Phytohormones and Elicitors Enhanced the Ecdysteroid and Glycosylflavone Content and Antioxidant Activity of Silene repens
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Treatment of S. repens Seedlings by Phytohormones and Elicitors
2.4. Total Extracts Preparation from S. repens Leaves and Roots
2.5. Solid-Phase Extraction (SPE) of Total Extract from S. repens Leaves and Roots
2.6. High-Performance Liquid Chromatography with Photodiode Array Detection and Electrospray Ionization Triple Quadrupole Mass Spectrometric Detection (HPLC-PDA-ESI-tQ-MS): Metabolite Profiling and Quantification
2.7. Antioxidant Activity of S. repens
HPLC-PDA Activity-Based Profiling
2.8. Statistical Analysis
3. Results and Discussion
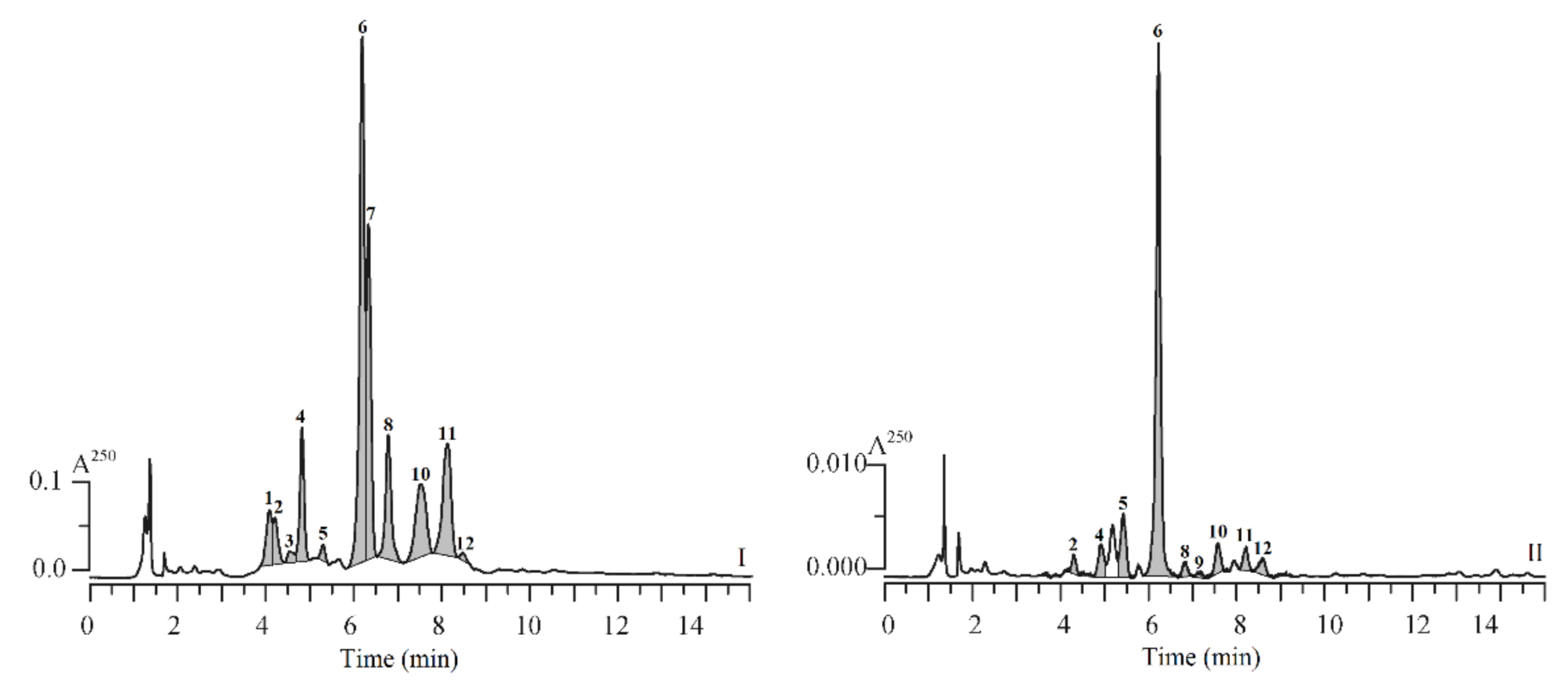
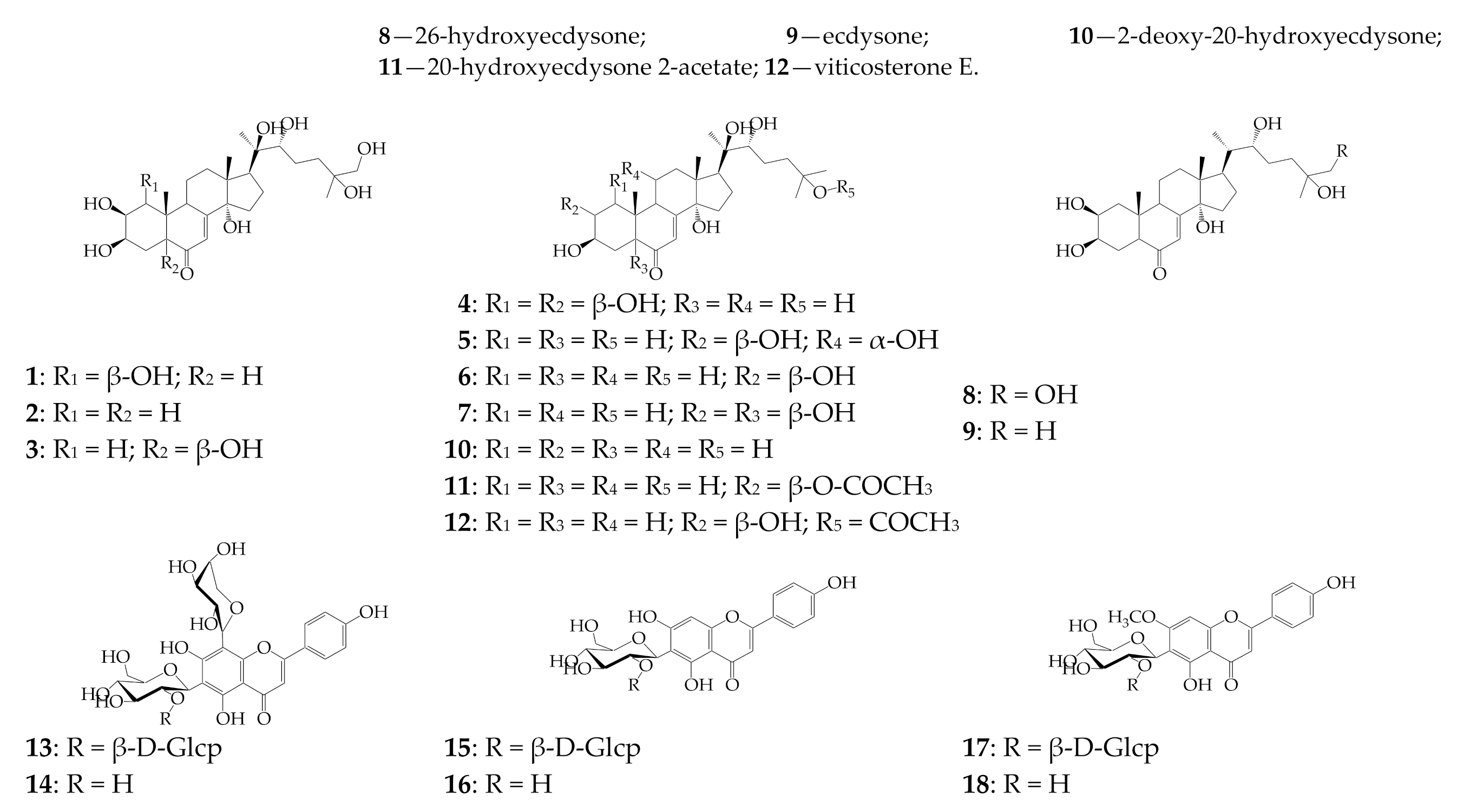
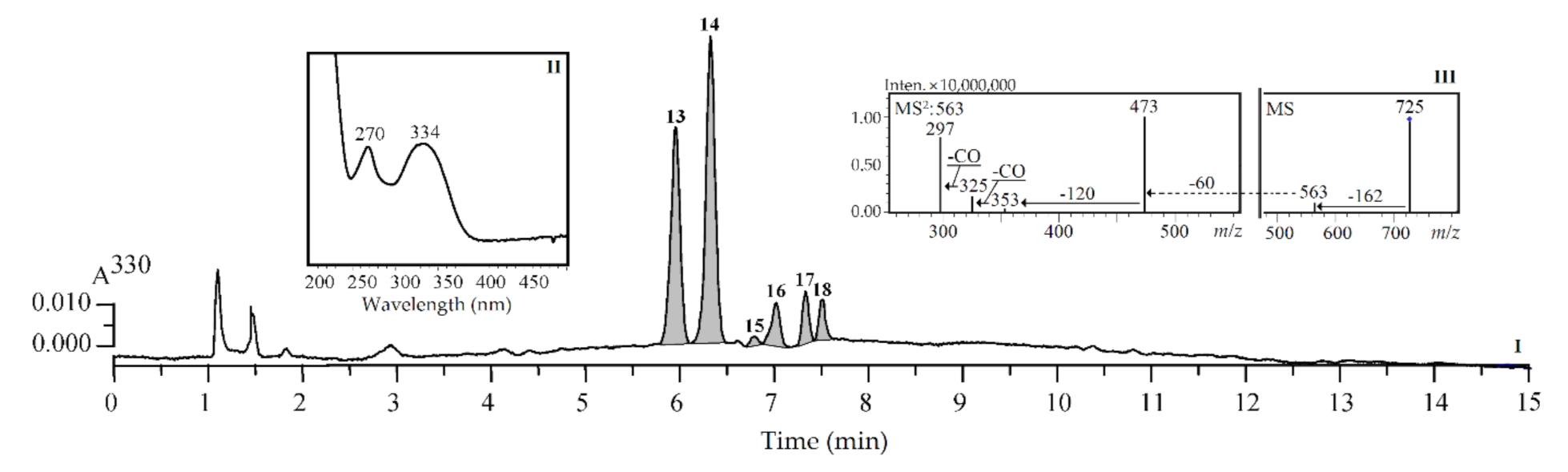
3.1. HPLC-PDA-tQ-ESI-MS Profiles of SPE Fractions of Silene repens: Qualitative Study
3.2. Effect of Phytohormones and Elicitors on Productivity and Content of Ecdysteroids in Introduced Seedlings of S. repens
3.3. Effect of Phytohormones and Elicitors on Content of Glycosylflavones in Introduced Seedlings of S. repens
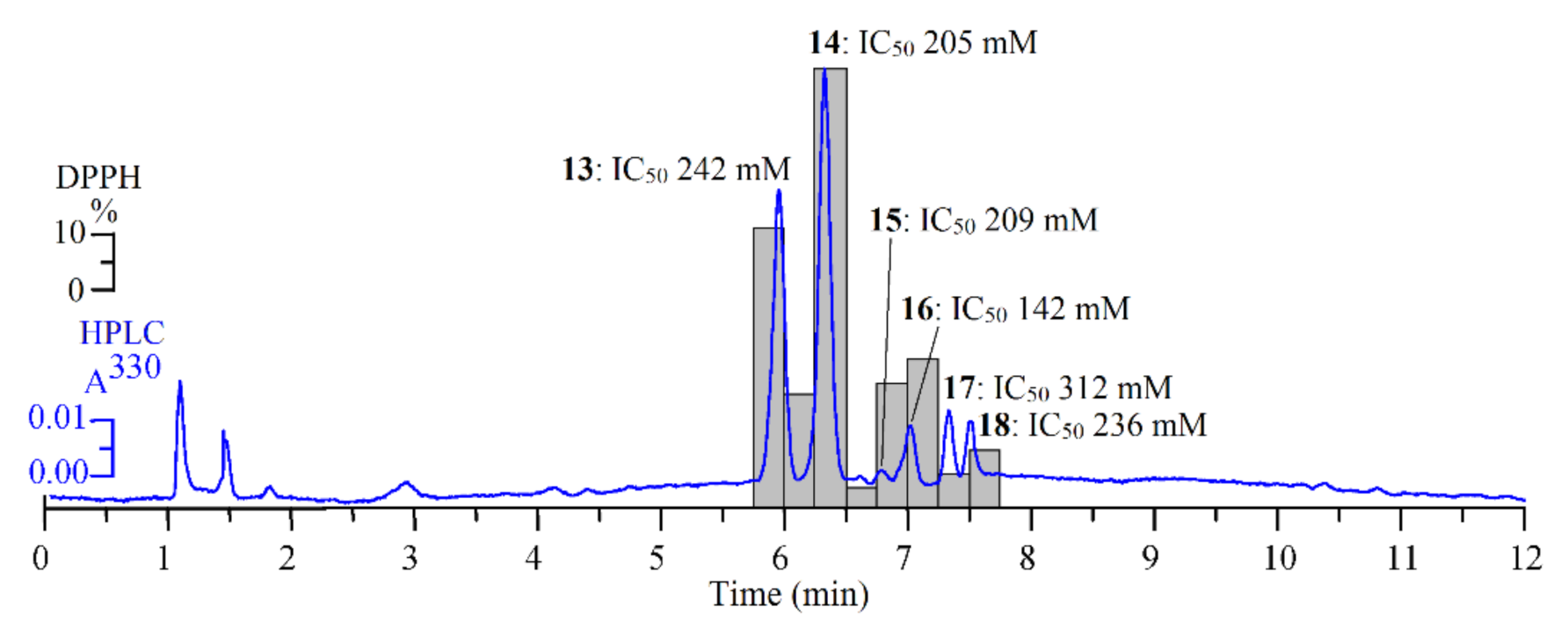
3.4. Antioxidant Activity of S. repens
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Afzal, J.; Saleem, M.H.; Batool, F.; Elyamine, A.M.; Rana, M.S.; Shaheen, A.; El-Esawi, M.A.; Javed, M.T.; Ali, Q.; Ashraf, M.A.; et al. Role of Ferrous Sulfate (FeSO4) in Resistance to Cadmium Stress in Two Rice (Oryza sativa L.) Genotypes. Biomolecules 2020, 10, 1693. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Wang, X.; Saleem, M.; Khan, M.; Afzal, J.; Fiaz, S.; Ali, S.; Ishaq, H.; Khan, A.; Rehman, N.; et al. Deciphering Plantago ovata Forsk Leaf Extract Mediated Distinct Germination, Growth and Physio-Biochemical Improvements under Water Stress in Maize (Zea mays L.) at Early Growth Stage. Agronomy 2021, 11, 1404. [Google Scholar] [CrossRef]
- Saleem, M.H.; Wang, X.; Ali, S.; Zafar, S.; Nawaz, M.; Adnan, M.; Fahad, S.; Shah, A.; Alyemeni, M.N.; Hefft, D.I.; et al. Interactive effects of gibberellic acid and NPK on morpho-physio-biochemical traits and organic acid exudation pattern in coriander (Coriandrum sativum L.) grown in soil artificially spiked with boron. Plant Physiol. Biochem. 2021, 167, 884–900. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.H.; Ali, S.; Rehman, M.; Hasanuzzaman, M.; Rizwan, M.; Irshad, S.; Shafiq, F.; Iqbal, M.; Alharbi, B.M.; Alnusaire, T.S.; et al. Jute: A Potential Candidate for Phytoremediation of Metals—A Review. Plants 2020, 9, 258. [Google Scholar] [CrossRef]
- Méndez-Hernández, H.A.; Ledezma-Rodríguez, M.; Avilez-Montalvo, R.N.; Juárez-Gómez, Y.L.; Skeete, A.; Avilez-Montalvo, J.; De-La-Peña, C.; Loyola-Vargas, V.M. Signaling Overview of Plant Somatic Embryogenesis. Front. Plant Sci. 2019, 10, 77. [Google Scholar] [CrossRef]
- Bürger, M.; Chory, J. Stressed Out About Hormones: How Plants Orchestrate Immunity. Cell Host Microbe 2019, 26, 163–172. [Google Scholar] [CrossRef]
- Bari, R.; Jones, J.D.G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef]
- Ku, Y.-S.; Sintaha, M.; Cheung, M.-Y.; Lam, H.-M. Plant Hormone Signaling Crosstalks between Biotic and Abiotic Stress Responses. Int. J. Mol. Sci. 2018, 19, 3206. [Google Scholar] [CrossRef]
- Ciura, J.; Kruk, J. Phytohormones as targets for improving plant productivity and stress tolerance. J. Plant Physiol. 2018, 229, 32–40. [Google Scholar] [CrossRef]
- Sundberg, E.; Østergaard, L. Distinct and Dynamic Auxin Activities During Reproductive Development. Cold Spring Harb. Perspect. Biol. 2009, 1, a001628. [Google Scholar] [CrossRef]
- Pierre-Jerome, E.; Drapek, C.; Benfey, P.N. Regulation of Division and Differentiation of Plant Stem Cells. Annu. Rev. Cell Dev. Biol. 2018, 34, 289–310. [Google Scholar] [CrossRef]
- Wani, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop. J. 2016, 4, 162–176. [Google Scholar] [CrossRef]
- Ebektas, Y.; Eeulgem, T. Synthetic plant defense elicitors. Front. Plant Sci. 2015, 5, 804. [Google Scholar] [CrossRef]
- Dedyukhina, E.G.; Kamzolova, S.V.; Vainshtein, M.B. Arachidonic acid as an elicitor of the plant defense response to phytopathogens. Chem. Biol. Technol. Agric. 2014, 1, 18. [Google Scholar] [CrossRef]
- Olennikov, D.N. Ecdysteroids, Flavonoids, and Phenylpropanoids from Silene nutans. Chem. Nat. Compd. 2019, 55, 127–130. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K. C-/O-GLYCOSYL FLAVONES OF SILENE ITALICA (Caryophyllaceae). Chem. Plant raw Mater. 2019, 3, 119–127. [Google Scholar] [CrossRef]
- Komarov, V.L. Flora of USSR; Publishing House of the USSR Academy of Sciences: Moscow, Russia, 1936; Volume VI, pp. 577–691. [Google Scholar]
- Olennikov, D.N. Ecdysteroids of Silene repens from Eastern Siberia. Chem. Nat. Compd. 2019, 55, 770–772. [Google Scholar] [CrossRef]
- Olennikov, D.N. Silenerepin—A New C-Glycosylflavone from Silene repens. Chem. Nat. Compd. 2020, 56, 423–426. [Google Scholar] [CrossRef]
- Das, N.; Mishra, S.K.; Bishayee, A.; Ali, E.S.; Bishayee, A. The phytochemical, biological, and medicinal attributes of phytoecdysteroids: An updated review. Acta Pharm. Sin. B 2021, 11, 1740–1766. [Google Scholar] [CrossRef]
- Thiem, B.; Kikowska, M.; Maliński, M.P.; Kruszka, D.; Napierała, M.; Florek, E. Ecdysteroids: Production in plant in vitro cultures. Phytochem. Rev. 2017, 16, 603–622. [Google Scholar] [CrossRef]
- Cahlíková, L.; Macáková, K.; Chlebek, J.; Hostalkova, A.; Kulhánková, A.; Opletal, L. Ecdysterone and its Activity on some Degenerative Diseases. Nat. Prod. Commun. 2011, 6, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Zhang, Y.; Pan, C.; Jia, Q.; Guo, F.; Li, Y.; Zhu, W.; Chen, K. Advances in studying of the pharmacological activities and structure–activity relationships of natural C-glycosylflavonoids. Acta Pharm. Sin. B 2013, 3, 154–162. [Google Scholar] [CrossRef][Green Version]
- Oualid, O.; Silva, A.M.S. Advances in C-glycosylflavonoid Research. Curr. Org. Chem. 2012, 16, 859–896. [Google Scholar] [CrossRef]
- Pérez-Balibrea, S.; Moreno, D.; García-Viguera, C. Improving the phytochemical composition of broccoli sprouts by elicitation. Food Chem. 2011, 129, 35–44. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, X.; Sandhu, A.K.; Gu, L. Effects of Exogenous Abscisic Acid on Yield, Antioxidant Capacities, and Phytochemical Contents of Greenhouse Grown Lettuces. J. Agric. Food Chem. 2010, 58, 6503–6509. [Google Scholar] [CrossRef]
- Gorelick, J.; Iraqi, R.H.; Bernstein, N. Ecdysteroid Content and Therapeutic Activity in Elicited Spinach Accessions. Plants 2020, 9, 727. [Google Scholar] [CrossRef]
- Davies, P.J. Regulatory Factors in Hormone Action: Level, Location and Signal Transduction. In Plant Hormones; Davies, P.J., Ed.; Springer: Dordrecht, The Netherlands, 2010; pp. 16–35. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I. Phytoecdysteroids from Silene jenisseensis. Chem. Nat. Compd. 2017, 53, 1199–1201. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I. New C,O-Glycosylflavones from the Genus Silene. Chem. Nat. Compd. 2020, 56, 1026–1034. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K. C-Glycosyl Flavones from Two Eastern Siberian Species of Silene. Chem. Nat. Compd. 2019, 55, 642–647. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K.; Kashchenko, N.I.; Nikolaev, V.M.; Kim, S.-W.; Vennos, C. Bioactive Phenolics of the Genus Artemisia (Asteraceae): HPLC-DAD-ESI-TQ-MS/MS Profile of the Siberian Species and Their Inhibitory Potential Against α-Amylase and α-Glucosidase. Front. Pharmacol. 2018, 9, 756. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K.; Vasilieva, A.G.; Fedorov, I.A. LC-MS Profile, Gastrointestinal and Gut Microbiota Stability and Antioxidant Activity of Rhodiola rosea Herb Metabolites: A Comparative Study with Subterranean Organs. Antioxidants 2020, 9, 526. [Google Scholar] [CrossRef]
- Yang, X.; Baburin, I.; Plitzko, I.; Hering, S.; Hamburger, M. HPLC-based activity profiling for GABAA receptor modulators from the traditional Chinese herbal drug Kushen (Sophora flavescens root). Mol. Divers. 2011, 15, 361–372. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Vasilieva, A.G.; Chirikova, N.K. Fragaria viridis Fruit Metabolites: Variation of LC-MS Profile and Antioxidant Potential During Ripening and Storage. Pharmaceuticals 2020, 13, 262. [Google Scholar] [CrossRef]
- Munkhzhargal, N.; Zibareva, L.N.; Lafont, R.; Pribytkova, L.N.; Pisareva, S.I. Investigation of ecdysteroid content and composition of Silene repens indigenous in Mongolia and introduced into western Siberia. Russ. J. Bioorg. Chem. 2010, 36, 923–928. [Google Scholar] [CrossRef]
- Zibareva, L.; Yeriomina, V.I.; Munkhjargal, N.; Girault, J.-P.; Dinan, L.; Lafont, R. The phytoecdysteroid profiles of 7 species of Silene (Caryophyllaceae). Arch. Insect Biochem. Physiol. 2009, 72, 234–248. [Google Scholar] [CrossRef]
- Mamadalieva, N.; Zibareva, L.N.; Saatov, Z.; Lafont, R. Phytoecdysteroids of Silene viridiflora. Chem. Nat. Compd. 2003, 39, 199–203. [Google Scholar] [CrossRef]
- Mamadalieva, N.; Zibareva, L.N.; Saatov, Z. phytoecdysteroids of Silene linicola. Chem. Nat. Compd. 2002, 38, 268–271. [Google Scholar] [CrossRef]
- Bathori, M.; Girault, J.-P.; Kalasz, H.; Mathe, I.; Dinan, L.N.; Lafont, R. Complex phytoecdysteroid cocktail of Silene otitis (Caryophyllaceae). Arch. Insect. Biochem. Physiol. 1999, 41, 1–8. [Google Scholar] [CrossRef]
- Tanveer, M.; Shahzad, B.; Sharma, A.; Biju, S.; Bhardwaj, R. 24-Epibrassinolide; an active brassinolide and its role in salt stress tolerance in plants: A review. Plant Physiol. Biochem. 2018, 130, 69–79. [Google Scholar] [CrossRef]
- Filek, M.; Sieprawska, A.; Oklestkova, J.; Rudolphi-Skórska, E.; Biesaga-Kościelniak, J.; Miszalski, Z.; Janeczko, A. 24-Epibrassinolide as a Modifier of Antioxidant Activities and Membrane Properties of Wheat Cells in Zearalenone Stress Conditions. J. Plant Growth Regul. 2018, 37, 1085–1098. [Google Scholar] [CrossRef]
- Machácková, I.; Vágner, M.; Sláma, K. Comparison between the effects of 20-hydroxyecdysone and phytohormones on growth and development in plants. Eur. J. Entomol. 1995, 92, 309–316. [Google Scholar]
- Tarkowská, D.; Krampolová, E.; Strnad, M. Plant Triterpenoid Crosstalk: The Interaction of Brassinosteroids and Phytoecdysteroids in Lepidium sativum. Plants 2020, 9, 1325. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, B. Structure-activity relationship studies of insect and plant steroid hormones. J. Pestic. Sci. 2015, 40, 146–151. [Google Scholar] [CrossRef]
- Zhao, Y. Auxin Biosynthesis and Its Role in Plant Development. Annu. Rev. Plant Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef]
- Boivin, S.; Fonouni-Farde, C.; Frugier, F. How Auxin and Cytokinin Phytohormones Modulate Root Microbe Interactions. Front. Plant Sci. 2016, 7, 1240. [Google Scholar] [CrossRef]
- Sasaki, H.; Yano, T.; Yamasaki, A. Reduction of High Temperature Inhibition in Tomato Fruit Set by Plant Growth Regulators. Jpn. Agric. Res. Q. JARQ 2005, 39, 135–138. [Google Scholar] [CrossRef]
- Van Der Plas, L.H.W.; Eijkelboom, C.; Hagendoorn, M.J.M. Relation between primary and secondary metabolism in plant cell suspensions. Plant Cell Tissue Organ Cult. 1995, 43, 111–116. [Google Scholar] [CrossRef]
- Rodrigues, C.; de Souza Vandenberghe, L.; De Oliveira, J.; Soccol, C.R. New perspectives of gibberellic acid production: A review. Crit. Rev. Biotechnol. 2011, 32, 263–273. [Google Scholar] [CrossRef]
- Gupta, R.; Chakrabarty, S.K. Gibberellic acid in plant: Still a mystery unresolved. Plant Signal. Behav. 2013, 8, e25504. [Google Scholar] [CrossRef]
- Qiao, X.; Jiang, S.; Li, X.; Li, F.; Zhao, D. Effects of phytohormones on plant regeneration and production of flavonoids in transgenic Saussurea involucrata hairy roots. Chin. J. Biotechn. 2011, 27, 69–75. [Google Scholar]
- Zengin, G.; Mahomoodally, M.F.; Aktumsek, A.; Ceylan, R.; Uysal, S.; Mocan, A.; Yilmaz, M.A.; Picot-Allain, C.M.N.; Ćirić, A.; Glamočlija, J.; et al. Functional constituents of six wild edible Silene species: A focus on their phytochemical profiles and bioactive properties. Food Biosci. 2018, 23, 75–82. [Google Scholar] [CrossRef]
- Morales, P.; Carvalho, A.M.; Sánchez-Mata, M.D.C.; Cámara, M.; Molina, M.; Ferreira, I.C.F.R. Tocopherol composition and antioxidant activity of Spanish wild vegetables. Genet. Resour. Crop. Evol. 2012, 59, 851–863. [Google Scholar] [CrossRef]
- Taskin, T.; Bitis, L. Antioxidant activity of Silene alba subsp divaricata and Stellaria media subsp. media from Caryophyllaceae. Spatula DD–Peer Rev. J. Complement. Med. Drug Discov. 2013, 3, 1–5. [Google Scholar] [CrossRef]
- Wolfender, J.-L.; Marti, G.; Thomas, A.; Bertrand, S. Current approaches and challenges for the metabolite profiling of complex natural extracts. J. Chromatogr. A 2015, 1382, 136–164. [Google Scholar] [CrossRef]

| Group | Concentration, mg/L | Leaves | Roots | |||
|---|---|---|---|---|---|---|
| Weight, мг a | Content of 6, mg/g b,c | Content of 7, mg/g b,c | Weight, mg a | Content of 6, mg/g b,c | ||
| Control 1 (water, spraying) | - | 60.0 ± 4.3 | 0.97 ± 0.02 | 0.41 ± 0.01 | 51.2 ± 4.0 | 0.44 ± 0.01 |
| Control 2 (watering) | - | 63.1 ± 5.4 | 0.92 ± 0.02 | 0.39 ± 0.01 | 50.3 ± 3.8 | 0.42 ± 0.01 |
| Epibrassinolide | 1 | 62.1 ± 4.2 | 0.99 ± 0.02 | 0.40 ±0.01 | 52.1 ± 4.0 | 0.42 ± 0.01 |
| 10 | 65.0 ± 4.3 | 2.10 ± 0.04 † | 1.14 ± 0.02 † | 54.4 ± 4.1 | 0.39 ± 0.00 † | |
| 100 | 82.7 ± 7.3 † | 2.18 ± 0.04 † | 1.16 ± 0.02 † | 61.3 ± 5.6 † | 0.41 ± 0.00 † | |
| Indole-3-butyric acid | 1 | 62.1 ± 5.1 | 0.96 ± 0.02 | 0.42 ± 0.01 | 49.1 ± 4.2 | 0.42 ± 0.01 |
| 10 | 63.4 ± 5.0 | 1.02 ± 0.02 † | 0.45 ± 0.01 | 50.1 ± 5.3 | 0.44 ± 0.01 | |
| 100 | 64.5 ± 4.2 | 1.05 ± 0.02 † | 0.47 ± 0.01 † | 58.2 ± 5.3 | 0.50 ± 0.01 † | |
| 4-Chlorophenylacetic acid | 1 | 62.1 ± 5.3 | 0.96 ± 0.02 | 0.39 ± 0.01 | 51.0 ± 5.1 | 0.45 ± 0.01 |
| 10 | 67.2 ± 5.2 | 1.01 ± 0.02 | 0.37 ± 0.01 | 57.3 ± 5.2 | 0.40 ± 0.01 † | |
| 100 | 79.6 ± 6.0 † | 1.06 ± 0.02 † | 0.39 ± 0.01 | 61.6 ± 5.7 † | 0.38 ± 0.00 † | |
| Gibberellic acids potassium salt | 1 | 61.0 ± 6.4 | 0.96 ± 0.02 | 0.41 ± 0.01 | 50.3 ± 5.1 | 0.42 ± 0.01 |
| 10 | 76.7 ± 10.2 † | 0.99 ± 0.02 | 0.40 ± 0.01 | 57.7 ± 6.8 | 0.51 ± 0.01 † | |
| 100 | 104.3 ± 10.1 † | 1.08 ± 0.02 † | 0.42 ±0.01 | 72.5 ± 10.2 † | 0.66 ± 0.02 † | |
| Arachidonic acid | 1 | 58.1 ± 4.0 | 0.94 ± 0.02 | 0.40 ± 0.01 | 52.1 ± 4.6 | 0.43 ± 0.01 † |
| 10 | 61.4 ± 4.1 | 0.91± 0.02 | 0.42 ± 0.01 | 67.2 ± 5.1 | 0.41 ± 0.02 † | |
| 100 | 82.4 ± 9.4 † | 0.89 ± 0.02 † | 0.41 ± 0.01 | 69.3 ± 5.2 † | 0.42 ± 0.02 † | |
| Ethyl arachidonate | 1 | 58.1 ± 5.0 | 0.98 ± 0.02 | 0.39 ± 0.01 | 50.4 ± 3.4 | 0.44 ± 0.01 |
| 10 | 85.6 ± 10.5 † | 0.87 ± 0.01 † | 0.35 ± 0.01 † | 70.7 ± 6.9 † | 0.42 ± 0.02 † | |
| 100 | 113.3 ± 11.4 † | 0.80 ± 0.01 † | 0.28 ± 0.01 † | 73.2 ± 9.1 † | 0.37 ± 0.02 † | |
| Group | Concentration, mg/L | Content of Glycosylflavones, mg/g ± S.D. a,b | ||||||
|---|---|---|---|---|---|---|---|---|
| 13 | 14 | 15 | 16 | 17 | 18 | Σ13–18 | ||
| Control 1 (water, spraying) | - | 0.60 ± 0.01 | 0.73 ± 0.02 | 0.02 ± 0.00 | 0.08 ± 0.00 | 0.15 ± 0.00 | 0.07 ± 0.00 | 1.65 |
| Control 2 (watering) | - | 0.63 ± 0.01 | 0.74 ± 0.02 | 0.02 ± 0.00 | 0.06 ± 0.00 | 0.17 ± 0.00 | 0.08 ± 0.00 | 1.70 |
| Epibrassinolide | 1 | 0.58 ± 0.01 | 0.79 ± 0.02 | tr. | 0.03 ± 0.00 | 0.10 ± 0.00 | 0.04 ± 0.00 | 1.54 |
| 10 | 0.77 ± 0.01 † | 1.14 ± 0.02 † | tr. | 0.02 ± 0.00 | 0.10 ± 0.00 | 0.03 ± 0.00 | 2.06 | |
| 100 | 0.79 ± 0.02 † | 1.28 ± 0.02 † | tr. | 0.03 ± 0.00 | 0.07 ± 0.00 | 0.03 ± 0.00 | 2.20 | |
| Indole-3-butyric acid | 1 | 0.61 ± 0.01 | 0.79 ± 0.02 † | 0.02 ± 0.00 | 0.10 ± 0.00 | 0.10 ± 0.00 | 0.08 ± 0.00 | 1.70 |
| 10 | 0.56 ± 0.01 | 1.12 ± 0.02 † | 0.02 ± 0.00 | 0.12 ± 0.00 † | 0.10 ± 0.00 | 0.10 ± 0.00 | 2.02 | |
| 100 | 0.61 ± 0.01 | 1.16 ± 0.02 † | 0.04 ± 0.00 | 0.15 ± 0.00 † | 0.12 ± 0.00 | 0.14 ± 0.00 † | 2.22 | |
| 4-Chlorophenylacetic acid | 1 | 0.65 ± 0.01 | 0.84 ± 0.02 † | 0.02 ± 0.00 | 0.07 ± 0.00 | 0.10 ± 0.00 | 0.06 ± 0.00 | 1.74 |
| 10 | 0.70 ± 0.02 † | 2.07 ± 0.04 † | 0.02 ± 0.00 | 0.12 ± 0.00 † | 0.07 ± 0.00 | 0.08 ±0.00 | 3.06 | |
| 100 | 0.75 ± 0.02 † | 2.97 ± 0.06 † | 0.11 ± 0.00 | 0.18 ± 0.00 † | 0.05 ± 0.00 | 0.11 ± 0.00 | 4.17 | |
| Gibberellic acids potassium salt | 1 | 0.54 ± 0.01 | 0.70 ± 0.02 | 0.04 ± 0.00 | 0.06 ± 0.00 | 0.17 ± 0.00 | 0.05 ± 0.00 | 1.56 |
| 10 | 0.32 ± 0.01 † | 0.38 ± 0.01 † | 0.02 ± 0.00 | 0.08 ± 0.00 | 0.57 ± 0.01 † | 0.06 ± 0.00 | 1.43 | |
| 100 | 0.30 ± 0.01 † | 0.27 ± 0.00 † | tr. | 0.10 ± 0.00 † | 0.63 ± 0.01 † | 0.07 ± 0.00 | 1.37 | |
| Arachidonic acid | 1 | 0.60 ± 0.1 | 0.78 ± 0.02 | 0.01 ± 0.00 | 0.06 ± 0.00 | 0.10 ± 0.00 | 0.06 ± 0.00 | 1.61 |
| 10 | 0.66 ± 0.01 | 1.16 ± 0.02 † | 0.02 ± 0.00 | 0.10 ± 0.00 † | 0.12 ± 0.00 | 0.12 ± 0.00 † | 2.18 | |
| 100 | 0.69 ± 0.01 † | 1.44 ± 0.03 † | 0.04 ± 0.00 | 0.12 ± 0.00 † | 0.15 ± 0.00 | 0.17 ± 0.00 † | 2.61 | |
| Ethyl arachidonate | 1 | 0.53 ± 0.01 | 0.63 ± 0.01 | 0.03 ± 0.00 | 0.07 ± 0.00 | 0.12 ± 0.00 | 0.04 ± 0.00 | 1.42 |
| 10 | 0.39 ± 0.01 † | 0.81 ± 0.02 † | 0.04 ± 0.00 | 0.12 ± 0.00 † | 0.10 ± 0.00 | 0.06 ± 0.00 | 1.52 | |
| 100 | 0.30 ± 0.00 † | 0.95 ± 0.02 † | 0.04 ± 0.00 | 0.17 ± 0.00 † | 0.11 ± 0.00 | 0.09 ± 0.00 | 1.66 | |
| Group | Concentration, mg/L | DPPH·, mg/g a ± SD | |||
|---|---|---|---|---|---|
| SPE-1 | SPE-2 | ||||
| Leaves | Roots | Leaves | Roots | ||
| Control 1 (water, spraying) | - | <1 | <1 | 46.52 ± 1.41 | <1 |
| Control 2 (watering) | - | <1 | <1 | 45.33 ± 1.09 | <1 |
| Epibrassinolide | 1 | <1 | <1 | 42.11 ± 1.43 † | <1 |
| 10 | <1 | <1 | 53.02 ± 1.48 † | <1 | |
| 100 | <1 | <1 | 52.40 ± 1.57 † | <1 | |
| Indole-3-butyric acid | 1 | <1 | <1 | 39.32 ± 1.09 † | <1 |
| 10 | <1 | <1 | 45.27 ± 1.03 | <1 | |
| 100 | <1 | <1 | 46.00 ± 1.29 | <1 | |
| 4-Chlorophenylacetic acid | 1 | <1 | <1 | 45.14 ± 0.85 † | <1 |
| 10 | <1 | <1 | 60.92 ± 1.40 † | <1 | |
| 100 | <1 | <1 | 78.20 ± 2.34 † | <1 | |
| Gibberellic acids potassium salt | 1 | <1 | <1 | 40.06 ± 1.02 † | <1 |
| 10 | <1 | <1 | 36.71 ± 0.86 † | <1 | |
| 100 | <1 | <1 | 33.75 ± 1.01 † | <1 | |
| Arachidonic acid | 1 | <1 | <1 | 38.63 ± 1.19 † | <1 |
| 10 | <1 | <1 | 55.23 ± 1.76 † | <1 | |
| 100 | <1 | <1 | 54.39 ± 1.57 † | <1 | |
| Ethyl arachidonate | 1 | <1 | <1 | 38.67 ± 1.00 † | <1 |
| 10 | <1 | <1 | 45.56 ± 1.23 † | <1 | |
| 100 | <1 | <1 | 44.14 ± 0.88 † | <1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashchenko, N.I.; Olennikov, D.N.; Chirikova, N.K. Phytohormones and Elicitors Enhanced the Ecdysteroid and Glycosylflavone Content and Antioxidant Activity of Silene repens. Appl. Sci. 2021, 11, 11099. https://doi.org/10.3390/app112311099
Kashchenko NI, Olennikov DN, Chirikova NK. Phytohormones and Elicitors Enhanced the Ecdysteroid and Glycosylflavone Content and Antioxidant Activity of Silene repens. Applied Sciences. 2021; 11(23):11099. https://doi.org/10.3390/app112311099
Chicago/Turabian StyleKashchenko, Nina I., Daniil N. Olennikov, and Nadezhda K. Chirikova. 2021. "Phytohormones and Elicitors Enhanced the Ecdysteroid and Glycosylflavone Content and Antioxidant Activity of Silene repens" Applied Sciences 11, no. 23: 11099. https://doi.org/10.3390/app112311099
APA StyleKashchenko, N. I., Olennikov, D. N., & Chirikova, N. K. (2021). Phytohormones and Elicitors Enhanced the Ecdysteroid and Glycosylflavone Content and Antioxidant Activity of Silene repens. Applied Sciences, 11(23), 11099. https://doi.org/10.3390/app112311099








