Perspective Compounds for Immobilization of Spent Electrolyte from Pyrochemical Processing of Spent Nuclear Fuel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Procedures
2.1.1. Synthesis of Low-Temperature MPP Compound
2.1.2. Synthesis of NAFP Glass
2.1.3. Synthesis of High-Temperature Ceramics Based on Bentonite
2.2. Methods
3. Results and Discussion
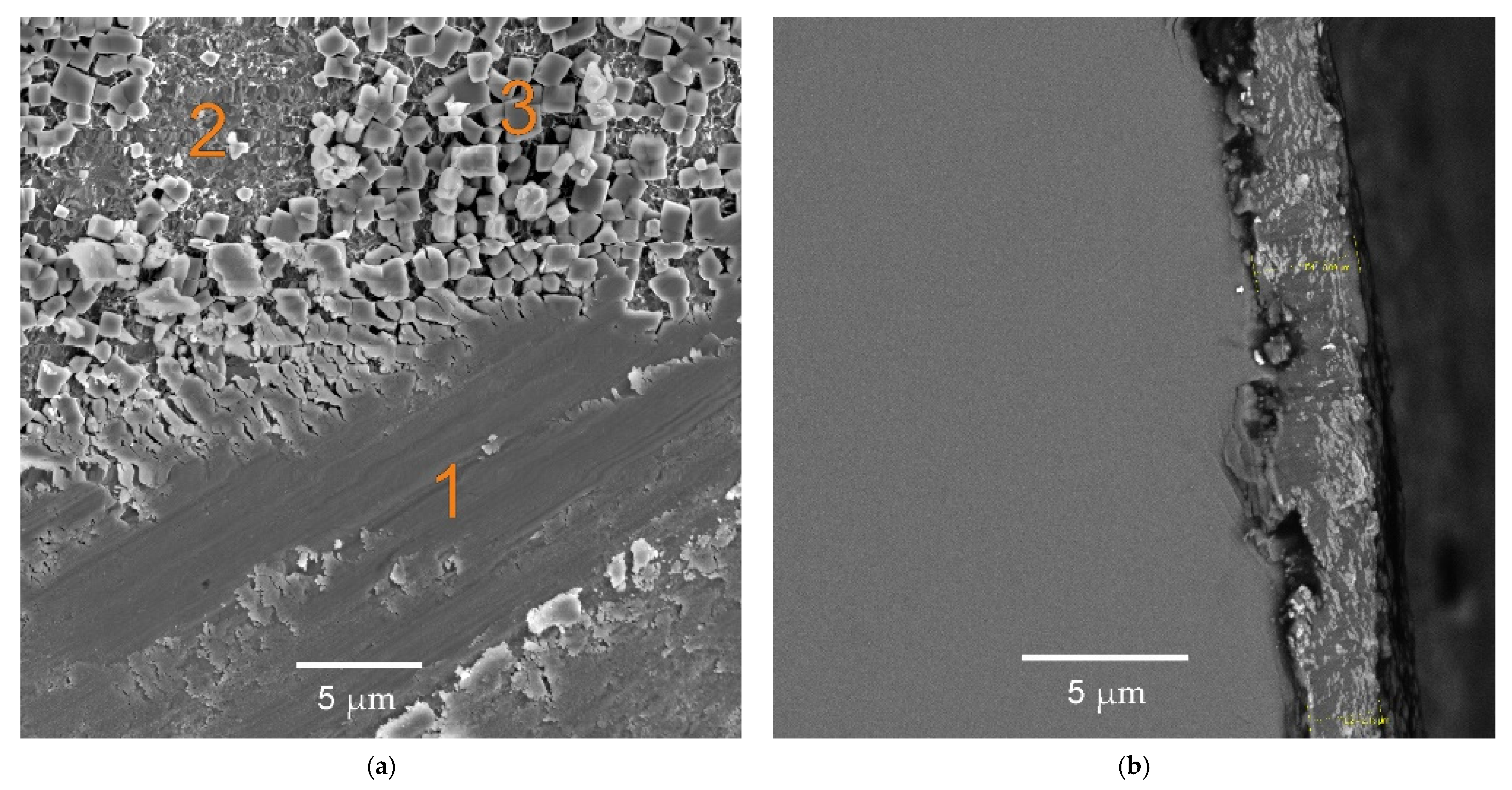
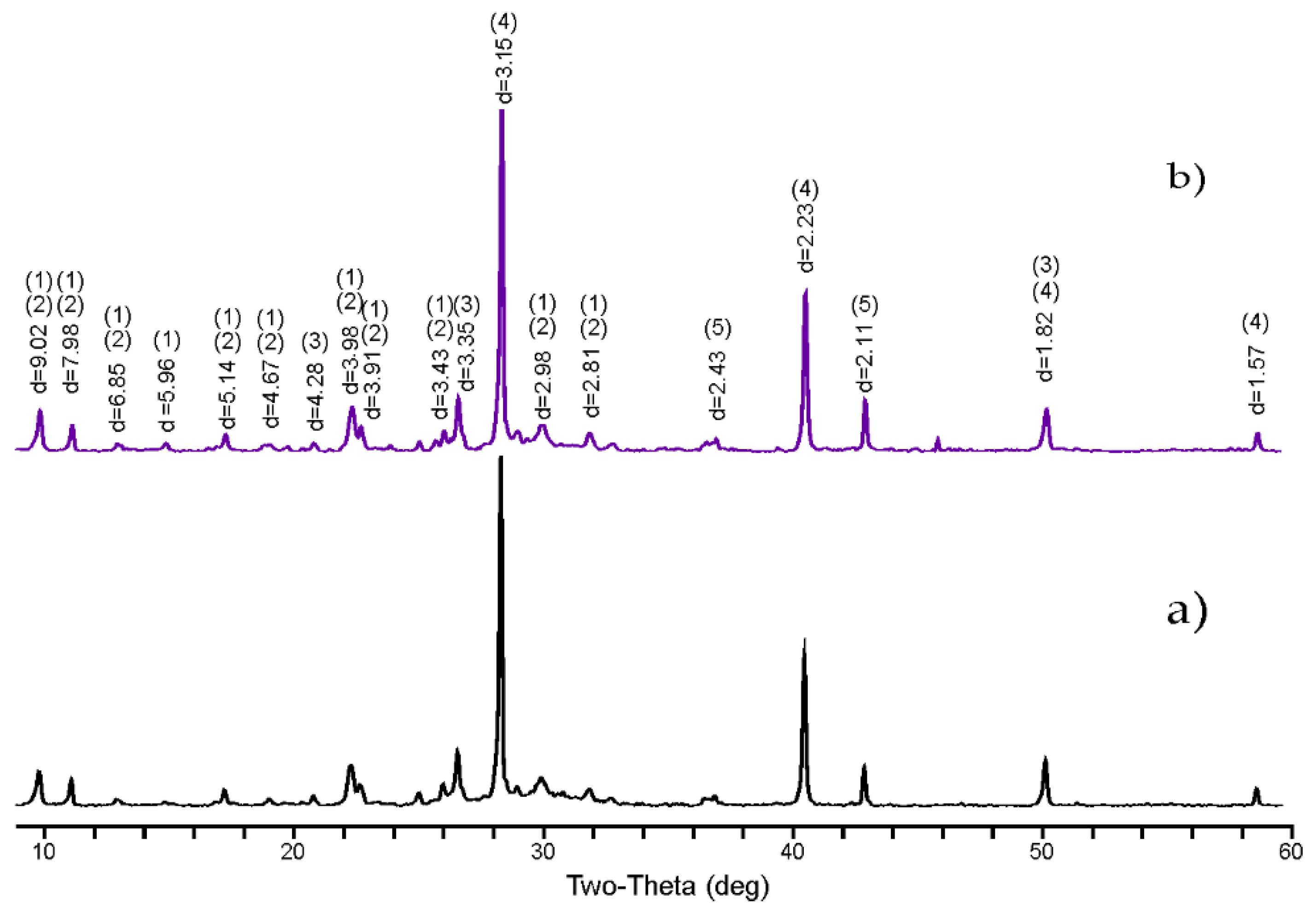
3.1. Characterization of the Synthesised Compounds Containing Chlorides
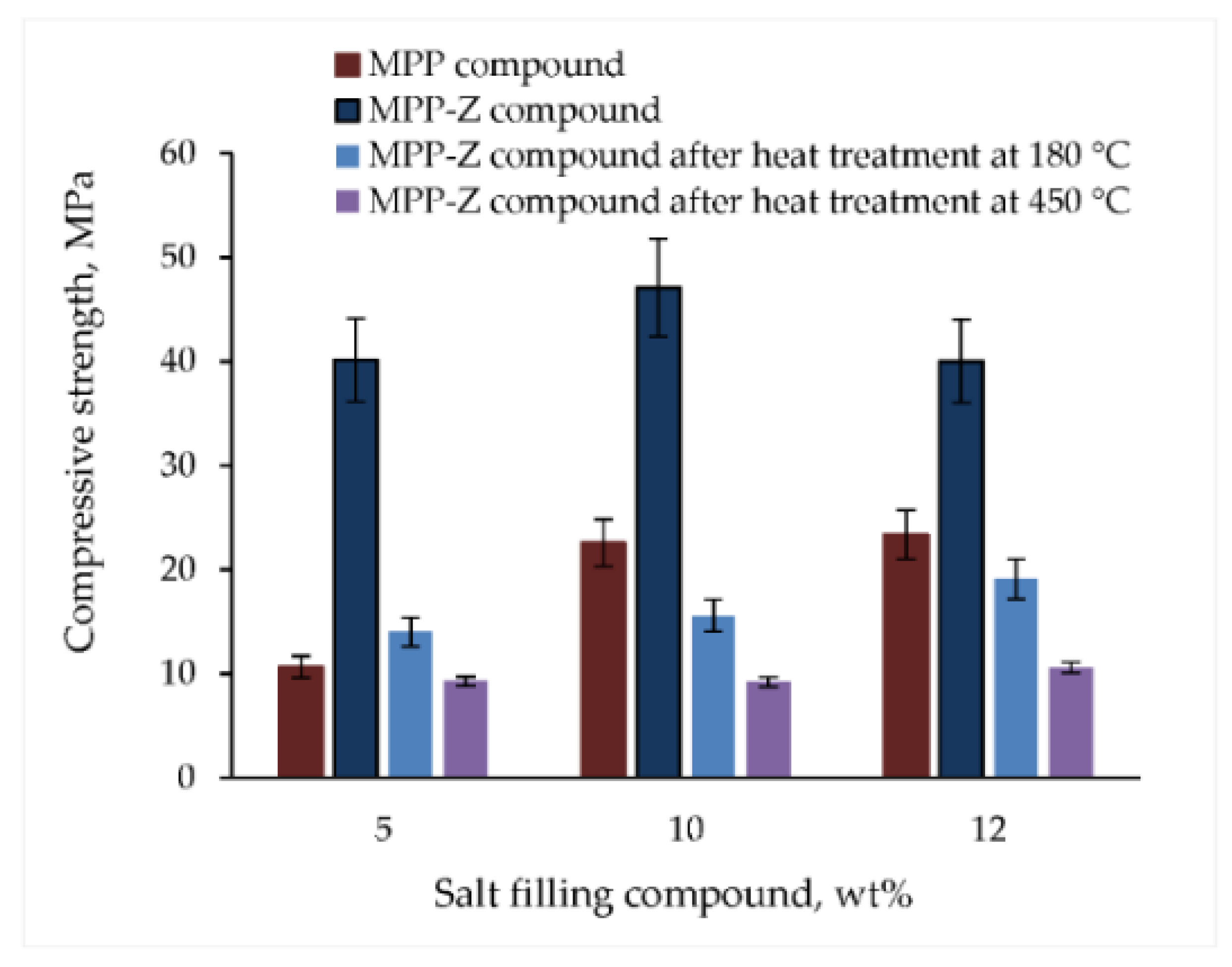
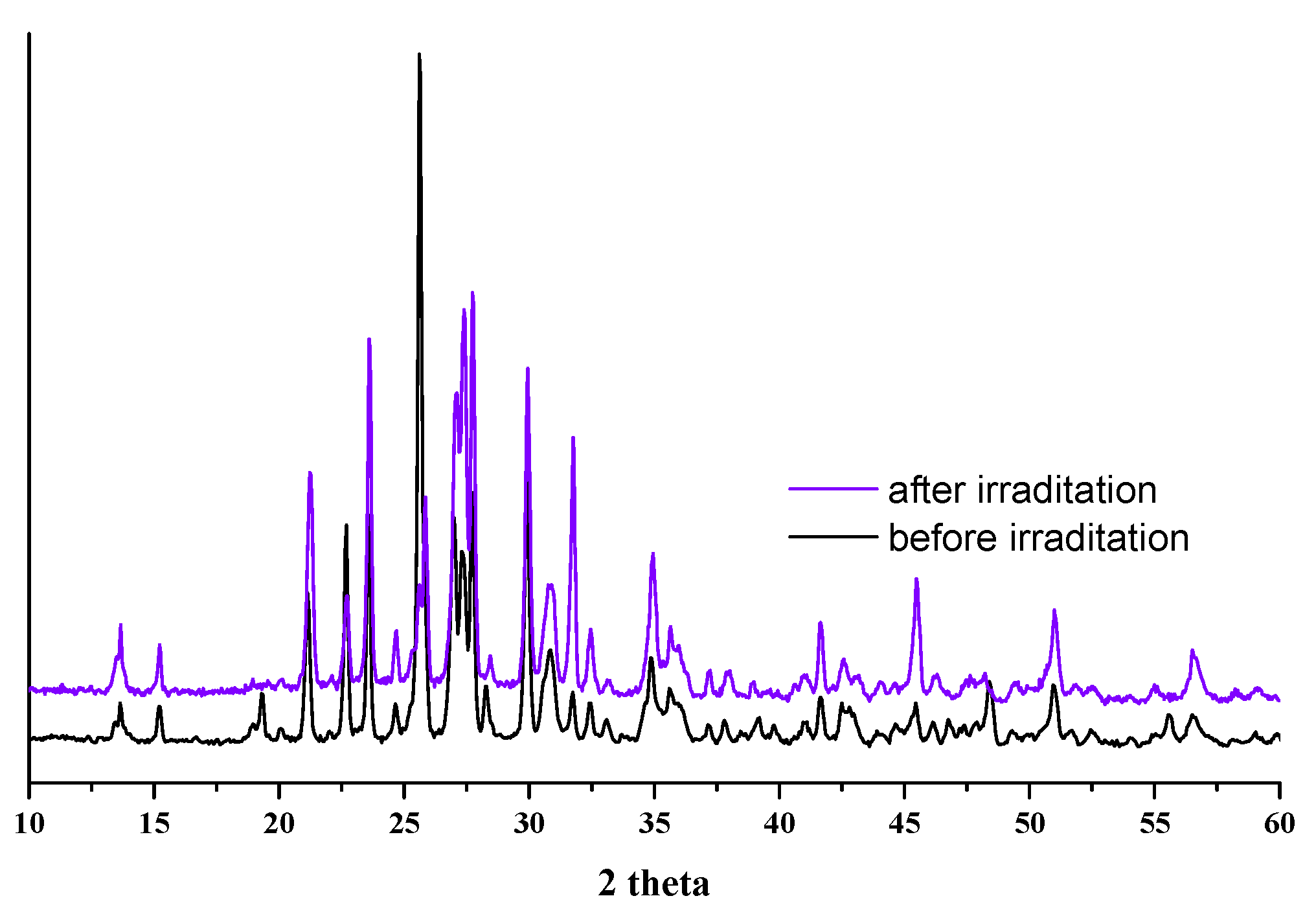
3.2. Mechanical, Thermal and Radiation Resistance of Materials
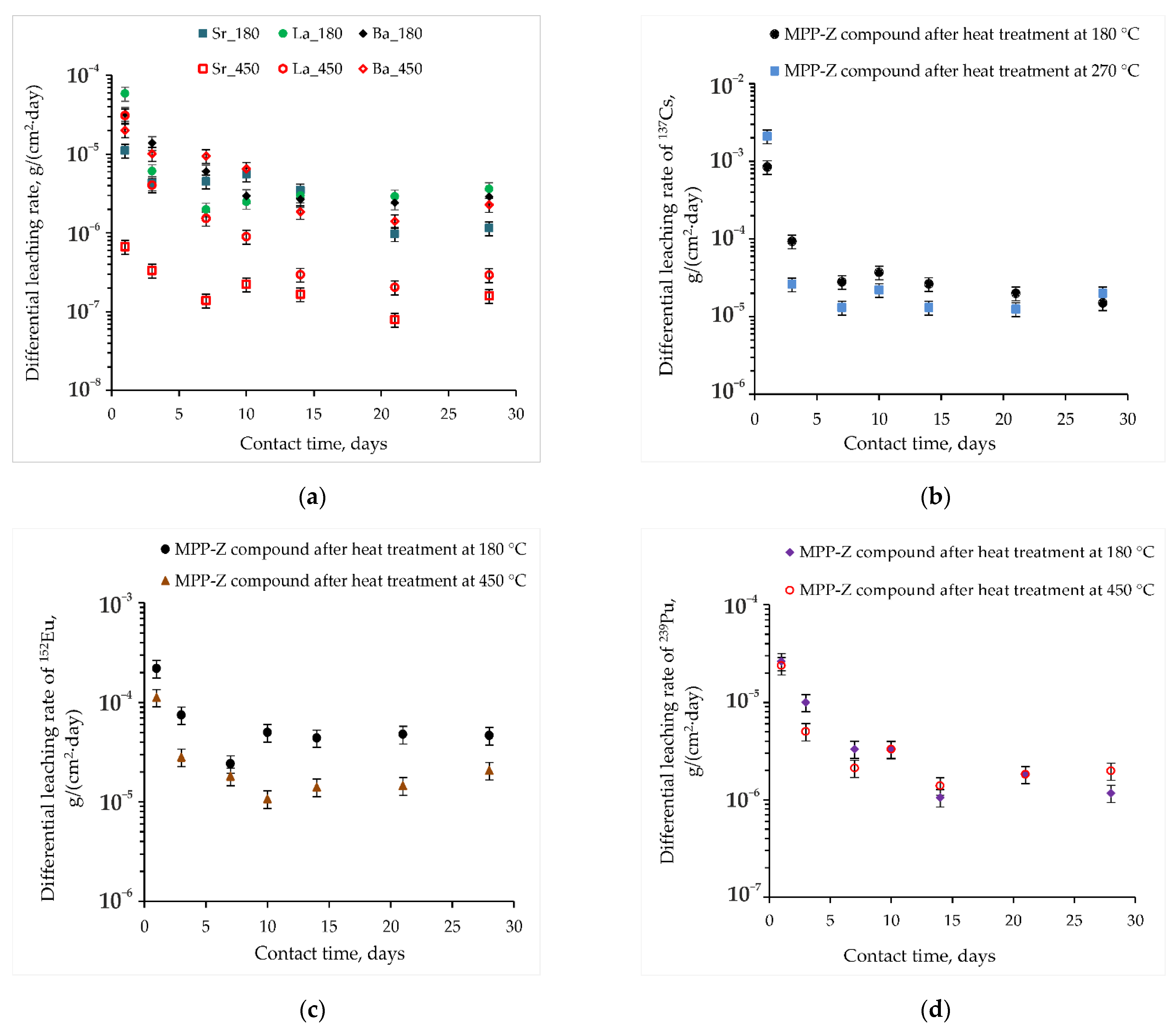
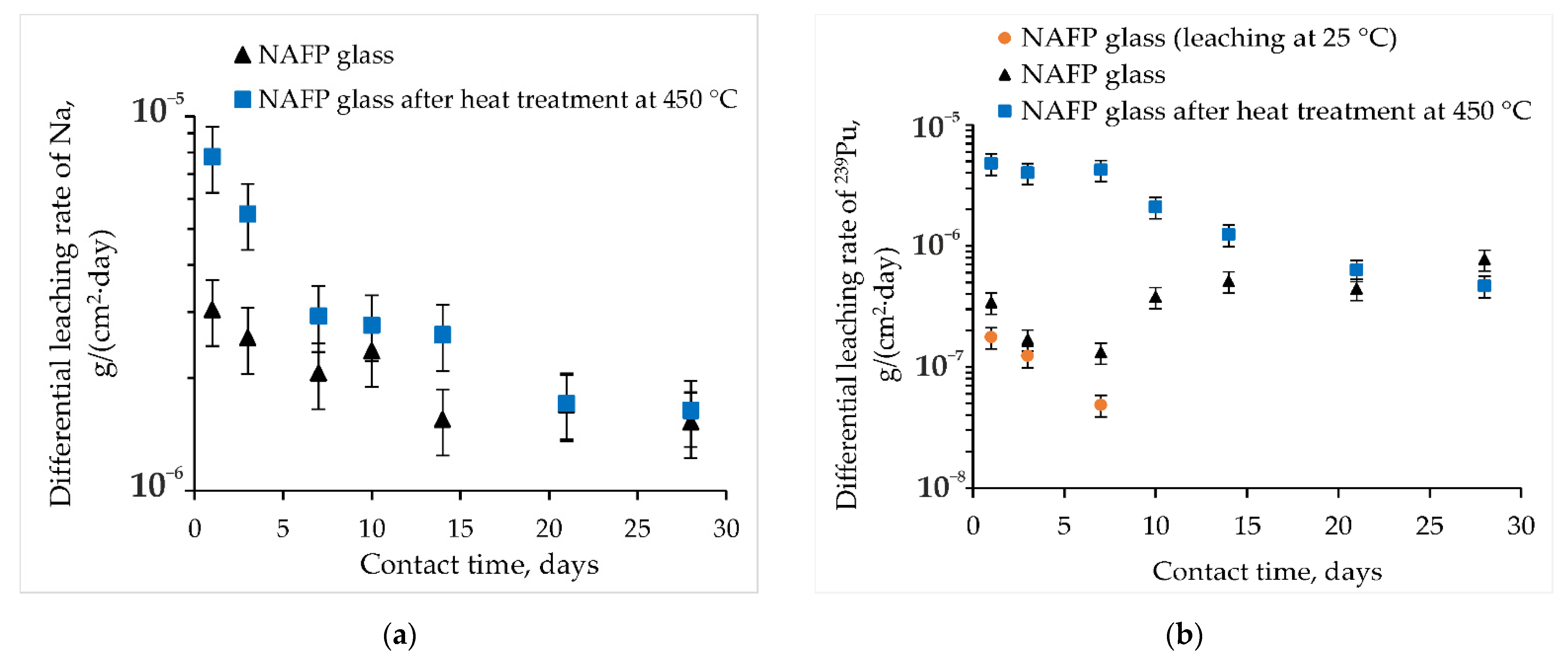
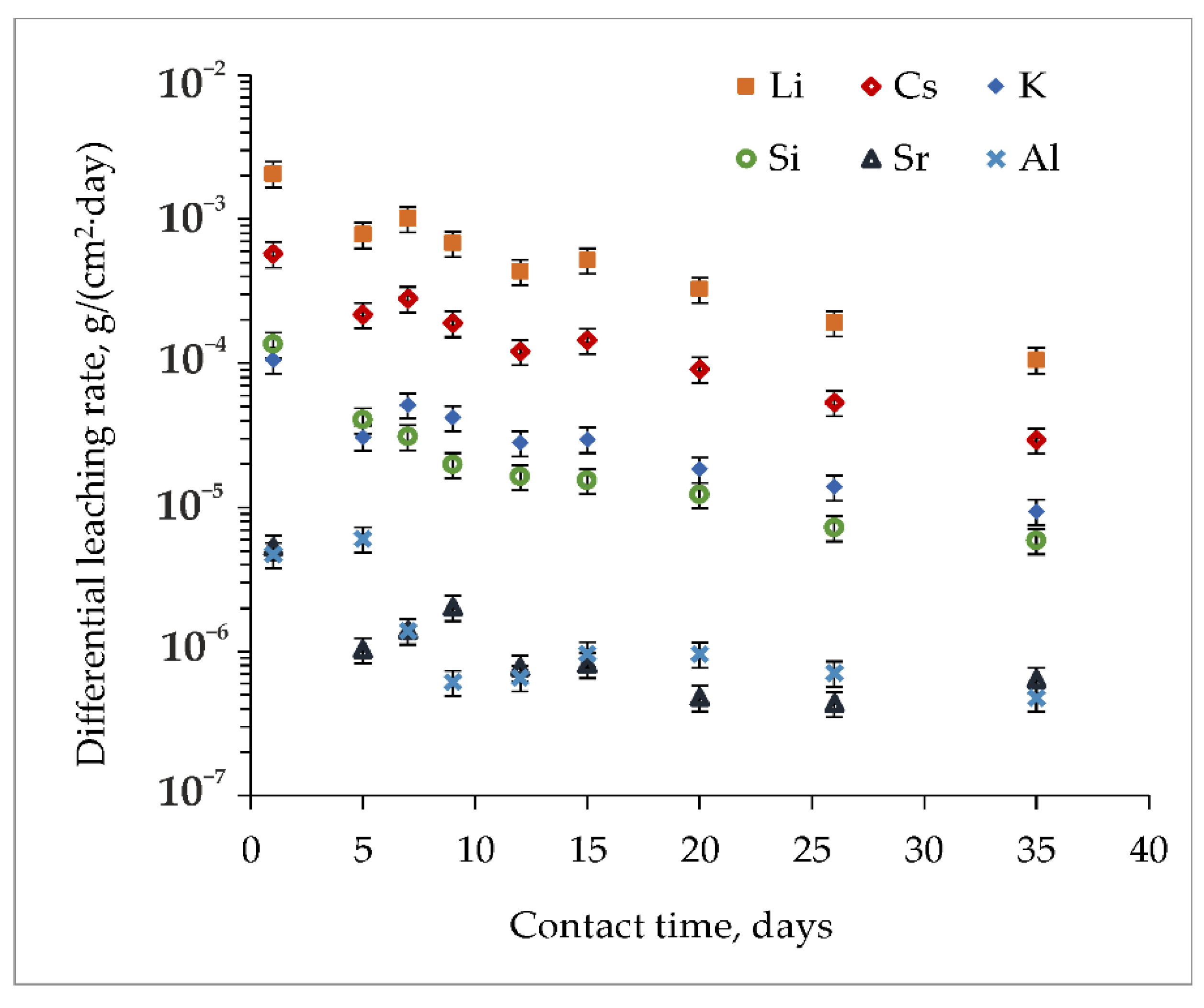
3.3. Hydrolytic Resistance of the Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shadrin, A.Y.; Dvoeglazov, K.N.; Maslennikov, A.G.; Kashcheev, V.A.; Tret’yakova, S.G.; Shmidt, O.V.; Vidanov, V.L.; Ustinov, O.A.; Volk, V.I.; Veselov, S.N.; et al. PH process as a technology for reprocessing mixed uranium–plutonium fuel from BREST-OD-300 reactor. Radiochemistry 2016, 58, 271–279. [Google Scholar] [CrossRef]
- Poluektov, P.P.; Schmidt, O.V.; Kascheev, V.A.; Ojovan, M.I. Modelling aqueous corrosion of nuclear waste phosphate glass. J. Nucl. Mater. 2017, 484, 357–366. [Google Scholar] [CrossRef]
- Choi, J.; Um, W.; Choung, S. Development of iron phosphate ceramic waste form to immobilize radioactive waste solution. J. Nucl. Mater. 2014, 452, 16–23. [Google Scholar] [CrossRef]
- Donald, I.W.; Metcalfe, B.L.; Fong, S.K.; Gerrard, L.A.; Strachan, D.M.; Scheele, R.D. A glass-encapsulated calcium phosphate wasteform for the immobilization of actinide-, fluoride-, and chloride-containing radioactive wastes from the pyrochemical reprocessing of plutonium metal. J. Nucl. Mater. 2007, 361, 78–93. [Google Scholar] [CrossRef]
- Pereira, C.; Hash, M.; Lewis, M.; Richmann, M. Ceramic-composite waste forms from the electrometallurgical treatment of spent nuclear fuel. JOM 1997, 49, 34–40. [Google Scholar] [CrossRef]
- Morrison, M.C.; Bateman, K.J.; Simpson, M.F. Scale Up of Ceramic Waste Forms for the EBR-II Spent Fuel Treatment Process. In Office of Scientific and Technical Information Technical Reports; Idaho National Laboratory: Idaho Falls, ID, USA, 2010; pp. 1–17. [Google Scholar]
- Priebe, S.; Bateman, K. The Ceramic Waste Form Process at Idaho National Laboratory. Nucl. Technol. 2008, 162, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Kuzin, M.A.; Makarov, A.O. Study of sorption properties of lithium and potassium inoculated NaA zeolite sorbent. Ecol. Ind. Russ. 2015, 19, 8–11. [Google Scholar] [CrossRef]
- Tomilin, S.V.; Lukinykh, A.N.; Lizin, A.A.; Bychkov, A.V.; Yakovlev, V.V.; Konovalov, V.I. Investigation of the incorporation of spent alkali chloride melt in ceramic. At. Energy 2007, 102, 217–222. [Google Scholar] [CrossRef]
- Park, H.S.; Ahn, B.-G.; Kim, H.-Y.; Kim, I.-T.; Cho, Y.-Z.; Lee, H. Solidification Method of Radioactive Waste Accompanying Chloride Recycling or Radioactive Iodide Removing and the Device Thereof. U.S. Patent No. 9,087,618 B2, 21 July 2015. [Google Scholar]
- Lee, K.R.; Riley, B.J.; Park, H.-S.; Choi, J.-H.; Han, S.Y.; Hur, J.-M.; Peterson, J.A.; Zhu, Z.; Schreiber, D.K.; Kruska, K.; et al. Investigation of physical and chemical properties for upgraded SAP (SiO2Al2O3P2O5) waste form to immobilize radioactive waste salt. J. Nucl. Mater. 2019, 515, 382–391. [Google Scholar] [CrossRef]
- Ringwood, A.E. Safe Disposal of High Level Nuclear Reactor Wastes: A New Strategy, 1st ed.; Australian National University Press: Canberra, Australia, 1978; pp. 1–63. [Google Scholar]
- Ringwood, A.E.; Oversby, V.M.; Kesson, S.E.; Sinclair, W.; Ware, N.; Hibberson, W.; Major, A. Immobilization of high-level nuclear reactor wastes in SYNROC: A current appraisal. Nucl. Chem. Waste Manag. 1981, 2, 287–305. [Google Scholar] [CrossRef]
- Lizin, A.A.; Tomilin, S.V.; Gnevashov, O.E.; Lukinykh, A.N.; Orlova, A.I. Orthophosphates of langbeinite structure for immobilization of alkali metal cations of salt wastes from pyrochemical processes. Radiochemistry 2012, 54, 542–548. [Google Scholar] [CrossRef]
- Laverov, N.P.; Yudintsev, S.V.; Stefanovsky, S.V.; Omel’yanenko, B.I.; Nikonov, B.S. Murataite as a universal matrix for immobilization of actinides. Geol. Ore Deposits 2006, 48, 335–356. [Google Scholar] [CrossRef]
- Lizin, A.A.; Tomilin, S.V.; Poglyad, S.S.; Pryzhevskaya, E.A.; Yudintsev, S.V.; Stefanovsky, S.V. Murataite: A matrix for immobilizing waste generated in radiochemical reprocessing of spent nuclear fuel. J. Radioanal. Nucl. Chem. 2018, 318, 2363–2372. [Google Scholar] [CrossRef]
- Matveenko, A.V.; Varlakov, A.P.; Zherebtsov, A.A.; Germanov, A.V.; Mikheev, I.V.; Kalmykov, S.N.; Petrov, V.G. Natural Clay Minerals as a Starting Material for Matrices for the Immobilization of Radioactive Waste from Pyrochemical Processing of SNF. Sustainability 2021, 13, 10780. [Google Scholar] [CrossRef]
- Kulikova, S.A.; Belova, K.Y.; Tyupina, E.A.; Vinokurov, S.E. Conditioning of Spent Electrolyte Surrogate LiCl-KCl-CsCl Using Magnesium Potassium Phosphate Compound. Energies 2020, 13, 1963. [Google Scholar] [CrossRef]
- Kulikova, S.A.; Vinokurov, S.E. The Influence of Zeolite (Sokyrnytsya Deposit) on the Physical and Chemical Resistance of a Magnesium Potassium Phosphate Compound for the Immobilization of High-Level Waste. Molecules 2019, 24, 3421. [Google Scholar] [CrossRef] [Green Version]
- Kulikova, S.A.; Danilov, S.S.; Belova, K.Y.; Rodionova, A.A.; Vinokurov, S.E. Optimization of the Solidification Method of High-Level Waste for Increasing the Thermal Stability of the Magnesium Potassium Phosphate Compound. Energies 2020, 13, 3789. [Google Scholar] [CrossRef]
- Vance, E.R.; Davis, J.; Olufson, K.; Chironi, I.; Karatchevtseva, I.; Farnan, I. Candidate waste forms for immobilisation of waste chloride salt from pyroprocessing of spent nuclear fuel. J. Nucl. Mater. 2012, 420, 396–404. [Google Scholar] [CrossRef]
- Stefanovsky, S.V.; Stefanovskaya, O.I.; Vinokurov, S.E.; Danilov, S.S.; Myasoedov, B.F. Phase composition, structure, and hydrolytic durability of glasses in the Na2O-Al2O3-(Fe2O3)-P2O5 system at replacement of Al2O3 by Fe2O3. Radiochemistry 2015, 57, 348–355. [Google Scholar] [CrossRef]
- Stefanovskii, S.V.; Stefanovskaya, O.I.; Semenova, D.V.; Kadyko, M.I.; Danilov, S.S. Phase Composition, Structure, and Hydrolytic Stability of Sodium-Aluminum(Iron) Phosphate Glass Containing Rare-Earth Oxides. Glas. Ceram. 2018, 75, 89–94. [Google Scholar] [CrossRef]
- Maslakov, K.I.; Teterin, Y.A.; Stefanovsky, S.V.; Kalmykov, S.N.; Teterin, A.Y.; Ivanov, K.E.; Danilov, S.S. XPS study of neptunium and plutonium doped iron-bearing and iron-free sodium-aluminum-phosphate glasses. J. Non-Cryst. Solids 2018, 482, 23–29. [Google Scholar] [CrossRef]
- Belousov, P.E.; Krupskaya, V.V.; Zakusin, S.V.; Zhigarev, V.V. Bentonite clays from 10th khutor deposite: Features of genesis, composition and adsorption properties. Rudn J. Eng. Res. 2017, 18, 135–143. [Google Scholar] [CrossRef] [Green Version]
- NP-019-15. Federal Norms and Rules in the Field of Atomic Energy Use “Collection, Processing, Storage and Conditioning of Liquid Radioactive Waste. Safety Requirements”; With Changes (Order No. 299 Dated 13.09.2021); Rostekhnadzor: Moscow, Russia, 2015; pp. 1–22.
- GOST R 52126-2003. Radioactive Waste. Long Time Leach Testing of Solidified Radioactive Waste Forms; Gosstandart 305: Moscow, Russia, 2003; pp. 1–8.
- Vinokurov, S.E.; Kulikova, S.A.; Krupskaya, V.V.; Myasoedov, B.F. Magnesium Potassium Phosphate Compound for Radioactive Waste Immobilization: Phase Composition, Structure, and Physicochemical and Hydrolytic Durability. Radiochemistry 2018, 60, 70–78. [Google Scholar] [CrossRef]
- Wagh, A.S. Chemically Bonded Phosphate Ceramics: Twenty-First Century Materials with Diverse Applications, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–422. ISBN 978-0-08-100380-0. [Google Scholar]
- Fu, M.; Yang, H.; Wu, C.; Ying, Z.; You, C.; Qian, J. Effects of Temperature on the Properties of α-High-level Radioactive Waste Immobilized, Hardened Magnesium Phosphate Cement. Cailiao Daobao/Mater. Rev. 2017, 31, 86–90. [Google Scholar] [CrossRef]
- Gardner, L.J.; Walling, S.A.; Lawson, S.M.; Sun, S.; Bernal, S.A.; Corkhill, C.L.; Provis, J.L.; Apperley, D.C.; Iuga, D.; Hanna, J.V.; et al. Characterization of and Structural Insight into Struvite-K, MgKPO4·6H2O, an Analogue of Struvite. Inorg. Chem. 2021, 60, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Doupovec, J.; Sitek, J.; Kákoš, J. Crystallization of iron phosphate glasses. J. Therm. Anal. 1981, 22, 213–219. [Google Scholar] [CrossRef]
- Weber, W.J. Radiation and Thermal Ageing of Nuclear Waste Glass. Procedia Mater. Sci. 2014, 7, 237–246. [Google Scholar] [CrossRef] [Green Version]

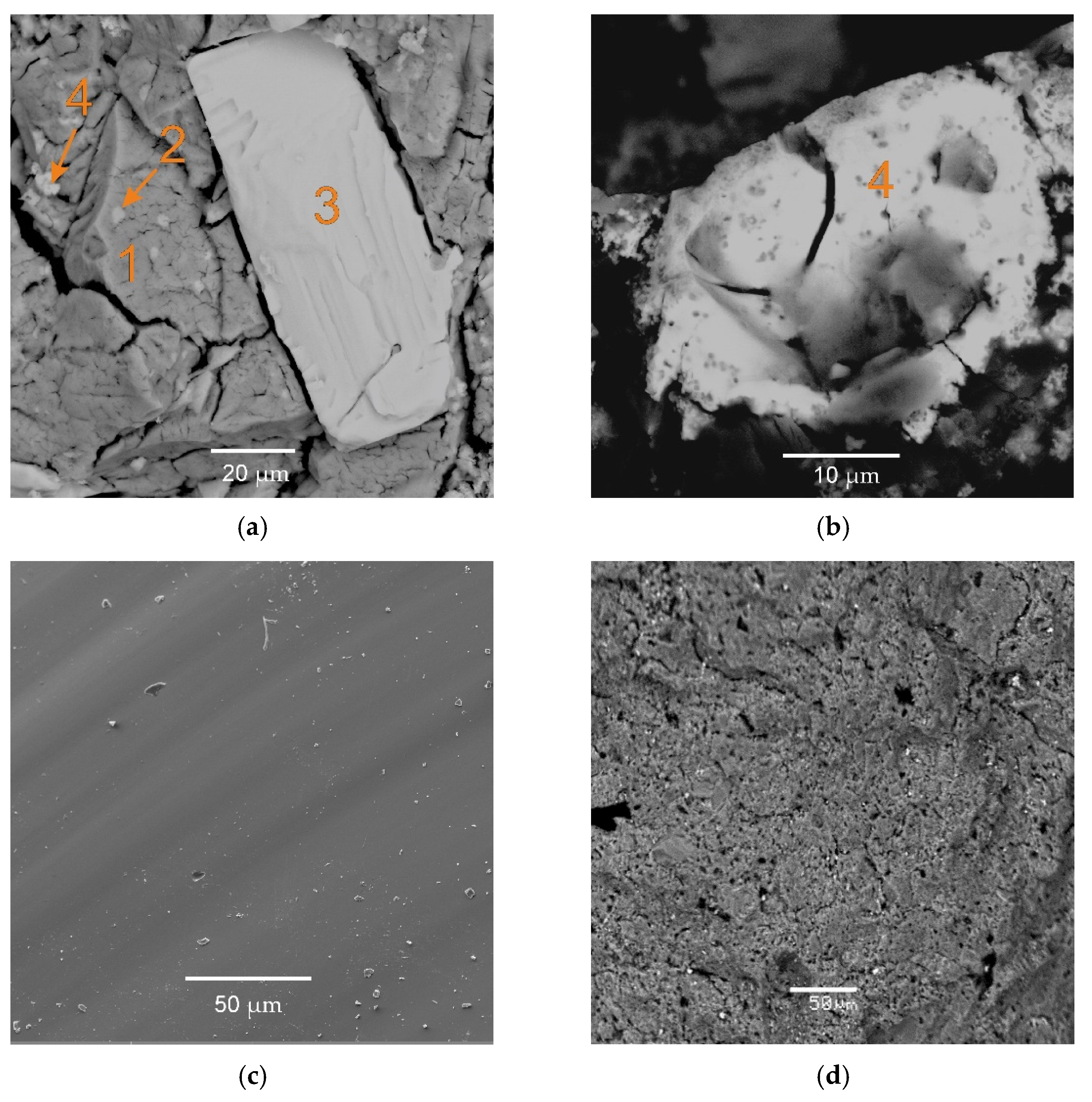
| Element | Phase #1 | Phase #2 | Phase #3 | Phase #4 |
|---|---|---|---|---|
| Mg | 16.1 | 16.2 | - | 7.9 |
| P | 18.0 | 17.2 | - | 21.4 |
| K | 11.4 | 8.4 | 41.0 | 7.6 |
| Cs | 0.1 | 2.5 | - | - |
| Sr | - | - | - | 0.9 |
| Ba | - | - | - | 1.0 |
| La | - | - | - | 10.9 |
| Cl | 0.9 | 0.8 | 55.0 | 3.4 |
| O | 53.6 | 54.8 | 3.9 | 46.9 |
| Element | Calculated | SEM-EDS | XRF |
|---|---|---|---|
| Na | 15.4 | 14.2 | 15.2 |
| P | 20.7 | 21.7 | 20.1 |
| Fe | 9.3 | 8.7 | 10.0 |
| Al | 4.5 | 5.4 | 4.8 |
| Cs | 0.3 | 0.2 | 0.3 |
| K | 1.6 | 1.8 | 1.6 |
| Li | 0.4 | - | - |
| La | 1.7 | 1.7 | 2.3 |
| Sr | 0.2 | 0.5 | 0.4 |
| Ba | 0.3 | 0.3 | 0.4 |
| Cl | 5.4 | 1.9 | 1.9 |
| O | 40.2 | 42.4 | 41.6 |
| Si | - | 1.2 | 1.4 |
| Element | Phase #1 | Phase #2 | Phase #3 |
|---|---|---|---|
| Na | 5.2 | 1.6 | 30.1 |
| P | 24.3 | 19.7 | 5.5 |
| Fe | 13.5 | 27.8 | 6.2 |
| Al | 4.7 | 3.7 | 0.9 |
| K | 2.1 | 2.2 | - |
| Cs | 0.4 | 0.3 | 0.2 |
| La | 2.5 | 3.1 | 0.8 |
| Sr | 0.3 | 0.3 | 0.1 |
| Ba | 0.6 | 0.6 | 0.4 |
| Cl | 2.1 | 0.4 | 35.0 |
| O | 43.4 | 39.4 | 20.6 |
| Si | 1.0 | 1.0 | 0.3 |
| Samples | Test Parameters | Compressive Strength, MPa |
|---|---|---|
| Initial sample | - | 51.3 ± 7.3 |
| After thermal tests | Thermal treatment at 450 °C | 50.9 ± 6.9 |
| After irradiation tests (irradiation by electrons) | 107 Gy | 41.5 ± 8.4 |
| 108 Gy | 48.3 ± 6.8 | |
| 109 Gy | 39.9 ± 5.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulikova, S.A.; Danilov, S.S.; Matveenko, A.V.; Frolova, A.V.; Belova, K.Y.; Petrov, V.G.; Vinokurov, S.E.; Myasoedov, B.F. Perspective Compounds for Immobilization of Spent Electrolyte from Pyrochemical Processing of Spent Nuclear Fuel. Appl. Sci. 2021, 11, 11180. https://doi.org/10.3390/app112311180
Kulikova SA, Danilov SS, Matveenko AV, Frolova AV, Belova KY, Petrov VG, Vinokurov SE, Myasoedov BF. Perspective Compounds for Immobilization of Spent Electrolyte from Pyrochemical Processing of Spent Nuclear Fuel. Applied Sciences. 2021; 11(23):11180. https://doi.org/10.3390/app112311180
Chicago/Turabian StyleKulikova, Svetlana A., Sergey S. Danilov, Anna V. Matveenko, Anna V. Frolova, Kseniya Y. Belova, Vladimir G. Petrov, Sergey E. Vinokurov, and Boris F. Myasoedov. 2021. "Perspective Compounds for Immobilization of Spent Electrolyte from Pyrochemical Processing of Spent Nuclear Fuel" Applied Sciences 11, no. 23: 11180. https://doi.org/10.3390/app112311180
APA StyleKulikova, S. A., Danilov, S. S., Matveenko, A. V., Frolova, A. V., Belova, K. Y., Petrov, V. G., Vinokurov, S. E., & Myasoedov, B. F. (2021). Perspective Compounds for Immobilization of Spent Electrolyte from Pyrochemical Processing of Spent Nuclear Fuel. Applied Sciences, 11(23), 11180. https://doi.org/10.3390/app112311180







