Variations in Essential Oil Chemical Composition and Biological Activities of Cryptomeria japonica (Thunb. ex L.f.) D. Don from Different Geographical Origins—A Critical Review
Abstract
:1. Introduction
2. Data Analysis
3. Yield and Chemical Composition of C. japonica EO
4. Antimicrobial Activity of C. japonica EO in Food Industry and Human Diseases
| Origin | Plant Organ | Target Species | Efficiency | Ref. |
|---|---|---|---|---|
| Portugal | Leaves, heartwood, bark | Mycobacterium tuberculosis, Botrytis cinerea, Fusarium circinatum, Cryphonectria parasitica, Aspergillus niger, Trichoderma harzianum, Cladosporium cladosporioides, Cladosporium sp., Candida albicans, Candida tropicalis, Saccharomyces cerevisiae, Cryptococcus neoformans, Aspergillus fumigatus, Microsporum gypseum, Trichophyton rubrum, Trichophyton mentagrophytes | Effective against M. Tuberculosis, T. harzianum, B. cinerea, C. cladosporioides and Cladosporium sp., MICs range 0.025–0.25 mg/mL | [32] |
| South Korea | Leaves plus twigs | Candida albicans, Candida pseudotropicalis, Candida glabrata, Candida tropicalis, Candida krusei, Candida parapsilosis, Cryptococcus neoformans, Aspergillus fumigatus | Effective, MICs 2.18 mg/mL or higher | [50] |
| Taiwan | Leaves, heartwood, sapwood, bark | Trametes versicolor, Lenzites betulina, Laetiporus sulphureus, Gloeophyllum trabeum, Fusarium oxysporum, Rhizoctonia solani, Ganoderma australe, Fusarium solani, Pestalotiopsis funereal, Collectotrichum gloeosporioides | Highly effective, IC50 range 0.039 > 0.500 mg/mL, except bark EO | [10] |
| Japan | Heartwood | Staphylococcus epidermis, Trichophyton rubrum | Effective, MIC = 0.313 mg/mL, except S. epidermis | [53] |
| South Korea | Leaves | Staphylococcus epidermis, Propionibacterium acne | Effective, MICs range 0.156–10 µL/mL | [38] |
| South Korea | Leaves | Escherichia coli, Staphylococcus aureus, Staphylococcus epidermis, Streptococcus pyogenes, Streptococcus mutans, Streptococcus sanguinis, Streptococcus sobrinus, Streptococcus ratti, Streptococcus criceti, Streptococcus anginosus, Streptococcus gordonii, Actinobacillus actinomycetemcomitans, Fusobacterium nucleatum, Prevotella intermedia, Porphylomonas gingivalis | Effective, MICs range 0.025–12.8 mg/mL, except E. coli | [37] |
| South Korea | Leaves plus twigs | Escherichia coli, Enterobacter aerogenes, Enterobacter cloacae, Citrobacter freundii, Acinetobacter calcoaceticus, Staphylococcus aureus, Bacillus subtilis, Klebsiella oxytoca, Klebsiella pneumoniae, Pseudomonas aeruginosa, Serratia marcescens | Ineffective, MICs > 21.8 mg/mL | [50] |
| Taiwan | Leaves, heartwood, twigs, bark | Legionella pneumophila | Ineffective, MBC > 2 mg/mL | [55] |
5. Acaricidal and Insecticidal Activities of C. japonica EO
| Plant Organ | Pests | Bioassay | Efficiency | Ref |
|---|---|---|---|---|
| Leaves | Lepisma saccharina | Contact assay (on treated filter papers) against the adult silverfish | Significant toxicity with LD50 of 0.087 mg/cm3 after 10 h | [34] |
| Repellent assay | More than 80% of repellency at 10 µg/cm3 after 4 h | |||
| Leaves | Aedes aegypti | Aqueous suspension of essential oil against the fourth-instar mosquito larvae | Significant larvicidal activity with LC50 of 37.5 µg/mL after 24 h | [58] |
| Bark | Significant larvicidal activity with LC50 of 48.1 µg/mL after 24 h | |||
| Sapwood | Significant larvicidal activity with LC50 of 82.7 µg/mL after 24 h | |||
| Heartwood | Significant larvicidal activity with LC50 of 72.0 µg/mL after 24 h | |||
| Leaves | Aedes albopictus | Aqueous suspension of essential oil against the fourth-instar mosquito larvae | Significant larvicidal activity with LC50 range of 51.2–57.9 µg/mL after 24 h | [21] |
| Leaves | Aedes aegypti | Repellent assay | 82% of repellency at 1.92 µg/cm3 after 20 min | [35] |
| Bark | More than 70% of repellency at 1.92 µg/cm3 after 20 min | |||
| Twigs | About 70% of repellency at 1.92 µg/cm3 after 20 min | |||
| Wood | More than 50% of repellency at 1.92 µg/cm3 after 20 min | |||
| Leaves | Aedes albopictus | Repellent assay | 71% of repellency at 1.92 µg/cm3 after 20 min | [35] |
| Bark | More than 60% of repellency at 1.92 µg/cm3 after 20 min | |||
| Twigs | More than 60% of repellency at 1.92 µg/cm3 after 20 min | |||
| Wood | More than 60% of repellency at 1.92 µg/cm3 after 20 min | |||
| Leaves | Anopheles gambiae | Aqueous suspension of essential oil against the third-instar mosquito larvae | Significant larvicidal activity with LC50 of 40.9 µg/mL after 24 h | [17] |
| Leaves | Coptotermes formosanus | Contact assay (on treated filter papers) against the adult termite | High mortality, with LD50 of 1.57 mg/g after 7 days | [36] |
| Bark | Inactive | [60] | ||
| Sapwood | Mortality of 100% with LC50 of 4.7 mg/g after 5 days | |||
| Heartwood | Mortality of 100% with LC50 of 2.8 mg/g after 5 days | |||
| Leaves | Reticulitermes chinensis | Contact assay (on treated filter papers) against the adult termite | High mortality, with LC50 of 0.9 µL/mL after 5 days | [40] |
| Bark | LC50 of 19.6 µL/mL after 5 days | |||
| Sapwood | LC50 of 158.3 µL/mL after 5 days | |||
| Heartwood | High mortality, with LC50 of 1.8 µL/mL after 5 days | |||
| Leaves | Tetranychus kanzawai | Contact assay (on treated leaf discs) against spider mites | Significant mortality, with LC50 of 1109 µg/mL after 96 h | [31] |
| Leaves | Tetranychus urticae | Contact assay (on treated leaf discs) against spider mites | Significant mortality, with LC50 of 1150 µg/mL after 96 h | [31] |
| Leaves | Reticulitermes chinensis | Contact assay (on treated filter papers) against the adult termite | High mortality, with LC50 of 0.9 µL/mL after 5 days | [40] |
| Bark | LC50 of 19.6 µL/mL after 5 days | |||
| Sapwood | LC50 of 158.3 µL/mL after 5 days | |||
| Heartwood | High mortality, with LC50 of 1.8 µL/mL after 5 days | |||
| Leaves | Tetranychus kanzawai | Contact assay (on treated leaf discs) against spider mites | Significant mortality, with LC50 of 1109 µg/mL after 96 h | [31] |
| Leaves | Tetranychus urticae | Contact assay (on treated leaf discs) against spider mites | Significant mortality, with LC50 of 1150 µg/mL after 96 h | [31] |
6. Other Biocidal Activities of C. japonica EO
7. Pharmacological Properties of C. japonica EO
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akash, M.; Jitendra, P.; Somenath, D.; Kumar, D.A. Essential oils and their application in food safety. Front. Sustain. Food Syst. 2021, 5, 133. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Isman, M.B. Bioinsecticides based on plant essential oils: A short overview. Z. Nat. C 2020, 75, 179–182. [Google Scholar] [CrossRef]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef]
- Liguo, F.; Yong-Fu, Y.; Mill, R.R. Taxodiaceae. In Flora of China; Zheng-Yi, W., Raven, P.H., Eds.; Science Press: Beijing, China; Missouri Botanical Garden: St. Louis, MO, USA, 1999; Volume 4, p. 56. [Google Scholar]
- Nagakura, J.; Shigenaga, H.; Akama, A.; Takahashi, M. Growth and transpiration of Japanese cedar (Cryptomeria japonica) and Hinoki cypress (Chamaecyparis obtusa) seedlings in response to soil water content. Tree Physiol. 2004, 24, 1203–1208. [Google Scholar] [CrossRef]
- Mizushina, Y.; Kuriyama, I. Cedar (Cryptomeria japonica) Oils. In Essential Oils in Food Preservation, Flavor and Safety, 1st ed.; Preedy, V.R., Ed.; Academic Press: London, UK, 2016; pp. 317–324. [Google Scholar]
- Dias, E.; Araújo, C.; Mendes, J.; Elias, R.; Mendes, C.; Melo, C. Espécies Florestais das Ilhas—Açores. In Árvores e Florestas de Portugal; Silva, J.S., Ed.; Público, Comunicação Social, SA/Fundação Luso-Americana/Liga para a Protecção da Natureza: Lisboa, Portugal, 2007; Volume 6, pp. 199–254. [Google Scholar]
- Hasegawa, Y.; Ueno, S.; Wei, F.J.; Matsumoto, A.; Uchiyama, K.; Ujino-Ihara, T.; Hakamata, T.; Fujino, T.; Kasahara, M.; Bino, T.; et al. Identification and genetic diversity analysis of a male-sterile gene (MS1) in Japanese cedar (Cryptomeria japonica D. Don). Sci. Rep. 2021, 11, 1496. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.S.; Lin, H.Y.; Chang, S.T. Chemical composition and antifungal activity of essential oils from different tissues of Japanese Cedar (Cryptomeria japonica). J. Agric. Food Chem. 2005, 53, 614–619. [Google Scholar] [CrossRef]
- Satyal, P.; Setzer, W. Chemical composition of Cryptomeria japonica leaf oil from Nepal. Am. J. Essent. Oil Nat. Prod. 2015, 3, 7–10. [Google Scholar]
- Ho, C.L.; Wang, E.I.; Yu, H.T.; Yu, H.M.; Su, Y.C. Compositions and antioxidant activities of essential oils of different tissues from Cryptomeria japonica D. Don. Q. J. Chin. For. 2010, 32, 63–76. [Google Scholar]
- Chung, M.J.; Cheng, S.S.; Lin, C.Y.; Chang, S.T. Profiling of volatile compounds from five interior decoration timbers in Taiwan using TD/GC–MS/FID. J. Wood Sci. 2018, 64, 823–835. [Google Scholar] [CrossRef] [Green Version]
- Stevanovic, Z.D.; Sieniawska, E.; Glowniak, K.; Obradovic, N.; Pajic-Lijakovic, I. Natural macromolecules as carriers for essential oils: From extraction to biomedical application. Front. Bioeng. Biotechnol. 2020, 8, 563. [Google Scholar] [CrossRef]
- Batish, D.R.; Singh, H.P.; Kohli, R.K.; Kaur, S. Eucalyptus essential oil as a natural pesticide. For. Ecol. Manag. 2008, 256, 2166–2174. [Google Scholar] [CrossRef]
- Lengai, G.M.W.; Muthomi, J.W.; Mbega, E.R. Phytochemical activity and role of botanical pesticides in pest management for sustainable agricultural crop production. Sci. Afr. 2020, 7, e00239. [Google Scholar] [CrossRef]
- Mdoe, F.P.; Cheng, S.S.; Lyaruu, L.; Nkwengulila, G.; Chang, S.T.; Kweka, E.J. Larvicidal efficacy of Cryptomeria japonica leaf essential oils against Anopheles gambiae. Parasites Vectors 2014, 7, 426. [Google Scholar] [CrossRef] [Green Version]
- Kusumoto, N.; Shibutani, S. Evaporation of volatiles from essential oils of Japanese conifers enhances antifungal activity. J. Essent. Oil Res. 2015, 27, 380–394. [Google Scholar] [CrossRef]
- Palermo, D.; Giunti, G.; Laudani, F.; Palmeri, V.; Campolo, O. Essential oil-based nano-biopesticides: Formulation and bioactivity against the confused flour beetle Tribolium confusum. Sustainability 2021, 13, 9746. [Google Scholar] [CrossRef]
- Salha, G.B.; Díaz, R.H.; Labidi, J.; Abderrabba, M. Deterpenation of Origanum majorana L. essential oil by reduced pressure steam distillation. Ind. Crop. Prod. 2017, 109, 116–122. [Google Scholar] [CrossRef]
- Cheng, S.S.; Chua, M.T.; Chang, E.H.; Huang, C.G.; Chen, W.J.; Chang, S.T. Variations in insecticidal activity and chemical compositions of leaf essential oils from Cryptomeria japonica at different ages. Bioresour. Technol. 2009, 100, 465–470. [Google Scholar] [CrossRef]
- Tsuruta, K.; Yoshida, Y.; Kusumoto, N.; Sekine, N.; Ashitani, T.; Takahashi, K. Inhibition activity of essential oils obtained from Japanese trees against Skeletonema costatum. J. Wood Sci. 2011, 57, 520–525. [Google Scholar] [CrossRef]
- Giacometti, J.; Bursać Kovačević, D.; Putnik, P.; Gabrić, D.; Bilušić, T.; Krešić, G.; Stulić, V.; Barba, F.J.; Chemat, F.; Barbosa-Cánovas, G.; et al. Extraction of biactive compounds and essential oils from mediterranean herbs by conventional and green innovative techniques: A review. Food Res. Int. 2018, 113, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Han, D.; Tian, M.; Row, K.H. Supercritical CO2 extraction of essential oils from Chamaecyparis obtusa. Nat. Prod. Commun. 2010, 5, 461–464. [Google Scholar] [CrossRef] [Green Version]
- Lopresto, C.G.; Meluso, A.; Di Sanzo, G.; Chakraborty, S.; Calabrò, V. Process-intensified waste valorization and environmentally friendly D-limonene extraction. Euro-Mediterr. J. Environ. Integr. 2019, 4, 31. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Bonesi, M.; Sanzo, G.D.; Verardi, A.; Lopresto, C.G.; Pugliese, A.; Menichini, F.; Balducchi, R.; Calabrò, V. Chemical Profile and Antioxidant properties of extracts and essential oils from Citrus × limon (L.) Burm. cv. Femminello Comune. Chem. Biodivers. 2016, 13, 571–581. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, J.; Yang, F.; Lei, C. Comparative analysis of essential oil components of two Cryptomeria species from China. Ind. Crop. Prod. 2011, 34, 1226–1230. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chang, T.C.; Chen, Y.H.; Chen, Y.J.; Cheng, S.S.; Chang, S.T. Monitoring the dynamic emission of biogenic volatile organic compounds from Cryptomeria japonica by enclosure measurement. Atmos. Environ. X 2015, 122, 163–170. [Google Scholar] [CrossRef]
- Uchida, S. The essential oil of sugi (Cryptomeria japonica) leaves. J. Am. Chem. Soc. 1916, 38, 687–699. [Google Scholar] [CrossRef] [Green Version]
- Oh, H.J.; Ahn, H.M.; So, K.H.; Kim, S.S.; Yun, P.Y.; Jeon, G.L.; Riu, K.Z. Chemical and antimicrobial properties of essential oils from three coniferous trees Abies koreana, Cryptomeria japonica, and Torreya nucifera. Appl. Biol. Chem. 2007, 50, 164–169. [Google Scholar]
- Yamashita, Y.; Hashimoto, N.; Kusumoto, N.; Saijo, H.; Goto, I.; Kobayashi, H.; Kurihara, Y.; Takahashi, K.; Ashitani, T. Acaricidal activity of components of Cryptomeria japonica against spider mites. J. Wood Sci. 2015, 61, 60–64. [Google Scholar] [CrossRef]
- Moiteiro, C.; Esteves, T.; Ramalho, L.; Rojas, R.; Alvarez, S.; Zacchino, S.; Bragança, H. Essential oil characterization of two Azorean Cryptomeria japonica populations and their biological evaluations. Nat. Prod. Commun. 2013, 8, 1785–1790. [Google Scholar] [CrossRef] [Green Version]
- Garcia, G.; Garcia, A.; Gibernau, M.; Bighelli, A.; Tomi, F. Chemical compositions of essential oils of five introduced conifers in Corsica. Nat. Prod. Res. 2017, 31, 1697–1703. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lai, W.C.; Chu, F.H.; Lin, C.T.; Shen, S.Y.; Chang, S.T. Essential oil from the leaves of Cryptomeria japonica acts as a silverfish (Lepisma saccharina) repellent and insecticide. J. Wood Sci. 2006, 52, 522–526. [Google Scholar] [CrossRef]
- Gu, H.J.; Cheng, S.S.; Lin, C.Y.; Huang, C.G.; Chen, W.J.; Chang, S.T. Repellency of essential oils of Cryptomeria japonica (Pinaceae) against adults of the mosquitoes Aedes aegypti and Aedes albopictus (Diptera:Culicidae). J. Agric. Food Chem. 2009, 57, 11127–11133. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.S.; Lin, C.Y.; Chung, M.J.; Chang, S.T. Chemical composition and antitermitic activity against Coptotermes formosanus Shiraki of Cryptomeria japonica leaf essential oil. Chem. Biodivers. 2012, 9, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.D.; Jeong, M.R.; Jeong, S.I.; Moon, S.E.; Kil, B.S.; Yun, S.I.; Lee, K.Y.; Song, Y.H. Chemical composition and antimicrobial activity of the essential oil of Cryptomeria japonica. Phytother. Res. 2007, 21, 295–299. [Google Scholar] [CrossRef]
- Yoon, W.J.; Kim, S.S.; Oh, T.H.; Lee, N.H.; Hyun, C.G. Cryptomeria japonica essential oil inhibits the growth of drug-resistant skin pathogens and LPS-induced nitric oxide and pro-inflammatory cytokine production. Pol. J. Microbiol. 2009, 58, 61–68. [Google Scholar]
- Kim, S.H.; Lee, S.Y.; Hong, C.Y.; Gwak, K.S.; Park, M.J.; Smith, D.; Choi, I.G. Whitening and antioxidant activities of bornyl acetate and nezukol fractionated from Cryptomeria japonica essential oil. Int. J. Cosmet. Sci. 2013, 35, 484–490. [Google Scholar] [CrossRef]
- Xie, Y.; Li, M.; Huang, Q.; Lei, C. Chemical composition and termiticidal activity of essential oils from different tissues of Chinese cedar (Cryptomeria fortunei). Nat. Prod. Commun. 2014, 9, 719–722. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, T.; Zhu, Q.; Ishikawa, H.; Ohnuki, K.; Kakino, K.; Horiuchi, N.; Shinotsuka, H.; Naito, T.; Matsumoto, T.; Minamisawa, N.; et al. Multiple uses of essential oil and by-products from various parts of the Yakushima native cedar (Cryptomeria japonica). J. Wood Chem. Technol. 2016, 36, 42–55. [Google Scholar] [CrossRef]
- Yasue, M.; Ogiyama, K.; Saito, M. The diterpene hydrocarbons in the leaves of Cryptomeria japonica. J. Jpn. For. Soc. 1976, 58, 285–290. [Google Scholar]
- Suzuki, Y.; Saijo, H.; Takahashi, K.; Kofujita, H.; Ashitani, T. Growth-inhibitory components in Sugi (Cryptomeria japonica) extracts active against Microcystis aeruginosa. Cogent Environ. Sci. 2018, 4, 1466401. [Google Scholar] [CrossRef]
- Hedden, P.; Sponsel, V. A century of gibberellin research. J. Plant Growth Regul. 2015, 34, 740–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, S.S.; Huang, C.G.; Chen, W.J.; Kuo, Y.H.; Chang, S.T. Larvicidal activity of tectoquinone isolated from red heartwood-type Cryptomeria japonica against two mosquito species. Bioresour. Technol. 2008, 99, 3617–3622. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, Q.; Yang, F.; Lei, C. Chemical variation in essential oil of Cryptomeria fortunei from various areas of China. Ind. Crop. Prod. 2012, 36, 308–312. [Google Scholar] [CrossRef]
- Kim, J.C.; Kim, K.J.; Kim, D.S.; Han, J.S. Seasonal variations of monoterpene emissions from coniferous trees of different ages in Korea. Chemosphere 2005, 59, 1685–1696. [Google Scholar] [CrossRef]
- Bang, K.W.; Lewis, G.; Villas-Boas, S.G. Leptospermum scoparium (Mānuka) and Cryptomeria japonica (Sugi) leaf essential oil seasonal chemical variation and their effect on antimicrobial activity. Preprints 2020, 2020080623. [Google Scholar] [CrossRef]
- Tajkarimi, M.M.; Ibrahim, S.A.; Cliver, D.O. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, B.K.; Kim, J.H.; Lee, S.H.; Hong, S.K. Comparison of chemical compositions and antimicrobial activities of essential oils from three conifer trees; Pinus densiflora, Cryptomeria japonica, and Chamaecyparis obtusa. J. Microbiol. Biotechnol. 2009, 19, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Matsushita, Y.I.; Hwang, Y.H.; Sugamoto, K.; Matsui, T. Antimicrobial activity of heartwood components of sugi (Cryptomeria japonica) against several fungi and bacteria. J. Wood Sci. 2006, 52, 552–556. [Google Scholar] [CrossRef]
- Yap, P.S.X.; Yusoff, K.; Lim, S.-H.E.; Chong, C.-M.; Lai, K.-S. Membrane disruption properties of essential oils—A double-edged sword? Processes 2021, 9, 595. [Google Scholar] [CrossRef]
- Takao, Y.; Kuriyama, I.; Yamada, T.; Mizoguchi, H.; Yoshida, H.; Mizushina, Y. Antifungal properties of Japanese cedar essential oil from waste wood chips made from used sake barrels. Mol. Med. Rep. 2012, 5, 1163–1168. [Google Scholar]
- Tsujimura, M.; Goto, M.; Tsuji, M.; Yamaji, Y.; Ashitani, T.; Kimura, K.; Ohira, T.; Kofujita, H. Isolation of diterpenoids from sugi wood-drying byproducts and their bioactivities. J. Wood Sci. 2019, 65, 19. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.W.; Chang, W.L.; Chang, S.T.; Cheng, S.S. Antibacterial activities of plant essential oils against Legionella pneumophila. Water Res. 2008, 42, 278–286. [Google Scholar] [CrossRef]
- Cheng, S.S.; Chang, S.T. Bioactivity and characterization of exudates from Cryptomeria japonica bark. Wood Sci. Technol. 2014, 48, 831–840. [Google Scholar] [CrossRef]
- Su, W.C.; Fang, J.M.; Cheng, Y.S. Flavonoids and lignans from leaves of Cryptomeria japonica. Phytochemistry 1995, 40, 563–566. [Google Scholar]
- Cheng, S.S.; Chang, H.T.; Chang, S.T.; Tsai, K.H.; Chen, W.J. Bioactivity of selected plant essential oils against the yellow fever mosquito Aedes aegypti larvae. Bioresour. Technol. 2003, 89, 99–102. [Google Scholar] [CrossRef]
- Gu, H.J.; Cheng, S.S.; Huang, C.G.; Chen, W.J.; Chang, S.T. Mosquito larvicidal activities of extractives from black heartwood-type Cryptomeria japonica. Parasitol. Res. 2009, 105, 1455–1458. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.S.; Chang, H.T.; Wu, C.L.; Chang, S.T. Anti-termitic activities of essential oils from coniferous trees against Coptotermes formosanus. Bioresour. Technol. 2007, 98, 456–459. [Google Scholar] [CrossRef]
- Arruda, F. Bio-Valorização de Resíduos de Cryptomeria japonica por Obtenção do Óleo Essencial e de Extratos Orgânicos e Determinação das suas Propriedades Biológicas. Master’s Thesis, Universidade dos Açores, Ponta Delgada, Portugal, 2019. [Google Scholar]
- Relf, V.; Good, B.; McCarthy, E.; de Waal, T. Evidence of Fasciola hepatica infection in Radix peregra and a mollusc of the family Succineidae in Ireland. Vet. Parasitol. 2009, 163, 152–155. [Google Scholar] [CrossRef]
- Rodrigues, A. Composição Química e Atividades Biológicas dos Metabolitos Secundários de Cryptomeria japonica (L.f.) D.Don Sobre a Lagarta da Pastagem Pseudaletia unipuncta Haworth (Lepidoptera: Noctuidae). Master’s Thesis, Universidade dos Açores, Ponta Delgada, Portugal, 2019. [Google Scholar]
- Tanaka, S.; Tomita, R.; Saijo, H.; Takahashi, K.; Ashitani, T. Growth-inhibitory activity of components in Cryptomeria japonica leaves against Robinia pseudoacacia. J. For. Res. 2020, 25, 192–197. [Google Scholar] [CrossRef]
- Kessler, A.; Baldwin, I.T. Defensive function of herbivore-induced plant volatile emissions in nature. Science 2001, 291, 2141–2144. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.W.; Lin, C.T.; Chu, F.H.; Chang, S.T.; Wang, S.Y. Neuropharmacological activities of phytoncide released from Cryptomeria japonica. J. Wood Sci. 2009, 55, 27–31. [Google Scholar] [CrossRef]
- Matsubara, E.; Ohira, T. Inhalation of Japanese cedar (Cryptomeria japonica) wood odor causes psychological relaxation after monotonous work among female participants. Biomed. Res. 2018, 39, 241–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohira, T.; Park, B.J.; Kurosumi, Y.; Miyazaki, Y. Evaluation of dried-wood odors: Comparison between analytical and sensory data on odors from dried sugi (Cryptomeria japonica) wood. J. Wood Sci. 2009, 55, 144–148. [Google Scholar] [CrossRef]
- Matsubara, E.; Kawai, S. Gender differences in the psychophysiological effects induced by VOCs emitted from Japanese cedar (Cryptomeria japonica). Environ. Health Prev. Med. 2018, 23, 10. [Google Scholar] [CrossRef] [Green Version]
- Misawa, M.; Kizawa, M. Antitussive effects of several volatile oils, especially of cedar leaf oil in guinea pig. Pharmacometrics 1990, 39, 81–93. [Google Scholar]
- Boyd, E.M.; Sheppard, E.P. The effect of steam inhalation of volatile oils on the output and composition of respiratory tract fluid. J. Pharmacol. Exp. Ther. 1968, 163, 250–256. [Google Scholar] [PubMed]
- Shimizu, K.; Fukunaga, S.; Yoshikawa, K.; Kondo, R. Screening of extracts of Japanese woods for melanin biosynthesis inhibition. J. Wood Sci. 2007, 53, 153–160. [Google Scholar] [CrossRef]
- Horiba, H.; Nakagawa, T.; Zhu, Q.; Ashour, A.; Watanabe, A.; Shimizu, K. Biological activities of extracts from different parts of Cryptomeria japonica. Nat. Prod. Commun. 2016, 11, 1337–1342. [Google Scholar] [CrossRef] [Green Version]
- Murata, K.; Tanaka, K.; Akiyama, R.; Noro, I.; Nishio, A.; Nakagawa, S.; Matsumura, S.; Matsuda, H. Anti-cholinesterase activity of crude drugs selected from the ingredients of incense sticks and heartwood of Chamaecyparis obtusa. Nat. Prod. Commun. 2018, 13, 803–806. [Google Scholar] [CrossRef] [Green Version]
- Freires, I.A.; Denny, C.; Benso, B.; de Alencar, S.M.; Rosalen, P.L. Antibacterial activity of essential oils and their isolated constituents against cariogenic bacteria: A systematic review. Molecules 2015, 20, 7329–7358. [Google Scholar] [CrossRef]
- Cha, J.D.; Kim, J.Y. Essential oil from Cryptomeria japonica induces apoptosis in human oral epidermoid carcinoma cells via mitochondrial stress and activation of caspases. Molecules 2012, 17, 3890–3901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsunaga, T.; Hasegawa, C.; Kawasuji, T.; Suzuki, H.; Saito, H.; Sagioka, T.; Takahashi, R.; Tsukamoto, H.; Morikawa, T.; Akiyama, T. Isolation of the antiulcer compound in essential oil from the leaves of Cryptomeria japonica. Biol. Pharm. Bull. 2000, 23, 595–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
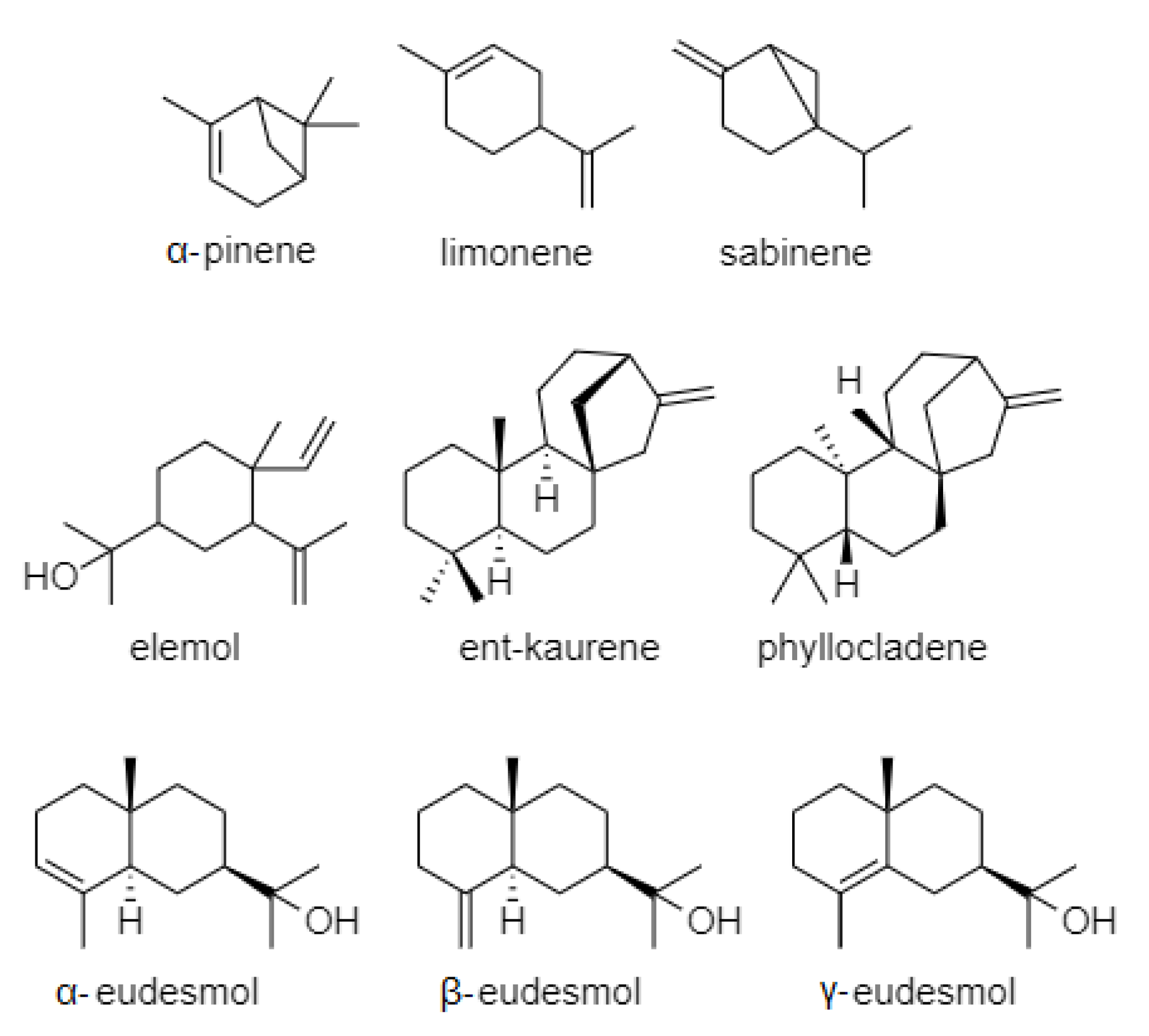


| Origin | Major Components of Cryptomeria japonica Essential Oil 1 | EOs Yield | Ref. |
|---|---|---|---|
| Taiwan_1 | ent-kaurene (40.6%), valencene (19.9%), eudesma-3,7 (11)-diene (8.4%), α-gurjunene (7.9%), β-eudesmol (5.9%), p-cymene (3.7%) | 27.38 mL/kg | [10] |
| Taiwan_2 | elemol (18.2%), ent-kaurene (11.6%), 3-carene (9.7%), sabinene (9.4%), 4-terpineol (9.1%), β-eudesmol (5.7%), α-pinene (5.6%), limonene (5.3%) | 24.6 mL/kg | [34] |
| Taiwan_3 | ent-kaurene (20.4%), elemol (19.1%), α+β eudesmol (11.8%), sabinene (10.2%), γ-eudesmol (6.3%), 4-terpineol (6.2%), α-pinene (4.8%) | 2.37% (w/w) | [21] |
| Taiwan_4 | ent-kaurene (21.7%), β-elemol (13.9%), 3-carene (13.1%), sabinene (10.3%), α-pinene (6.5%), 4-terpineol (6.0%), r-eudesmol (4.5%) | –– | [35] |
| Taiwan_5 | ent-kaurene (19.1%), α-pinene (16.5%), elemol (16.3%), 3-carene (9.5%), α-eudesmol (8.9%), γ-eudesmol (5.4%) | 39.3 mL/kg | [12] |
| Taiwan_6 | ent-kaurene (27.6%), sabinene (11.9%), 4-terpineol (8.2%), β-elemol (7.1%), cedrol (6.8%), γ-terpinene (4.8%) | 2.14% (w/w) | [28] |
| Taiwan_7 | ent-kaurene (23.3%), β-elemol (18.3%), α-pinene (8.5%), γ-eudesmol (8.2%), limonene (6.8%), α-eudesmol (6.5%), β-eudesmol (4.8%), bornyl acetate (3.8%) | 1.6% (w/w) | [36] |
| South Korea_1 | elemol (11.2%), 4-terpineol (9.8%), sabinene (8.9%), 10 (15)-cadinen-4-ol (7.2%), α-terpineol (6.1%), α-pinene (6.1%), γ-terpinene (4.7%) | 0.70% 2 | [37] |
| South Korea_2 | ent-kaurene (17.2%), elemol (10.9%), γ-eudesmol (9.4%), sabinene (8.9%), α-eudesmol (5.3%), β-eudesmol (5.1%), 4-terpineol (4.1%) | 0.6% (v/w) 2 | [38] |
| South Korea_3 | ent-kaurene (26.3%), γ-eudesmol (19.0%), α-eudesmol (7.9%), elemol (6.9%), β-eudesmol (6.0%), sabinene (5.1%), 4-terpineol (4.6%), α-pinene (3.0%) | 0.84% (v/w) | [30] |
| South Korea_4 | ent-Kaurene (19.4%), α-terpineol (13.4%), α-eudesmol (12.2%), elemol (10.9%), γ-eudesmol (10.6%), α-pinene (9.8%), 4-terpineol (5.7%) | 4.7% (w/w) | [39] |
| China_1 | β-elemol (20.1%), ent-kaurene (14.8%), α-pinene (8.0%), β-phellandrene (6.0%), β-elemene (5.9%), α-eudesmol (5.6%), γ-eudesmol (4.1%) | 1.15% (w/w) | [27] |
| China_2 | ent-kaurene (30.6%), α-eudesmol (12.5%), elemol (11.6%), β-eudesmol (11.4%), γ-eudesmol (11.3%), α-pinene (1.8%), 4-terpineol (1.7%) | 1.5% (w/w) | [40] |
| Japan_1 | α-pinene (17.0%), sabinene (12.0%), elemol (9.6%), ent-kaurene (6.7%), γ-terpinene (6.1%), γ-eudesmol (4.9%) | –– | [18] |
| Japan_2 | ent-kaurene (22.5%), elemol (22.4%), 4-terpineol (21.0%), γ-eudesmol (17.2%), β-eudesmol (8.0%), α-pinene (2.0%) | –– | [31] |
| Japan_3 | α-pinene (13.1%), ent-kaurene (9.2%), thujopsene (8.8%), β-pinene (8.2%), limonene (5.1%), camphene (3.6%), cedrol (3.3%) | 2.2 mL/kg | [41] |
| Nepal | ent-kaurene (42.1%), elemol (20.3%), γ-eudesmol (7.0%), β-eudesmol (5.0%), α-eudesmol (4.7%), sabinene (4.3%), α-pinene (4.2%) | 0.5% (w/w) | [11] |
| Portugal (Azores) | α-pinene (9.6–9.5%), (+)-phyllocladene (3.5–26.5%), ent-kaurene (0.2–20.6%), sabinene (0.5–19.9%), limonene (1.4–11.5%), elemol (0.2–12.7%), α-eudesmol (1.6–7.1%) | 0.5–1.9% (w/w) 2 | [32] |
| France (Corsica) | sabinene (19.6%), α-pinene (19.1%), β-elemol (10.7%), limonene (9.0%), ent-kaurene (6.5%), 4-terpineol (6.4%), myrcene (4.3%) | 0.61% (w/w) | [33] |
| Principal Components | |||
|---|---|---|---|
| PC 1 | PC 2 | PC 3 | |
| α-Pinene | 0.481 | 0.837 | −0.182 |
| Sabinene | 0.784 | 0.334 | −0.238 |
| β-Pinene | −0.046 | 0.871 | −0.279 |
| β-Myrcene | 0.750 | 0.579 | −0.179 |
| 3-Carene | 0.641 | −0.421 | −0.358 |
| Limonene | 0.694 | 0.577 | −0.058 |
| γ-Terpinene | 0.816 | 0.098 | −0.251 |
| α-Terpineol | 0.893 | 0.005 | −0.111 |
| Bornyl acetate | 0.619 | 0.132 | −0.117 |
| Elemol | −0.126 | −0.006 | 0.714 |
| α+β Eudesmol | −0.277 | −0.378 | 0.651 |
| γ-Eudesmol | −0.165 | −0.240 | 0.767 |
| Ent-kaurene | −0.539 | −0.655 | −0.174 |
| Phyllocladene | 0.034 | 0.613 | −0.178 |
| Eigenvalue | 4.56 | 3.44 | 2.00 |
| % of variance | 46.79 | 16.67 | 10.05 |
| Cumulative % | 46.79 | 61.45 | 71.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, A.; Arruda, F.; Medeiros, J.; Baptista, J.; Madruga, J.; Lima, E. Variations in Essential Oil Chemical Composition and Biological Activities of Cryptomeria japonica (Thunb. ex L.f.) D. Don from Different Geographical Origins—A Critical Review. Appl. Sci. 2021, 11, 11097. https://doi.org/10.3390/app112311097
Lima A, Arruda F, Medeiros J, Baptista J, Madruga J, Lima E. Variations in Essential Oil Chemical Composition and Biological Activities of Cryptomeria japonica (Thunb. ex L.f.) D. Don from Different Geographical Origins—A Critical Review. Applied Sciences. 2021; 11(23):11097. https://doi.org/10.3390/app112311097
Chicago/Turabian StyleLima, Ana, Filipe Arruda, Jorge Medeiros, José Baptista, João Madruga, and Elisabete Lima. 2021. "Variations in Essential Oil Chemical Composition and Biological Activities of Cryptomeria japonica (Thunb. ex L.f.) D. Don from Different Geographical Origins—A Critical Review" Applied Sciences 11, no. 23: 11097. https://doi.org/10.3390/app112311097
APA StyleLima, A., Arruda, F., Medeiros, J., Baptista, J., Madruga, J., & Lima, E. (2021). Variations in Essential Oil Chemical Composition and Biological Activities of Cryptomeria japonica (Thunb. ex L.f.) D. Don from Different Geographical Origins—A Critical Review. Applied Sciences, 11(23), 11097. https://doi.org/10.3390/app112311097








