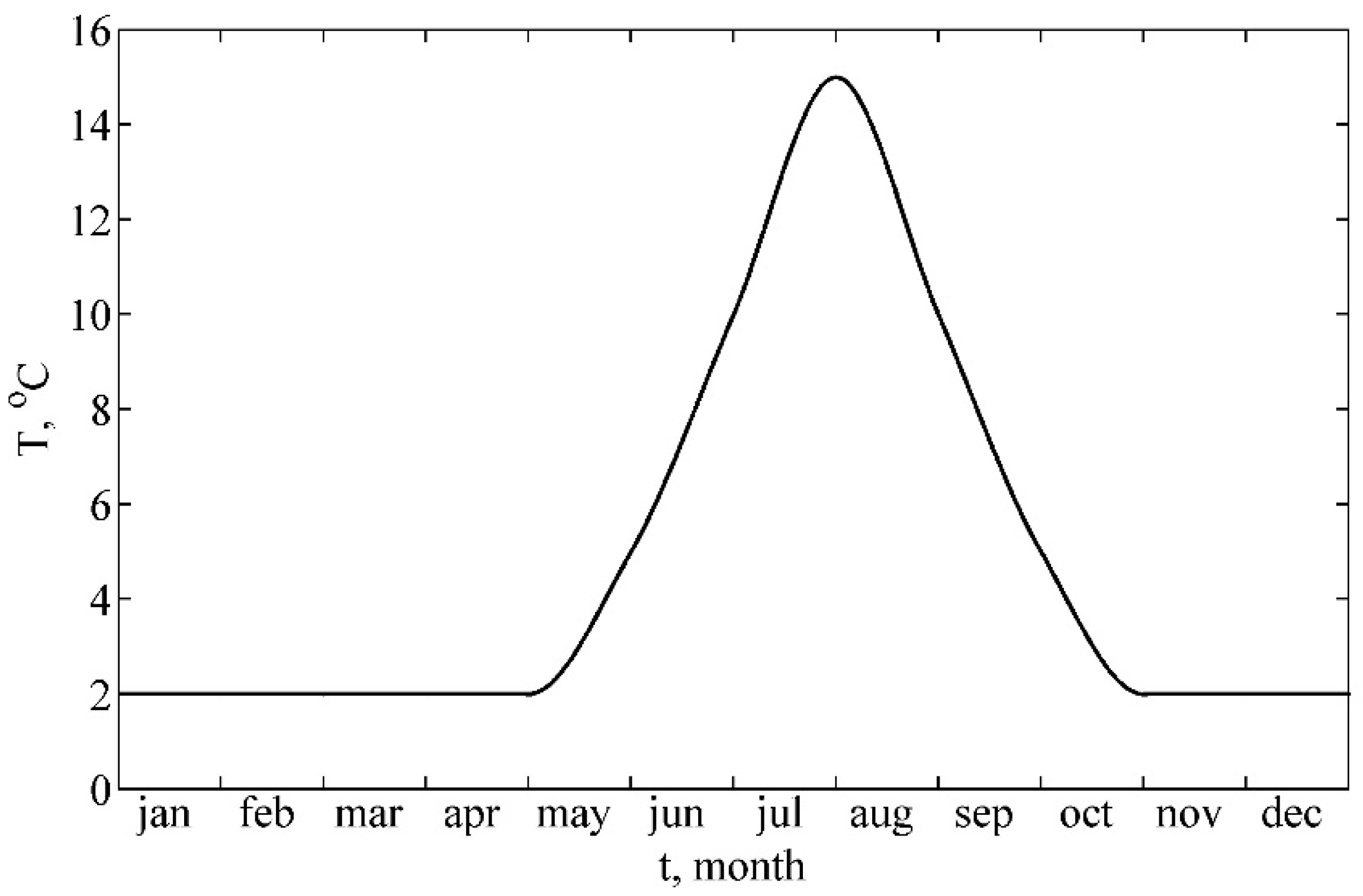
2.5.2. Initial Conditions
3. Results
3.1. Thermophysical Modeling Results
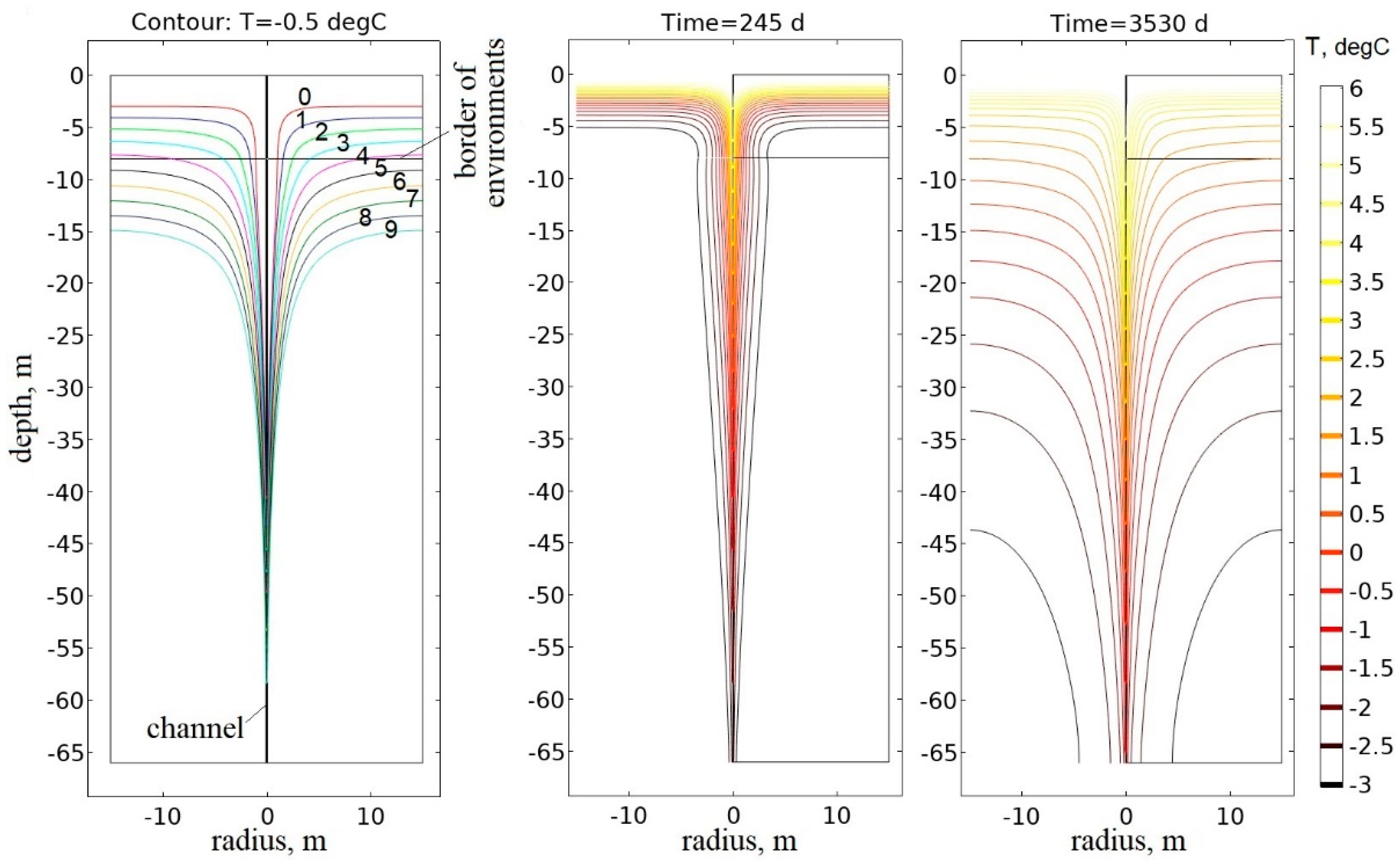
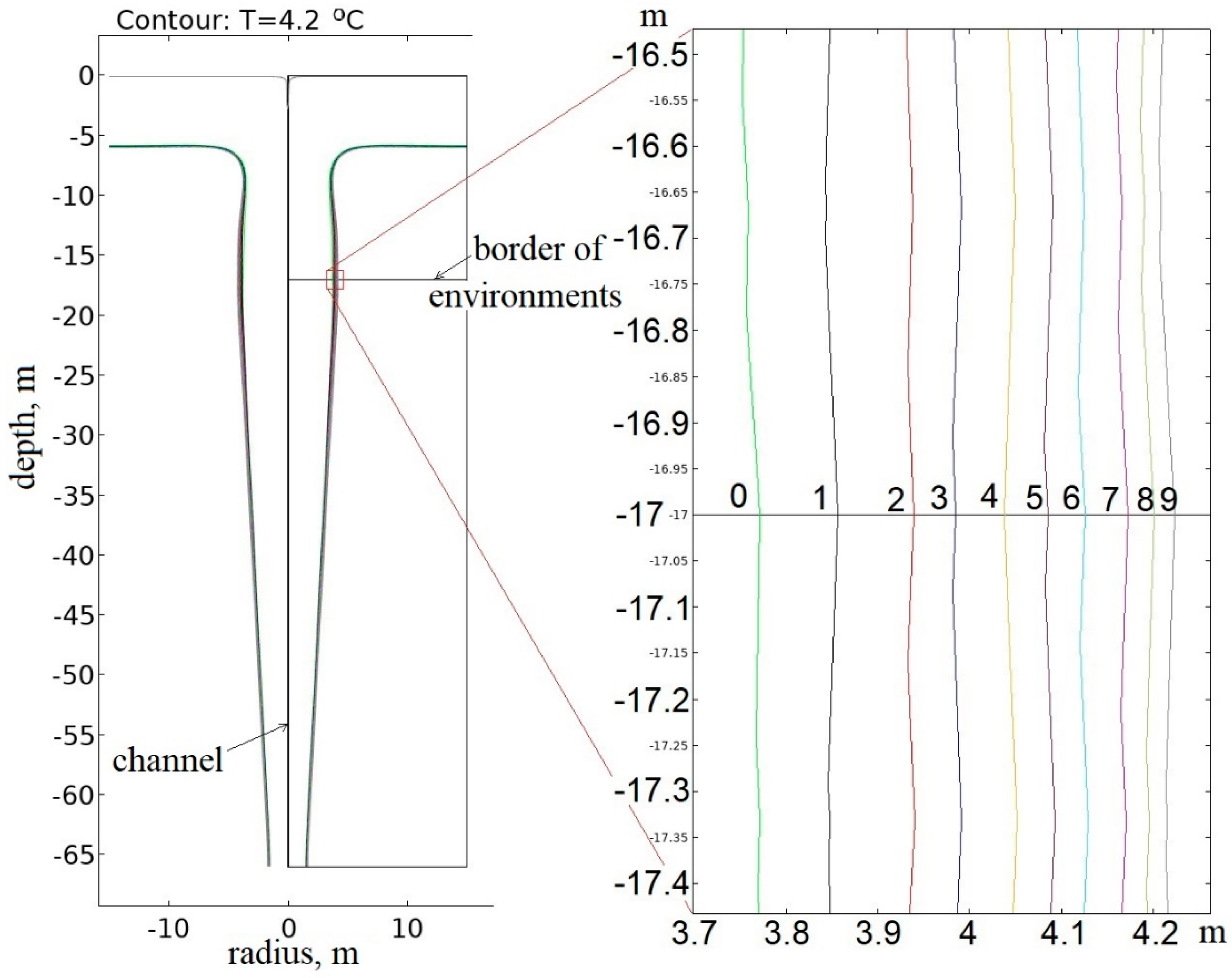
3.1.1. Thermophysical Modeling Results for the Frozen Rock Mass
3.1.2. Thermophysical Modeling Results for the Thawed Rock Mass
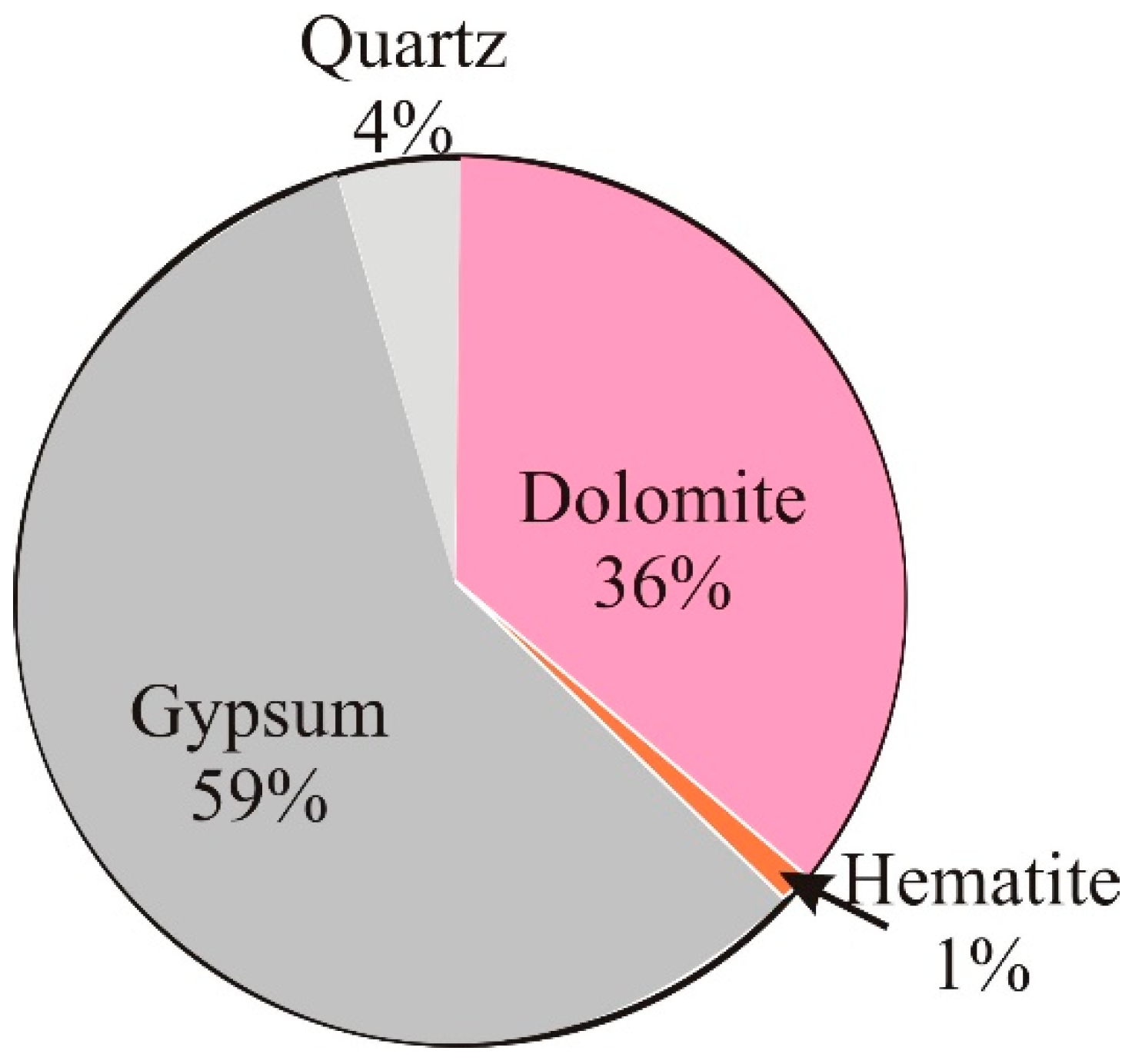
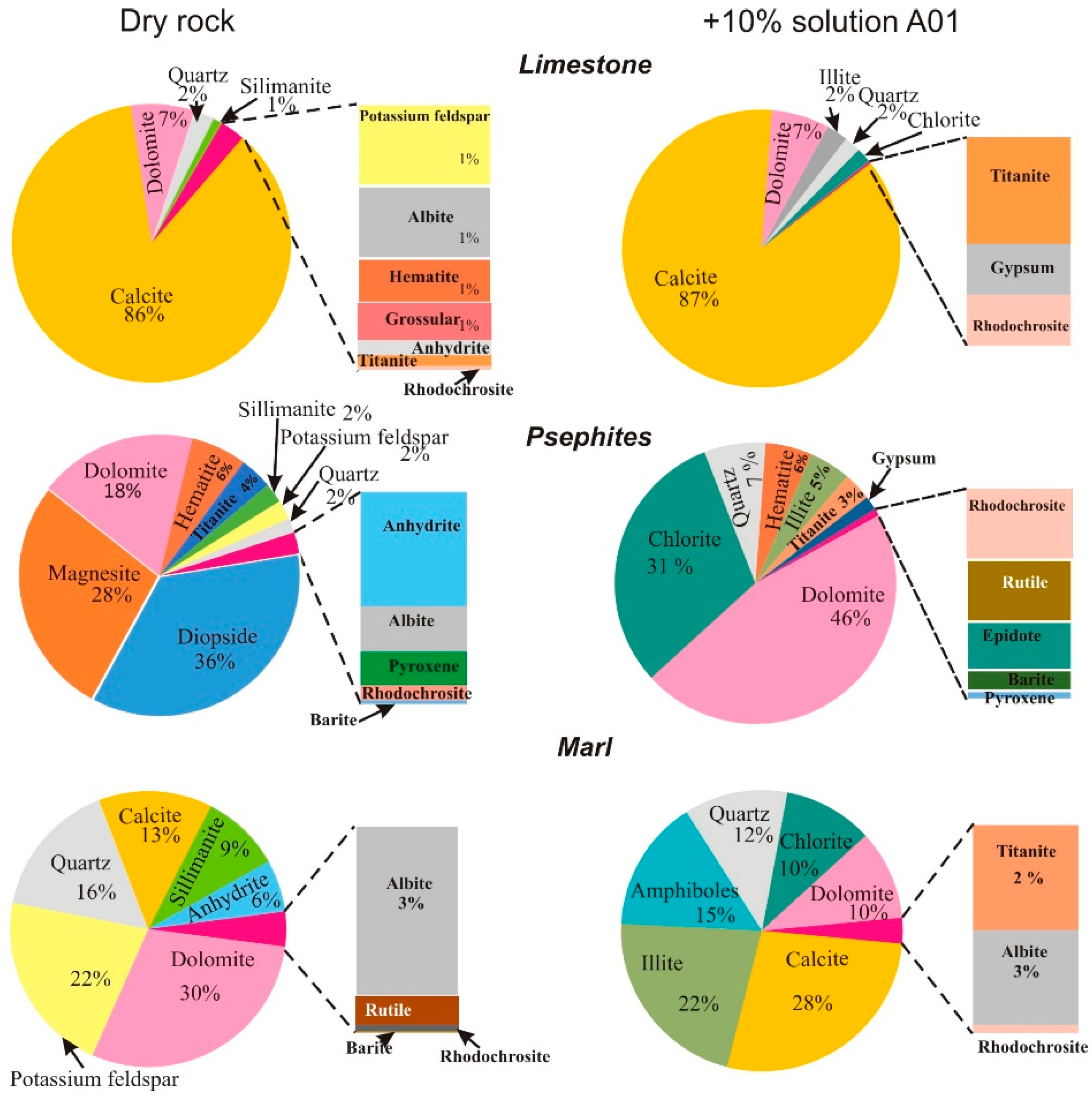
3.2. Thermodynamic Modeling Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lessard, F.; Bussière, B.; Côté, J.; Benzaazoua, M.; Boulanger-Martel, V.; Marcoux, L. Integrated environmental management of pyrrhotite tailings at Raglan Mine: Part 2 desulphurized tailings as cover material. J. Clean. Prod. 2018, 186, 883–893. [Google Scholar] [CrossRef]
- Knutsson, R.; Viklander, P.; Knutsson, S.; Laue, J. How to avoid permafrost while depositing tailings in cold climate. Cold Reg. Sci. Technol. 2018, 153, 86–96. [Google Scholar] [CrossRef]
- Hollesen, J.; Elberling, B.; Jansson, P. Modelling temperature-dependent heat production over decades in High Arctic coal waste rock piles. Cold Reg. Sci. Technol. 2011, 65, 258–268. [Google Scholar] [CrossRef]
- Nazarova, L.A.; Nazarov, L.A.; Dzhamanbaev, M.D.; Chynybaev, M.K. Evolution of thermohydrodynamic fields at tailings dam at Kumtor mine (Kyrgyz Republic). J. Min. Sci. 2015, 51, 17–22. [Google Scholar] [CrossRef]
- Ben Ali, H.E.; Neculita, C.M.; Molson, J.W.; Maqsoud, A.; Zagury, G.J. Performance of passive systems for mine drainage treatment at low temperature and high salinity: A review. Miner. Eng. 2019, 134, 325–344. [Google Scholar] [CrossRef]
- Ali, H.E.B.; Neculita, C.M.; Molson, J.W.; Maqsoud, A.; Zagury, G.J. Salinity and low temperature effects on the performance of column biochemical reactors for the treatment of acidic and neutral mine drainage. Chemosphere 2019, 243, 125303. [Google Scholar] [CrossRef] [PubMed]
- Edahbi, M.; Plante, B.; Benzaazoua, M. Environmental challenges and identification of the knowledge gaps associated with REE mine wastes management. J. Clean. Prod. 2019, 212, 1232–1241. [Google Scholar] [CrossRef]
- Tolvanen, A.; Eilu, P.; Juutinen, A.; Kangas, K.; Kivinen, M.; Markovaara-Koivisto, M.; Naskali, A.; Salokannel, V.; Tuulentie, S.; Similä, J. Mining in the Arctic environment—A review from ecological, socioeconomic and legal perspectives. J. Environ. Manag. 2019, 233, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Salerno, F.; Gruber, S.; Freppaz, M.; Williams, M.; Fratianni, S.; Giardino, M. Review: Impacts of permafrost degradation on inorganic chemistry of surface fresh water. Glob. Planet. Chang. 2018, 162, 69–83. [Google Scholar] [CrossRef]
- Colombo, N.; Salerno, F.; Martin, M.; Malandrino, M.; Giardino, M.; Serra, E.; Godone, D.; Said-Pullicino, D.; Fratianni, S.; Paro, L.; et al. Influence of permafrost, rock and ice glaciers on chemistry of high-elevation ponds (NW Italian Alps). Sci. Total Environ. 2019, 685, 886–901. [Google Scholar] [CrossRef] [PubMed]
- Bortnikova, S.B.; Silantyeva, N.V.; Zapolsky, A.N.; Yurkevich, N.V.; Saeva, O.P.; Shevko, A.Y.; Shuvaeva, O.V.; Edelev, A.V. Assessment of acid base accounting of mine waste rocks and mobility of potentially toxic elements of the Razdolinsky ore field (Krasnoyarsk Territory). Bull. Tomsk. Polytech. Univ. Geo Assets Eng. 2018, 329, 55–72. (In Russian) [Google Scholar]
- Korneeva, T.V.; Yurkevich, N.V.; Aminov, P.G. Geochemical features of migration flows in the impact zone of mining technogenesis (Mednogorsk). Bull. Tomsk. Polytech. Univ. Geo Assets Eng. 2017, 328, 85–94. (In Russian) [Google Scholar]
- Korneeva, T.V.; Yurkevich, N.V.; Saeva, O.P. Geochemical modeling of heavy metals behavior in technogenic systems. Bull. Tomsk. Polytech. Univ. Geo Assets Eng. 2018, 329, 89–101. (In Russian) [Google Scholar]
- Yurkevich, N.V.; Abrosimova, N.A.; Bortnikova, S.B.; Karin, Y.G.; Saeva, O.P. Geophysical investigations for evaluation of environmental pollution in a mine tailings area. Toxicol. Environ. Chem. 2017, 99, 1328–1345. [Google Scholar] [CrossRef]
- Elberling, B. Temperature and oxygen control on pyrite oxidation in frozen mine tailings. Cold Reg. Sci. Technol. 2005, 41, 121–133. [Google Scholar] [CrossRef]
- MEND Project 1.61.2. Acid Mine Drainage in Permafrost Regions: Issues, Control Strategies and Research Requirements; Technical Report; Department of Indian and Northern Affairs Canada: Calgary, AB, Canada, 1996; 103p. Available online: http://mend-nedem.org/mend-report/acid-mine-drainage-in-permafrost-regions-issues-control-strategies-and-research-requirements/ (accessed on 1 November 2021).
- Pham, H.N. Heat Transfer in Waste Rock Piles Construted in a Contiuous Permafrost Region. Ph.D. Dissertation, Department of Civil and Environmental Engineering, University of Alberta, Edmonton, AB, Canada, 2013; 363p. [Google Scholar] [CrossRef]
- Stevens, C.W.; Shapka-Fels, T.; Rykaart, M. Thermal Cover Design for Mine Waste Facilities in Cold Regions. Conference: Thermal Cover Design for Mine Waste Facilities in Cold Regions at: Tailings and Mine Waste 2018, Keystone, Colorado. Available online: https://www.srk.com/en/publications/thermal-cover-design-for-mine-waste-facilities-in-cold-regions (accessed on 1 November 2021).
- Kilizhekov, O.K. Regularities of Localization and Features of Exploration of Buried Diamond Placers in the Sredne-Markhinsky Region (Yakutsk Diamond-Bearing Province). Dissertation of the Candidate of Geological and Mineralogical Sciences. 2017. Available online: https://www.igm.nsc.ru/images/diss/kilizhekov/ref_kilizhekov.pdf (accessed on 19 September 2021).
- Buiskikh, A.A.; Zamoshch, M.N. Prediction of thermal regime within a tailing dump under permafrost. J. Min. Sci. 2010, 46, 28–33. [Google Scholar] [CrossRef]
- Vafai, K.; Kim, J. On the limitations of the Brinkman-Forchheimerextended Darcy equation. Int. J. Heat Fluid Flow 1995, 16, 11–15. [Google Scholar] [CrossRef]
- Amiri, A.; Vafai, K. Transient analysis of incompressible flow through a packed bed. Int. J. Heat Mass Transf. 1998, 41, 4259–4279. [Google Scholar] [CrossRef]
- The COMSOL Multiphysics Reference Guide. Available online: http://www.lmn.pub.ro/~daniel/ElectromagneticModelingDoctoral/Books/COMSOL4.3/mph/COMSOLMultiphysicsReferenceGuide.pdf (accessed on 19 September 2021).
- Karpov, I.K. Physicochemical Modeling on a Computer in Geochemistry; Nauka: Novosibirsk, Russia, 1981; 247p. [Google Scholar]
- Karpov, I.K.; Chudnenko, K.V.; Kulik, D.A. Modeling chemical mass transfer in gheochemical processes: Thermodynamic relations, conditions of equilibria, and numerical algorithms. Am. J. Sci. 1997, 297, 767–806. [Google Scholar] [CrossRef]
- Chudnenko, K.V. Thermodynamic Modeling in Geochemistry: Theory, Algorithms, Software, Applications; Academic Publishing House “Geo”: Novosibirsk, Russia, 2010; 287p. [Google Scholar]


| Characteristic | t 1/1 | t 1/2 | t 3/1 | t 3/2 |
|---|---|---|---|---|
| Mineralogical Composition: | Technogenic Psephite, Magnetite, Pyrite | Limestone with an Admixture of Pyrite and Gypsum | Technogenic Psephite, Magnetite, Pyrite | Limestone with an Admixture of Pyrite and Gypsum |
| λ, W/m/K | 1.94 | 2.10 | 2.37 | 2.91 |
| C, kJ/(m3·K) | 2490 | 2381 | 1996 | 1946 |
| c = C/ρ, J/kg/K | 1406 | 972 | 1018 | 828 |
| ρ, g/cm3 | 1.77 | 2.45 | 1.96 | 2.35 |
| k, m2 | 10−12 | 10−12 | 10−12 | 10−12 |
| Ti, °C | +4 | +4 | −4 | −4 |
| Sample | 2/55 | 3/60 | 4/11 | 4/25 | 5/65 | 6/38 |
|---|---|---|---|---|---|---|
| Rock Type | Limestone | Marl | Tails | Tails | Marl | Limestone |
| Well | t2 | t3 | t4 | t4 | t5 | t6 |
| Depth, m | 55 | 60 | 11 | 25 | 65 | 38 |
| Component concentration, wt.% | ||||||
| SiO2 | 43.21 | 15.80 | 24.77 | 21.90 | 33.48 | 5.81 |
| TiO2 | 0.39 | 0.11 | 0.61 | 0.72 | 0.54 | 0.06 |
| Al2O3 | 9.11 | 2.18 | 2.60 | 2.54 | 9.78 | 1.34 |
| Fe2O3 | 3.42 | 1.20 | 6.17 | 4.72 | 5.18 | 0.60 |
| MnO | 0.03 | 0.02 | 0.09 | 0.09 | 0.10 | 0.01 |
| MgO | 5.45 | 3.18 | 21.87 | 20.28 | 6.00 | 5.08 |
| CaO | 14.36 | 40.30 | 19.20 | 21.15 | 17.67 | 45.72 |
| Na2O | 0.15 | 0.12 | 0.08 | 0.09 | 0.35 | 0.11 |
| K2O | 3.89 | 0.82 | 0.43 | 0.44 | 3.45 | 0.29 |
| P2O5 | 0.05 | 0.04 | 0.25 | 0.28 | 0.13 | 0.03 |
| BaO | 0.02 | <0.01 | 0.05 | 0.07 | 0.02 | <0.01 |
| SO3 | 1.97 | 1.59 | 1.48 | 1.34 | 3.44 | 0.20 |
| V2O5 | 0.01 | <0.01 | 0.01 | 0.01 | 0.01 | <0.01 |
| Cr2O3 | <0.01 | <0.01 | 0.09 | 0.08 | <0.01 | <0.01 |
| NiO | <0.01 | <0.01 | 0.08 | 0.06 | <0.01 | <0.01 |
| LOI | 18.15 | 34.52 | 22.26 | 26.29 | 19.68 | 40.99 |
| SUM | 100.21 | 99.86 | 100.05 | 100.06 | 99.84 | 100.23 |
| The main phases of the mineral composition | Quartz calcite dolomite ankerite | Calcite ankerite anorthite quartz beaverite hematite pyrite | Magnetite quartz pyrite witherite calcite | Magnetite pyrite, calcite, quartz | Pyrite impurities calcite quartz dolomite | Calcite dolomite ankerite |
| Settling Pond | Storage Pond | |||||
|---|---|---|---|---|---|---|
| Parameter | Units | A01 | A20 | A14 | A25H | A27B |
| pH | units pH | 7.3 | 6.3 | 5.3 | 7.7 | 7.7 |
| HCO3− | mg/dm3 | 47 | 27 | 24 | 160 | 170 |
| SO4− | mg/dm3 | 1900 | 1700 | 1800 | 1600 | 1700 |
| NO3− | mg/dm3 | 230 | 180 | 190 | 130 | 130 |
| NO2− | mg/dm3 | 15 | 20 | 27 | 0.04 | 0.003 |
| PO43− | mg/dm3 | 16 | 16 | 11 | 6.5 | 8.1 |
| Cl− | mg/dm3 | 520 | 400 | 440 | 200 | 210 |
| NH4+ | mg/dm3 | 8.6 | 7.0 | 5.2 | 0.5 | 0.5 |
| Ca | mg/dm3 | 400 | 400 | 370 | 220 | 210 |
| Mg | mg/dm3 | 390 | 400 | 370 | 300 | 290 |
| K | mg/dm3 | 36 | 31 | 33 | 16 | 17 |
| Na | mg/dm3 | 59 | 61 | 62 | 23 | 22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yurkevich, N.; Fadeeva, I.; Shevko, E.; Yannikov, A.; Bortnikova, S. Modeling the Process of Thawing of Tailings Dam Base Soils by Technological Waters. Appl. Sci. 2021, 11, 11089. https://doi.org/10.3390/app112311089
Yurkevich N, Fadeeva I, Shevko E, Yannikov A, Bortnikova S. Modeling the Process of Thawing of Tailings Dam Base Soils by Technological Waters. Applied Sciences. 2021; 11(23):11089. https://doi.org/10.3390/app112311089
Chicago/Turabian StyleYurkevich, Nataliya, Irina Fadeeva, Elizaveta Shevko, Alexey Yannikov, and Svetlana Bortnikova. 2021. "Modeling the Process of Thawing of Tailings Dam Base Soils by Technological Waters" Applied Sciences 11, no. 23: 11089. https://doi.org/10.3390/app112311089
APA StyleYurkevich, N., Fadeeva, I., Shevko, E., Yannikov, A., & Bortnikova, S. (2021). Modeling the Process of Thawing of Tailings Dam Base Soils by Technological Waters. Applied Sciences, 11(23), 11089. https://doi.org/10.3390/app112311089







