Abstract
The present study examines the sorption of Cs (I) and Sr (II) on organic sorbents in the pH range from 2 to 10, as well as the mechanisms of their binding. In order to determine the influence of the physical properties and the quantity of functional groups of the organic sorbents on sorption, experiments were carried out on organic materials of varying degrees of metamorphism: high-moor peat, hard and brown coals and shungite. A detailed description of their mineral composition, cation exchange capacity, buffering capacity and elemental composition of sorbents is provided. XRD, XRF, SEM and BET adsorption methods were used for assaying. As a result of the conducted research, it can be concluded that Sr (II) showed a higher sorption per unit specific surface area than Cs (I) in the studied range of concentrations and pH values. Sr (II) sorption decreases in the following order: high-moor peat > brown coal > shungite > hard coal. The sorption of Cs (I) is highest on brown coal and lesser for high-moor peat, shungite and hard coal. It is suggested that Cs (I) and Sr (II) can be fixed on carboxyl functional groups and Cs (I), possibly, in insignificant amounts on phenolic hydroxyls of all four studied organic sorbents.
Keywords:
sorption; cesium; strontium; organic sorbents; peat; brown coal; hard coal; lignite; shungite 1. Introduction
Ensuring the safety of nuclear legacy facilities involves not only the disposal of high-activity waste in underground storage facilities, but also the isolation and reclamation of millions of tons of intermediate and low-level waste placed in near-surface storage facilities [,]. The main problem for many such facilities is the proximity of rivers and other watercourses, which gives rise to the need to use waterproofing [] and filtration barriers for effective sorption of radionuclides.
Wastewater treatment with a low content of heavy metals using organic sorbents is known to be an efficient and relatively cheap method, and is used as an alternative to synthetic resins and other more expensive materials [,,,]. The advantage of organic sorbents is the ability to utilize the waste material by incineration, which can significantly reduce the volume of waste of the first and second classes.
High-moor peat is formed on high moors and waterlogged areas as a result of decomposition of plant debris. The main peat-forming agents in such bogs are sphagnum mosses [].
Coal is a combustible mineral, which mainly consists of organic matter, converted in the process of lithification under the influence of temperature and pressure.
Shungite are quite rare rock and distributed in the northwestern part of Russia. It usually contains more than 30% of the so-called shungite substance with the admixture of terrigenous and clayey material. It is in fact a polymorphic modification of carbon, which occupies an intermediate position between anthracite and graphite. Shungite consists of molecular structures with different allotropy, connected by amorphous carbon. Shungites are formed from organic bottom sediments of a high level of carbonatization with a fullerene content of 1 ppm to 10 ppm. The study of shungite began more than 150 years ago, which is associated with its special properties. Currently, shungites are used in various fields: as catalysts, in construction, as fire- and acid-resistant materials, in medicine, in the chemical industry, in metallurgy, etc. [,,].
The sorption properties of peat, hard and brown coal are primarily determined by the composition and number of functional groups which, under certain conditions, are capable of interacting with metals. The mechanisms of metal sorption on shungite remain unclear. Most of the published works provide data on the sorption of metals on shungite containing up to 70% of impurity minerals, mainly clayey [,], which probably determines the high sorption properties of such shungite rocks. In this work, sorption experiments were carried out on enriched monomineral shungite, consisting of 99% pure shungite.
The purpose of this study was to investigate the sorption properties of the natural organic sorbents with various degrees of lithification in relation to the 137Cs and 90Sr radionuclides. Experiments were carried out under identical conditions over a wide pH range. A detailed study of the mineral composition and functional groups of the studied sorbents made it possible to establish the regularities and some mechanisms of sorption of Cs (I) and Sr (II) on organic sorbents.
2. Materials and Methods
For the research, the following samples of natural sorbents were selected: peat from the “Staroselsky Mokh” high-moor bog (Tver region), brown coal from the Pavlovsky deposit (Primorsky Territory), hard coal from the Chernogorsky deposit (Republic of Khakassia) and shungite from the Zazhoginsky deposit (Republic of Karelia). All samples were dried at 90 °C and milled to a fraction of particle size smaller than 69 μm.
X-ray diffraction patterns were obtained with an X-ray diffractometer Ultima-IV (Rigaku, Tokyo, Japan) acquired with the funding of the Moscow State University Development Program (Cu-Kα radiation, semiconductor 1D detector D/Tex-Ultra, scan range 3–65°2θ, scan speed 3°2θ/min and step—0.02°2θ). Non-textured powder specimens were prepared by sieving the sample powder into a sample holder and cutting up the excess material with a razor. The mineral composition was analyzed according to the method of [], the quantitative composition was estimated using the Rietveld method [] with the Profex software (Version 3.14.3, Nicola Doeblin, Solothurn, Switzerland, 2019) [].
Chemical analysis was performed using the X-ray fluorescence method in accordance with standard procedure using the spectrometer Axios mAX (PANalytical, Almelo, The Netherlands) at IGEM RAS (Moscow). The samples were dried at 110 °C and prepared by fusion with lithium borate at 1200 °C. The iron content was determined only as total Fe2O3, regardless of the actual valence state of the Fe.
The effective cation exchange capacity (CEC) was determined as the sum of exchangeable Ca2+, Mg2+, Na+, K+ and exchangeable acidity. Exchangeable Ca2+, Mg2+, Na+, K+ were displaced by an ammonium ion from an unbuffered 1M NH4Cl solution []. The displaced cations were determined by inductively-coupled plasma optical emission spectrometry (Aglient 5110).
Exchangeable acidity was determined in 1M KCl solution (the ratio of the solid:liquid phase was 1:25) by titration using a Mettler Toledo DL 58 autotitrator.
The microstructure of the sorbents was studied with a scanning electron microscope (SEM) LEO1450VP (Carl Zeiss, Oberkochen, Germany). Samples for SEM were prepared in the form of individual particles and aggregates. A sample in powder form was deposited on double-sided electrically conductive adhesive tape. Then the excess sample particles were removed using compressed air. As a result, a monolayer of individual particles and aggregates on an adhesive tape was obtained. The studied surface was coated with a thin gold film, 5–10 nm thick, under vacuum. A conductive coating is required to avoid the electrical charging of the sample during analysis.
The total buffering capacity of sorbents to acid and to base was determined by potentiometric titration from the initial titration point (pHitp) to pH 3.25 and to pH 10 by 0.02 N HCl and 0.02 N NaOH solutions, respectively. 5 mL of distilled water was added to 0.5 g of sorbent and the resulting suspension was titrated in a conditioned atmosphere (atmospheric air devoid of CO2), with constant mixing. An additional portion of titrate was added after 2 min. The volume of titrant portion used during titration of NaOH and HCl for high-moor peat and brown coal was 0.2 mL and 0.05 mL, respectively. For the titration of the other sorbents, 0.05 mL of acid or alkali was added to the samples.
The buffer capacity of the sorbent with respect to acid or base, β, at each titration point was estimated by the following formula:
where is the change in the concentration of H+ or OH− during titration with acid and base, respectively, and is the change in pH after adding each portion of the titrant. The obtained values were summed up to determine the buffer capacity of the samples in the intervals equal to 0.25 pH units and to determine the total buffering from pHitp to pH 3.25 and pH 10 by titration with acid and base, respectively.
Since the studied samples contain insignificant amounts of clay minerals, and the time of interaction of the titrant with the sample was only 2 min, it can be assumed that the reactions of protonation or deprotonation of functional groups took place on the surface of the mineral phase. Therefore, it can be assumed that the number of protons or hydroxyls spent on titration approximately corresponds to the number of surface functional groups. The maxima on the buffer diagrams calculated over the pH ranges indicate the number of functional groups capable of protonation or deprotonation in the corresponding pH ranges.
The evaluation of the SSA was carried out using the Analyzer Quadrasorb SI/Kr (Quantachrome Instruments, Boynton Beach, FL, USA). Adsorption was performed at the temperature of liquid nitrogen (77.35 K). The samples were pre-dried in a vacuum at 100 °C for 4 h. This is the temperature of dehydration and the use of a higher temperature can damage the structure of aluminosilicates and, therefore, change the state of the pore space.
The studied samples are characterized by different values of the specific surface area; therefore, the calculations of the buffering capacity and sorption capacity of the sorbents were normalized per 1 m2 of surface.
The elemental composition of the sorbents was determined on an Elemental CHNS analyzer Vario EL III (Elementar, Langenselbold, Germany).
The sorption experiments were performed in 0.01 mol/L NaClO4, at a solid to solution ratio of 1 g/L for Cs (I) and 0.5 g/L for Sr (II), at room temperature in the pH range 2.0–10. the experiments were performed at concentrations of Cs (I) 10−6 M (with the addition of a 137Cs marker to a stable CsCl solution) and Sr (II) 10−8 M (90Sr). The initial pH values were set by adding small amounts of diluted solutions of NaOH or HClO4. After 48 h, the equilibrium pH of the samples was measured and used for further calculations. The solution was separated from the solid phase by centrifugation at 40,000× g for 15 min (Allegra 64R, Beckman Coulter). After that the solution was measured by liquid scintillation spectroscopy Quantulus-1220 (Perkin Elmer, Waltham, MA, USA). The radioactivity of 90Sr samples was measured after establishing equilibrium with the daughter isotope 90Y. Experiment duration (48 h) was chosen as an obviously sufficient period to reach equilibrium according to literature data [,,]. Additionally, to ensure the completeness of sorption, aliquots were repeatedly obtained (after 2 weeks) and measured -activity values varied within the instrumental error (less than 2%).
3. Results
3.1. Composition of the Sorbents
The results of mineralogical studies (Table 1) indicate that all samples contain an admixture of mineral phases. Since XRD analysis determined only the crystalline phases (Figure 1), the mineral composition was recalculated taking into account organic matter. The values of losses of ignition were obtained as the content of organic matter (Table 1). Despite the amount of loss of ignition, in addition to organic matter, includes the proportion of all volatile components, such as carbonates and structural water of clay minerals, this approach makes it possible to closely estimate the total composition of both organic and mineral phases. The main part of the impurities is represented by clay minerals and quartz. The hard coal sample contains 6.5% of kaolinite and 1.9% of quartz. Chlorite, siderite, ankerite and magnetite are found in insignificant amounts. In addition to 2.8% of quartz, the brown coal sample contains 11.3% of kaolinite, 4.9% of smectite, and 1.5% of illite. Chlorite and feldspars are detected in small quantities. The high-moor peat sample contains 9% of quartz, 1.6% of illite and minor amounts of feldspars and chlorite as impurities. Shungite is almost entirely composed of organic matter and contains less than 1% of impurities (quartz, illite and pyrite).

Table 1.
Mineral composition of the sorbents, %.
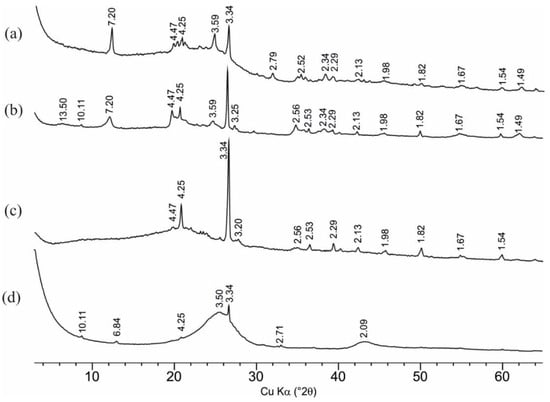
Figure 1.
Powder X-ray diffraction patterns of natural sorbents: (a)—hard coal; (b)—brown coal; (c)—peat; (d)—shungite.
3.2. Chemical Composition and Acid-Base Properties of the Sorbents
The chemical composition of the sorbents corresponds well with their mineral composition. The largest amounts of aluminum were found in brown and hard coals, which can be explained by the high content of aluminosilicates in these samples. The increased Si content in these samples is also associated with the highest content of clay minerals (Table 1 and Table 2). The increased content of Mg in the composition of brown coal and Si in high-moor peat, most likely, can be explained by the presence of smectite and quartz in the samples, respectively (Table 1 and Table 2).

Table 2.
Chemical composition of the sorbents, %.
The ignition residue content of the studied samples increases in the following order: shungite (0.93%) < hard coal (9.6%) < high-moor peat (13%) < brown coal (22%), which is in good agreement with the data on the elemental composition of organic matter (Table 3).

Table 3.
Elemental composition of the organic matter in the sorbents, %.
The largest amount of C, in the composition of the organic matter, was found in shungite; the smallest in high-moor peat (Table 3). Based on the sum of N, C, S and H, expressed in mass percent, it can be concluded that the minimum number of oxygen atoms in the composition of organic matter is contained in the shungite material, and the maximum is in high-moor peat (Table 3).
The atomic ratio H:C in the studied samples decreases in the following order: high-moor peat > brown coal > hard coal > shungite (Table 3). A decrease in the H:C atomic ratio indicates an increase in the degree of aromaticity of high molecular weight organic compounds [,]. It follows from the above that high-moor peat and brown coal should contain more aliphatic functional groups in comparison with hard coal and shungite.
This assumption is confirmed by the data from the determination of the buffer properties of the sorbents with respect to acid and base. Buffering to both acid and base was higher in the high-moor peat sample and in brown coal compared to the other two sorbents (Table 4).

Table 4.
Total buffering of the samples in relation to acid and base.
3.3. Capacitive and Surface Properties of Sorbents
The maximum value of the effective cation exchange capacity (CEC) corresponds to the sample of brown coal and high-moor peat and is 18.2 and 8.8 meq/100 g, respectively (Table 5). The effective CEC value of coal is 4.6 meq/100 g. The shungite sample has practically no CEC. The specific surface area (SSA) in the studied samples decreases in the following order: hard coal > shungite > brown coal > peat (Table 5). The average measured pore size for the samples of brown and hard coals was about 5 nm, while for peat and shungite it was about 3 nm. As seen from Figure 2, the presence of micropores is observed only in the samples of hard coal and shungite. Moreover, the volume of micropores in the shungite sample is about half of the total volume of the sample. The pore size in the coal sample can be divided into three main groups: 1–3, 3–5, and >3.5 microns. The main pore volume of shungite is represented by mesopores with an average size of 5 microns. The porosity size of brown coal is uniformly distributed in the range from 3 to 10 mn; larger pores are presented in an insignificant quantity.

Table 5.
CEC and surface properties of sorbents.
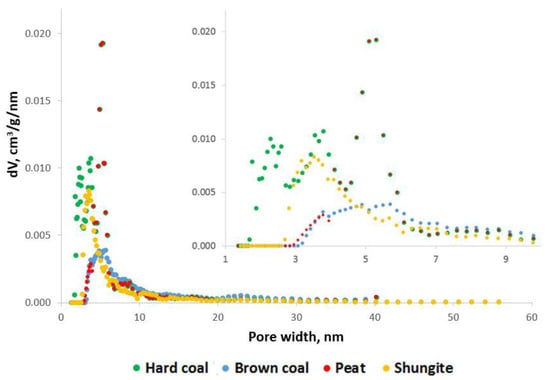
Figure 2.
Pore size distribution.
Characterization of the samples with SEM showed that the samples of shungite, hard and brown coal are represented by detrital grains up to 60–70 microns in size, having a close to cubic shape (Figure 3). Brown and hard coal grains have a rough surface, shungite grains have a flat surface. Grains of hard coal and shungite are densely covered with particles of a flattened and isometric shape, no more than 1–2 microns in size, apparently represented by clay minerals. Peat is represented by plant debris with a dendritic structure, the surface of which is covered with a uniform network of pores with a diameter of 5–10 microns. The morphology of the pores is varied; there are pores of round, elongated, irregular or papilla shapes (Figure 3).

Figure 3.
Scanning electron microscope images [].
3.4. Sorption of Cs (I) and Sr (II)
Since the studied sorbents have different specific surface areas, in order to compare their sorption characteristics, the sorption value was calculated not only as a percentage per unit mass (Figure 4a and Figure 5a), but also normalized per surface unit (Figure 4b and Figure 5b). As a result of the experiments carried out, the following regularities are observed. Sorption of Cs (I) increases with increasing pH, and the highest sorption capacity is observed in samples of brown coal (up to 40%) and high-moor peat (up to 24%). For the hard coal and shungite samples it is less Cs (I)—up to 20% and 11%, respectively (Figure 4a).
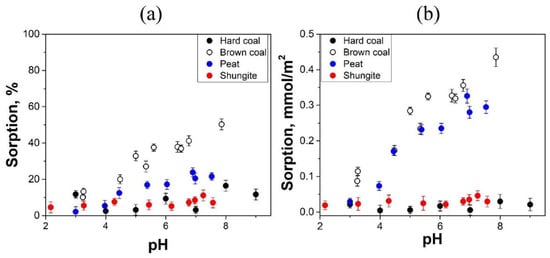
Figure 4.
Dependence of the amount of sorbed Cs (I), in % (a) and in mmol/m2 (b), on the pH of the equilibrium solution.
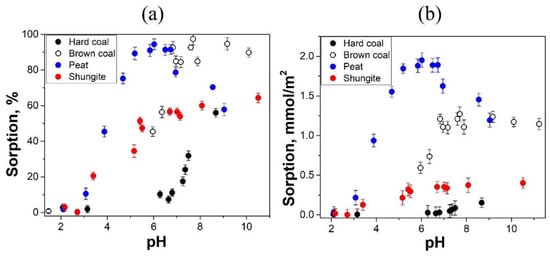
Figure 5.
Dependence of the amount of sorbed Sr (II), in % (a) and in mmol/m2 (b), on the pH of the equilibrium solution.
The amount of Cs (I) sorbed on 1 m2 of the surface of brown coal and high-moor peat turned out to be significantly higher than that on hard coal and shungite (Figure 4b).
Thus, for the studied samples, a correlation is observed between the value of sorption, CEC and SSA of the sample.
The maximum sorption of Sr (II) reaches 95–100% on high-moor peat and brown coal, and 56% and 64% on shungite and hard coal, respectively. Sorption of Sr (II) on high-moor peat significantly increases with increasing pH and reaches a maximum at equilibrium pH values in the range from 5.2 to 6.7. At pH > 6.7, Sr (II) sorption decreases (Figure 5a,b).
A significant increase in the Sr (II) sorption on brown coal occurs in the pH range from 5.9 to 7.7. A further increase in pH does not lead to an increase in the sorption of Sr (II) ions. Sorption of Sr (II) on shungite and on hard coal increases in the pH ranges of 2.1–5.4 and 6.3–8.7, respectively (Figure 5a,b).
The maximum amount of Sr (II) per 1 m2 is sorbed on high-moor peat. Brown coal sorbs half as much Sr (II). Shungite sorbs much less Sr (II) than both high-moor peat and brown coal, but almost two times more than hard coal (Figure 5b).
4. Discussion
It follows from the results of the experiments that the number of moles sorbed per 1 m2 of SSA of sorbent for all the studied organic sorbents is greater for Sr (II) than for Cs (I). In this case, the sorption of Sr (II) decreases in the order: high-moor peat > brown coal > shungite > hard coal. Cs (I) is sorbed in maximum amounts on brown coal and in smaller amounts on high-moor peat, shungite and hard coal. The high rates of cesium sorption on shungite in previous works [] apparently indicate a strong influence of the admixture of clay minerals and the importance of a detailed study of the mineral composition of the studied rocks.
Since impurities of clay minerals (illite and smectite), which have a high sorption capacity, are present in small amounts (Table 1), it can be assumed that their presence insignificantly affects the results of experiments, and the binding occurs mainly on organic surfaces.
The maximum sorption of Cs (I) and Sr (II) cations on all studied sorbents was less than the number of functional groups on the surface of the sorbents (Table 4 and Table 6). It can be assumed that not all functional groups are involved in sorption. Firstly, in different pH ranges, not all functional groups will be de-protonated and capable of sorption of cations, and secondly, the bond strength of the metal with the sorbent under different pH ranges can vary.

Table 6.
Maximum sorption of metal ions.
Sr (II) and other polyvalent cations have a higher affinity for organic matter than Cs (I) [,,,]. Therefore, all the studied samples sorb more moles of Sr (II) than Cs (I) per 1 m2 of surface, despite the fact that 1 mole of Sr (II) requires twice as many sorption sites as does 1 mole of Cs (I) (Table 6).
Maxima on the diagrams of the buffering capacity of organic sorbents (Figure 6) can be used for the estimation of the number of functional groups and their ability to participate in protonation–deprotonation reactions in the studied range of pH values. The main functional groups on organic sorbents are carboxyl (-COOH), phenolic (-OH), methoxyl (-OCH3), amino groups, etc. Carboxyl groups and phenolic hydroxyls predominate in humic substances [,,].
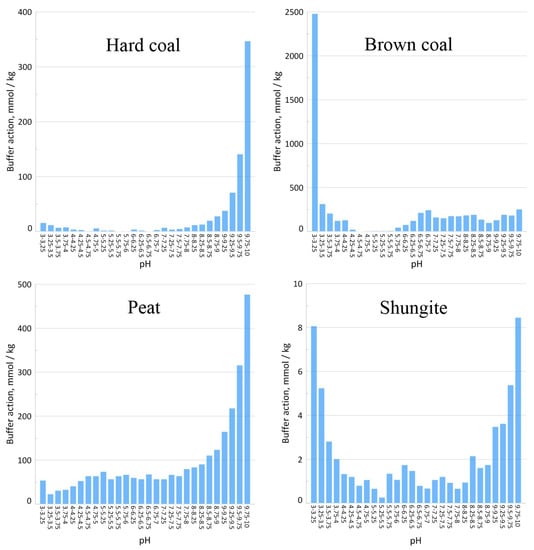
Figure 6.
Buffering to acid and base according to pH intervals.
Carboxyl functional groups are deprotonated in the pH range from 4 to 8, the pKa of which varies from 4 to 5. Phenolic hydroxyls are capable of deprotonation at pH > 8 and are characterized by pKa values of 10–11 [,,,]. Substituted phenols are titrated at lower pH values.
Hard coal has an insignificant buffering capacity for both acid and base (Table 4). On the diagram of the buffering capacity of this sample, maxima are distinguished in the pH intervals of 3.75–4.75 and 6–6.5, provided by the titration of carboxyl groups, and in the pH range of 7.5–8.75, in which phenolic hydroxyls are titrated (Figure 6). Large buffering values in the pH range of 7.5–8.75 in comparison with the acid range are in good agreement with the conclusions about the increase in the degree of aromaticity of organic matter, made on the basis of a change in the H: C atomic ratio (Table 3).
Brown coal and high-moor peat are characterized by the highest buffering values among the studied sorbents (Table 4). The maximum buffering capacity of brown coal corresponds to the pH ranges 3.74–4.25, 6.25–7.25, 7.75–8.0 and 9.25–9.75 (Figure 6). The diagrams of the buffering capacity of high-moor peat show maxima in the ranges of pH values of 4.25–5.25; 6.5–6.25; 6.5–6.75 and 7.75–10 (Figure 6). The first three ranges on the described buffering diagrams are provided by the deprotonation of carboxyl groups, and the last one—by the titration of phenolic hydroxyls of humic substances and lignin.
Shungite has the lowest buffering capacity for both acid and base among the studied sorbents (Table 4, Figure 6). Based on the structure of shungite described above, it can be assumed that the shungite material contains the smallest number of functional groups capable of interacting with metals in comparison with the other studied sorbents.
An increase in the sorption of Cs (I) ions with increasing pH is characteristic of all the studied sorbents and is explained, firstly, by the deprotonation of carboxyl and phenolic functional groups.
A significant increase in the sorption of Sr (II) ions on high-moor peat occurs with an increase in pH values up to ≈ 5 and is maintained at a constant level in the pH range of 5–6. Based on the titration results shown in Figure 6, it can be concluded that the main role in the fixation of Sr (II) ions on high-moor peat is played by carboxyl functional groups with pKa of ≈ 4–7. The main role in the Sr (II) sorption on the surface of brown coal is played by functional groups of the same nature, but with higher pKa values of 6 to 7 (Figure 6).
A decrease in the sorption of Sr (II) on high-moor peat at pH > 6 can probably be explained by a decrease in the bond strength of the cation with the sorbent. It is possible that phenolic hydroxyls do not play a significant part in the sorption of Sr (II) on the surface of the high-moor peat. Similar dependences on pH were obtained when studying the sorption of 110mAg, 60Co and 65Zn by humic substances extracted from peat [].
Since the amount of Sr (II) sorbed on brown coal turned out to be much less than the number of functional groups potentially capable for sorption, the observed plateau on the sorption curve (Figure 4b) can be explained with the assumption that phenolic hydroxyls do not take part in sorption, and that the carboxyl groups available for interaction are completely filled.
The sorption of Sr (II) on shungite and hard coal slightly increases with increasing pH (Figure 5). Considering that the organic matter in the composition of these sorbents has a high degree of aromaticity (Table 3 and Table 4) and a relatively small total amount of carboxyl and phenolic functional groups, it is not possible to determine the role of carboxyl groups and phenolic hydroxyls at low sorption values.
From the data obtained, it can be concluded that the main mechanism for fixing metal ions is their interaction with negatively charged functional (deprotonated) groups of organic matter. Cs (I) and Sr (II) can be fixed on the surface of organic sorbents in an exchange form. Sr (II) can form stable complexes with oxygen-containing functional groups of organic matter [,,].
Since experiments on the sorption of metals on organic sorbents were carried out with a wide range of pH values, it is obvious that the surface properties will change with a change in pH. Thus, under alkaline conditions, the leaching of humic substances increases. It has been shown that alkaline treatment of coals leads to an increase in the surface area and total pore volume [].
In addition, humic substances undergo conformational and configurational changes during the pH change from 2 to 10 [,]. Such changes can lead to a situation where some of the functional groups, capable of ionization at a given pH value, occur inside the molecule at a different pH level and thereby become inaccessible to the sorbate.
5. Conclusions
The sorption properties of the natural organic sorbents in relation to the 137Cs and 90Sr radionuclides depend on various degree of lithification, pH, the quantity and properties of available oxygen-containing functional groups.
As a result of the conducted research, it can be concluded that Sr (II) shows a higher sorption than Cs (I) in the studied range of concentrations and pH values.
The maximum sorption of Sr (II) reaches 95–100% on high-moor peat and brown coal, and lower values for shungite and hard coal. Sorption of Sr (II) significantly increases with increasing pH and reaches a maximum at pH 5 to 7 and decreases at pH values higher than 7. The maximum sorption of Sr (II) decreases in the order: high-moor peat > brown coal > shungite > hard coal.
Sorption of Cs (I) increases with increasing pH, and the highest sorption capacity is observed in samples of brown coal (up to 40%) and high-moor peat (up to 24%). The maximum sorption of Cs (I) decreases in the raw: brown coal > high-moor peat > shungite > hard coal.
Sorption values, normalized per surface unit, showed a difference compared with percentage per unit mass. The amount of Cs (I) sorbed on 1 m2 of the surface of brown coal and high-moor peat turned out to be significantly higher than that on hard coal and shungite. The maximum amount of Sr (II) per 1 m2 is sorbed on high-moor peat, while brown coal sorbs half as much Sr (II). Shungite sorbs much less Sr (II) than both high-moor peat and brown coal, but almost two times more than hard coal.
From the data obtained, it can be concluded that the main mechanism for fixing metal ions is their interaction with negatively charged functional (deprotonated) groups of organic matter. Cs (I) and Sr (II) can be fixed on the surface of organic sorbents in an exchange form. Sr (II) can form stable complexes with oxygen-containing functional groups of organic matter. It is suggested that Cs (I) can be fixed on carboxyl functional groups and, possibly, in insignificant amounts on phenolic hydroxyls of all four studied organic sorbents. The main role in the fixation of Sr (II) on high-moor peat and brown coal would then be played by carboxyl functional groups with pKa values of ≈ 4–7 and ≈ 6–7, respectively. The organic matter in the composition of hard coal and shungite has a high degree of aromaticity and a relatively small total amount of carboxyl and phenolic functional groups, which determines the low sorption capacity of these sorbents. It is therefore difficult to distinguish, with certainty, the role of carboxyl groups and phenolic hydroxyls at low sorption values of Cs (I) and Sr (II) on these sorbents.
Author Contributions
P.B.: prepared material, conceived design of the experiments and wrote the paper; A.S. and A.R.: carried out sorption experiments with radionuclides; S.Z.: carried out X-ray diffraction analysis; E.T.: analyzed specific surface area and porosity; I.T. and Y.I.: carried out CEC determination, buffering capacity to acid and base and interpretation of the results; V.K.: collected the data for mineral analysis and interpretation. All authors have read and agreed to the published version of the manuscript.
Funding
Sorption experiments and cation exchange capacity of the studied sorbents were conducted with the support of the Russian Science Foundation, project № 18-77-00015. Mineral composition and buffering properties of the sorbents were studied with the support of the Russian Foundation for Basic Research, project No. 18-29-12115. Experimental studies were partially performed using the equipment acquired with the funding of Moscow State University Development Program (X-ray Diffractometer Ultima-IV, Rigaku and Scanning Electron Microscope LEO 1450VP, Carl Zeiss). This research was performed according to the development program of the Interdisciplinary Scientific and Educational School of M.V. Lomonosov Moscow State University “future planet and global environmental change” and RF President stipendium to ASS (SP-4892.2021.2).
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- Krupskaya, V.V.; Biryukov, D.V.; Belousov, P.E.; Lekhov, V.A.; Romanchuk, A.Y.; Kalmykov, S.N. Use of natural clay materials to increase nuclear and radiation safety of nuclear legacy facilities. Radioact. Waste 2018, 2, 30–43. (In Russian) [Google Scholar]
- Krupskaya, V.V.; Zakusin, S.V.; Lekhov, V.A.; Dorzhieva, O.V.; Belousov, P.E.; Tyupina, E.A. Buffer Properties of Bentonite Barrier Systems for Radioactive Waste Isolation in Geological Repository in the Nizhnekanskiy Massif. Radioact. Waste 2020, 1, 35–55. (In Russian) [Google Scholar] [CrossRef]
- Ilina, O.A.; Krupskaya, V.V.; Vinokurov, S.E.; Kalmykov, S.N. State-of-Art in the Development and Use of Clay Materials as Engineered Safety Barriers at Radioactive Waste Conservation and Disposal Facilities in Russia. Radioact. Waste 2019, 4, 71–84. [Google Scholar] [CrossRef]
- Platonov, V.V.; Kalmykov, S.N.; Pislyak, V.G.; Tananaev, I.G. Use of humic materials for solving radioecological problems. Bull. Far East. Branch Russ. Acad. Sci. 2016, 3, 72–79. [Google Scholar]
- Samoilov, V.I.; Saduakasova, A.T.; Zelenin, V.I.; Kulenova, N.A. Investigation of the process of sorption of uranium from lake water using natural sorbents and their modification products. Min. Inf. Anal. Bull. 2016, 4, 283–291. [Google Scholar]
- Belousov, P.; Semenkova, A.; Egorova, T.; Romanchuk, A.; Zakusin, S.; Dorzhieva, O.; Tyupina, E.; Izosimova, Y.; Tolpeshta, I.; Chernov, M.; et al. Cesium Sorption and Desorption on Glauconite, Bentonite, Zeolite, and Diatomite. Minerals 2019, 9, 625. [Google Scholar] [CrossRef]
- Semenkova, A.; Belousov, P.; Rzhevskaia, A.; Izosimova, Y.; Maslakov, K.; Tolpeshta, I.; Romanchuk, A.; Krupskaya, V. U(VI) sorption onto natural sorbents. J. Radioanal. Nucl. Chem. 2020, 326, 293–301. [Google Scholar] [CrossRef]
- Dobrovolskaya, T.G.; Golovchenko, A.V.; Zvyagintsev, D.G.; Inisheva, L.I.; Kurakov, A.V.; Smagin, A.V.; Zenova, G.M.; Lysak, L.V.; Semenova, T.A.; Stepanov, A.L.; et al. Functioning of Microbial Complexes in High-Moor Peatlands–Analysis of the Reasons for the Slow Destruction of Peat; KMK Scientific Publishing Association: Moscow, Russia, 2013; p. 128. [Google Scholar]
- Khromushin, V.A.; Tchesnova, T.V.; Platonov, V.V.; Khadartsev, A.A.; Kireev, S.S. The shungite as natural nanotechnologie (literature review). J. New Med. Technol. E J. 2014, 1, 1–8. [Google Scholar] [CrossRef]
- Lukin, A.E. On genesis of shungites. Geol. J. 2005, 4, 27–47. [Google Scholar]
- Polunina, I.A.; Goncharova, I.S.; Visotskii, V.V.; Petukhova, G.A.; Polunin, K.E.; Ulyanov, A.V.; Buryak, A.K. Modification of shungite material for use in sorption and membrane technology. Sorpt. Chromatogr. Process. 2017, 8, 181–185. [Google Scholar]
- Andryushchenko, N.D.; Safonov, A.V.; Konevnik, Y.V.; Kondrashova, A.A.; Proshin, I.M.; Zakharova, E.V.; Babich, T.L.; Ivanov, P.V. Sorption characteristics of materials of the filtration barrier in upper aquifers contaminated with radionuclides. Radiochemistry 2017, 4, 414–424. [Google Scholar] [CrossRef]
- Moore, D.M.; Reynolds, R.C., Jr. X-ray Diffraction and the Identification and Analysis of Clay Minerals, 2nd ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 1999; p. 378. [Google Scholar]
- Post, J.E.; Bish, D.L. Rietveld refinement of crystal structures using powder X-ray diffraction data. Rev. Miner. Geochem. 1989, 20, 277–308. [Google Scholar]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Cryst. 2015, 48, 1573–1580. [Google Scholar] [CrossRef]
- Vorobyova, L.A. Chemical Analysis of Soils; Moscow State University: Moscow, Russia, 1998. [Google Scholar]
- Bezhin, N.A.; Dovhyi, I.I.; Kapranov, S.V.; Bobko, N.I.; Milyutin, V.V.; Kaptakov, V.O.; Kozlitin, E.A.; Tananaev, I.G. Separation of radiostrontium from seawater using various types of sorbents. J. Radioanal. Nucl. Chem. 2021, 328, 1199–1209. [Google Scholar] [CrossRef]
- Campus, A.; February, R.; Yusan, S.; Erenturk, S. Adsorption Characterization of Strontium on PAN/Zeolite Composite Adsorbent. World J. Nucl. Sci. Technol. 2011, 1, 6–12. [Google Scholar] [CrossRef]
- Khandaker, S.; Kuba, T.; Kamida, S.; Uchikawa, Y. Adsorption of cesium from aqueous solution by raw and concentrated nitric acid-modified bamboo charcoal. J. Environ. Chem. Eng. 2017, 5, 1456–1464. [Google Scholar] [CrossRef]
- Orlov, D.S. Humic Acids of Soils and the General Theory of Humification, 1st ed.; Moscow State University: Moscow, Russia, 1990; p. 323. [Google Scholar]
- Stevenson, F.J. Humus Chemistry, Genesis, Composition, Reaction; John Wiley: New York, NY, USA, 1994; p. 444. [Google Scholar]
- Sanzharova, N.I.; Sysoeva, A.A.; Isamov, N.N.; Aleksakhin, R.M.; Kuznetsov, V.K.; Zhigareva, T.L. The role of chemistry in the rehabilitation of agricultural lands subjected to radioactive contamination. Russ. Chem. 2005, 3, 26–34. [Google Scholar]
- Singh, B.K.; Jain, A.; Kumar, S.; Tomar, B.S.; Tomar, R.; Manchanda, V.K.; Ramanathan, S. Role of magnetite and humic acid in radionuclide migration in the environment. J. Contam. Hydrol. 2009, 106, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Çelebi, O.; Erten, H.N. Adsorption Behavior of Radionuclides, 137Cs and 140Ba, onto Solid Humic Acid. In Survival and Sustainability, Environmental Earth Sciences; Gökçekus¸, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1065–1085. [Google Scholar] [CrossRef]
- Berns, A.E.; Flath, A.; Mehmood, K.; Hofmann, D.; Jacques, D.; Sauter, M.; Vereecken, H.; Engelhardt, I. Numerical and Experimental Investigations of Cesium and Strontium Sorption and Transport in Agricultural Soils. Vadose Zone J. 2017, 17, 170126. [Google Scholar] [CrossRef]
- Sposito, G. The Chemistry of Soils; Oxford University Press: New York, NY, USA; Oxford, UK, 1989; p. 279. [Google Scholar]
- Perdue, E.M. Acidic functional groups of humic substances. In Humic Substances in Soil, Sediments and Water; Aiken, G.R., Ed.; John Wiley: New York, NY, USA, 1985; pp. 493–526. [Google Scholar]
- Hayes, M.S.B. Influence of the acid/base status on the formation and interaction of acids and bases in soils. In Proceedings of the Transaction of the 13 Congress of the International Society of Soil Science, Hamburg, Germany, 13–20 August 1986; pp. 93–109. [Google Scholar]
- Senesi, N.; Loffredo, E. The Chemistry of Soil Organic Matter. In Soil Physical Chemistry; Sparks Donald, L., Ed.; CRC Press: Boca Raton, FL, USA; Boston, MA, USA; London, UK; New York, NY, USA; Washington, DC, USA, 1998; pp. 239–271. [Google Scholar]
- Helal, A.A.; Helal, A.; Salim, N.Z.; Khalifa, S.M. Sorption of radionuclides on peat humin. J. Radioanal. Nucl. Chem. 2006, 267, 363–368. [Google Scholar] [CrossRef]
- Buckau, G. Effects of Humic Substances on the Migration of Radionuclides: Complexation and Transport of Actinides. In Second Technical Progress Report; Institut für Nukleare Entsorgungstechnik: Germany, Karlsruhe, 1999; p. 408. [Google Scholar]
- Tanaka, T.; Nagao, S.; Sakamoto, Y.; Ohnuki, T.; Ni, S.; Senoo, M. Distribution Coefficient in the Sorption of Radionuclides onto Ando Soil in the Presence of Humic Acid/Influence of the Molecular Size of Humic Acid. J. Nucl. Sci. Technol. 1997, 34, 829–834. Available online: https://www.tandfonline.com/loi/tnst20 (accessed on 10 November 2021). [CrossRef][Green Version]
- Chesnokov, N.V.; Mikova, N.M.; Ivanov, I.P.; Kuznetsov, B.N. Obtaining carbon sorbents by chemical modification of fossil coals and plant biomass. J. Sib. Fed. Univ. Chem. 2014, 7, 42–53. [Google Scholar]
- Sparks, D.L. Environmental Soil Chemistry; Academic Press: Cambridge, MA, USA, 2003. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).