Plant Species Classification and Diversity of the Understory Vegetation in Oak Forests of Swat, Pakistan
Abstract
1. Introduction
2. Materials and Methods
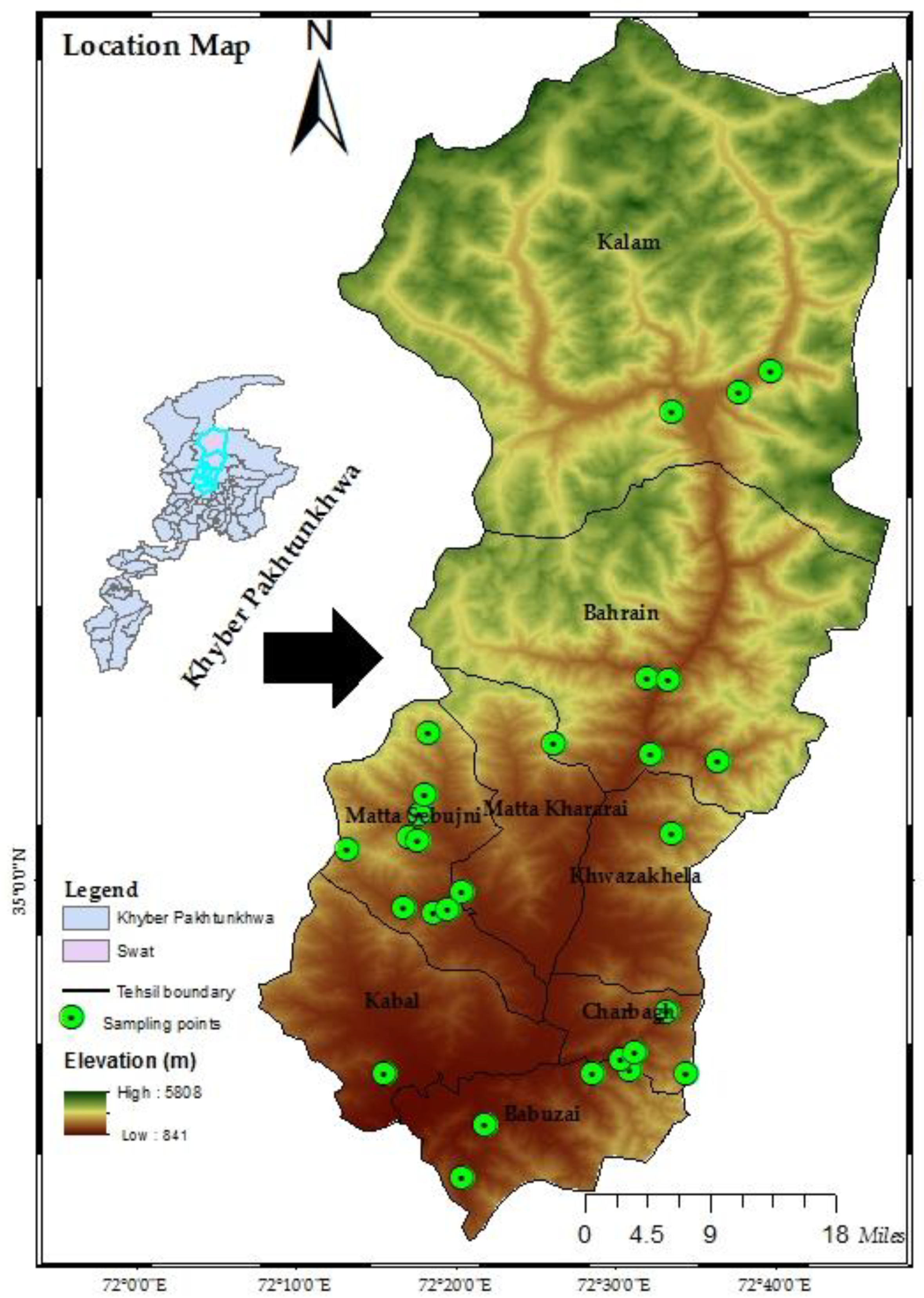
2.1. Study Site
2.2. Field Investigation
2.3. Soil Analysis
2.4. Data Analysis
3. Results
3.1. Understory Species Composition
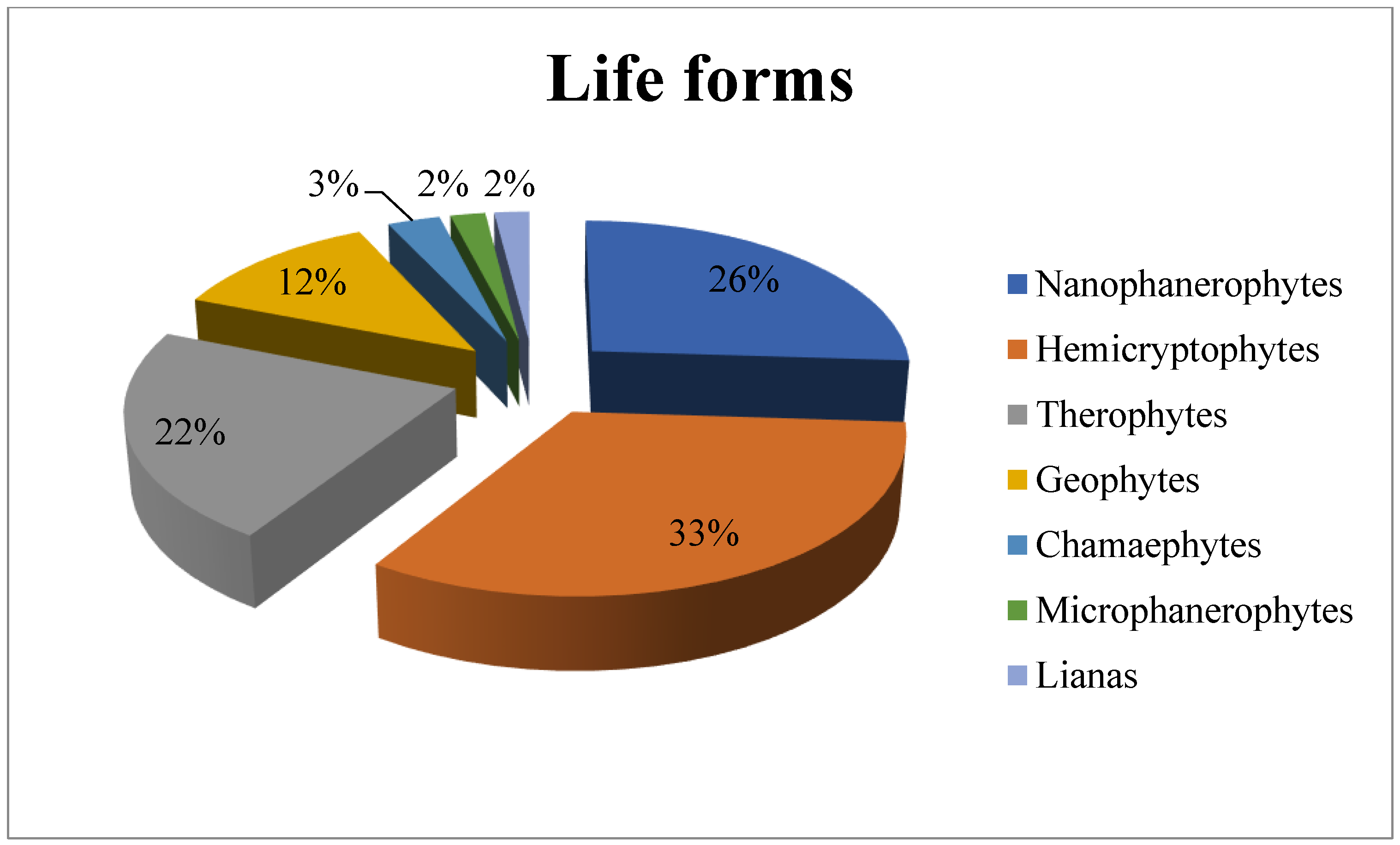
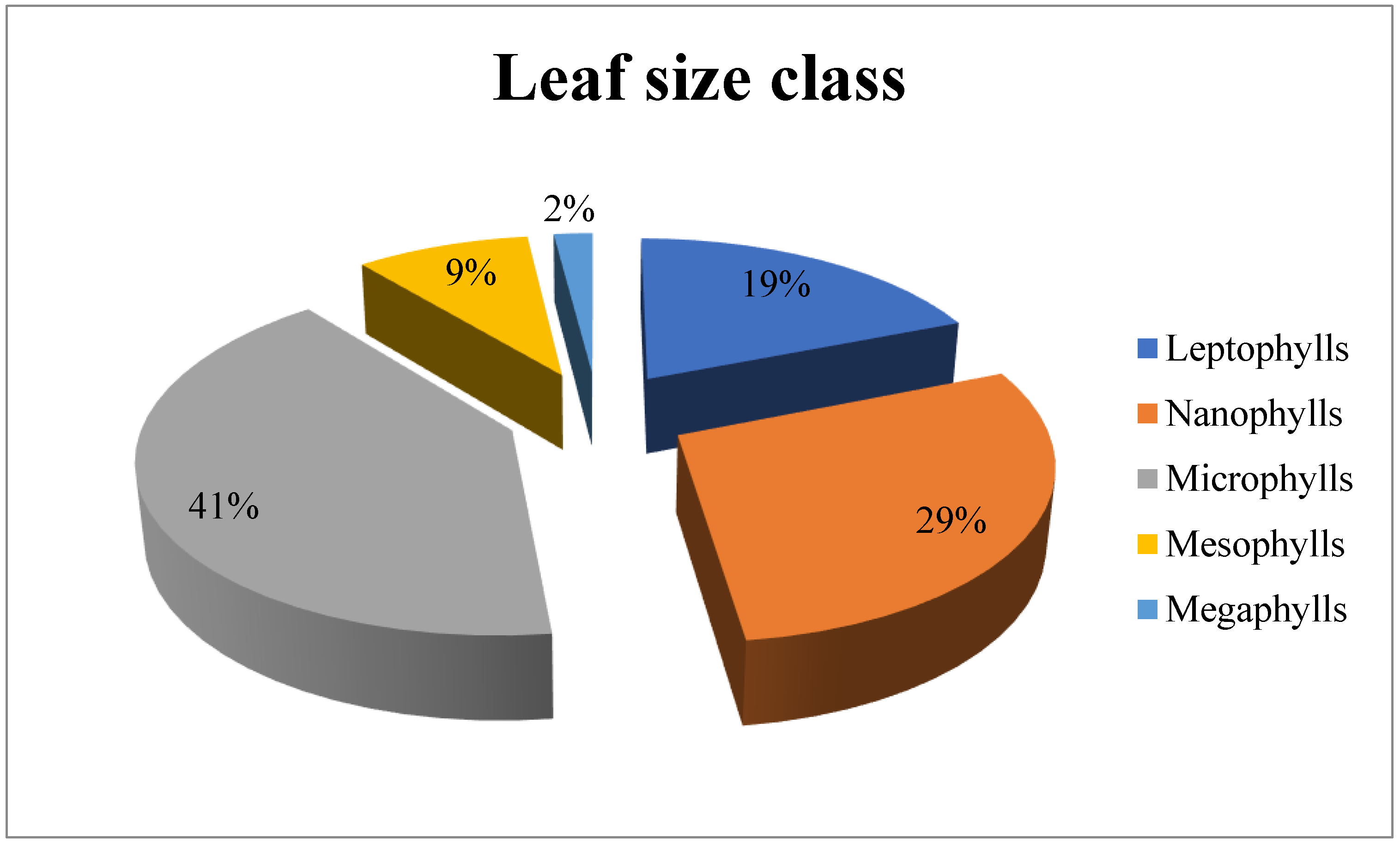
3.2. Leaf Size Spectra
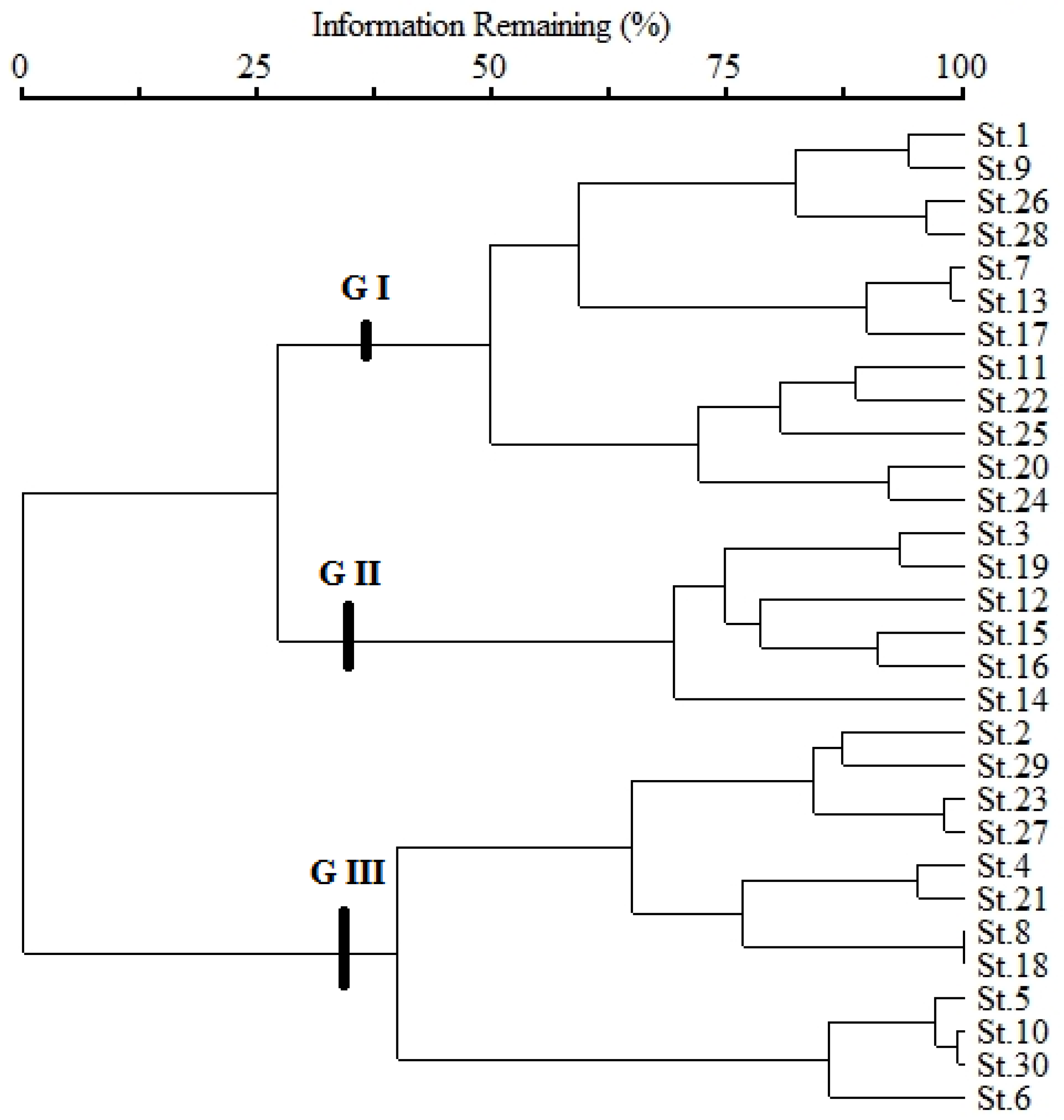
3.3. Understory Classification
3.4. Understory Species Diversity and Richness
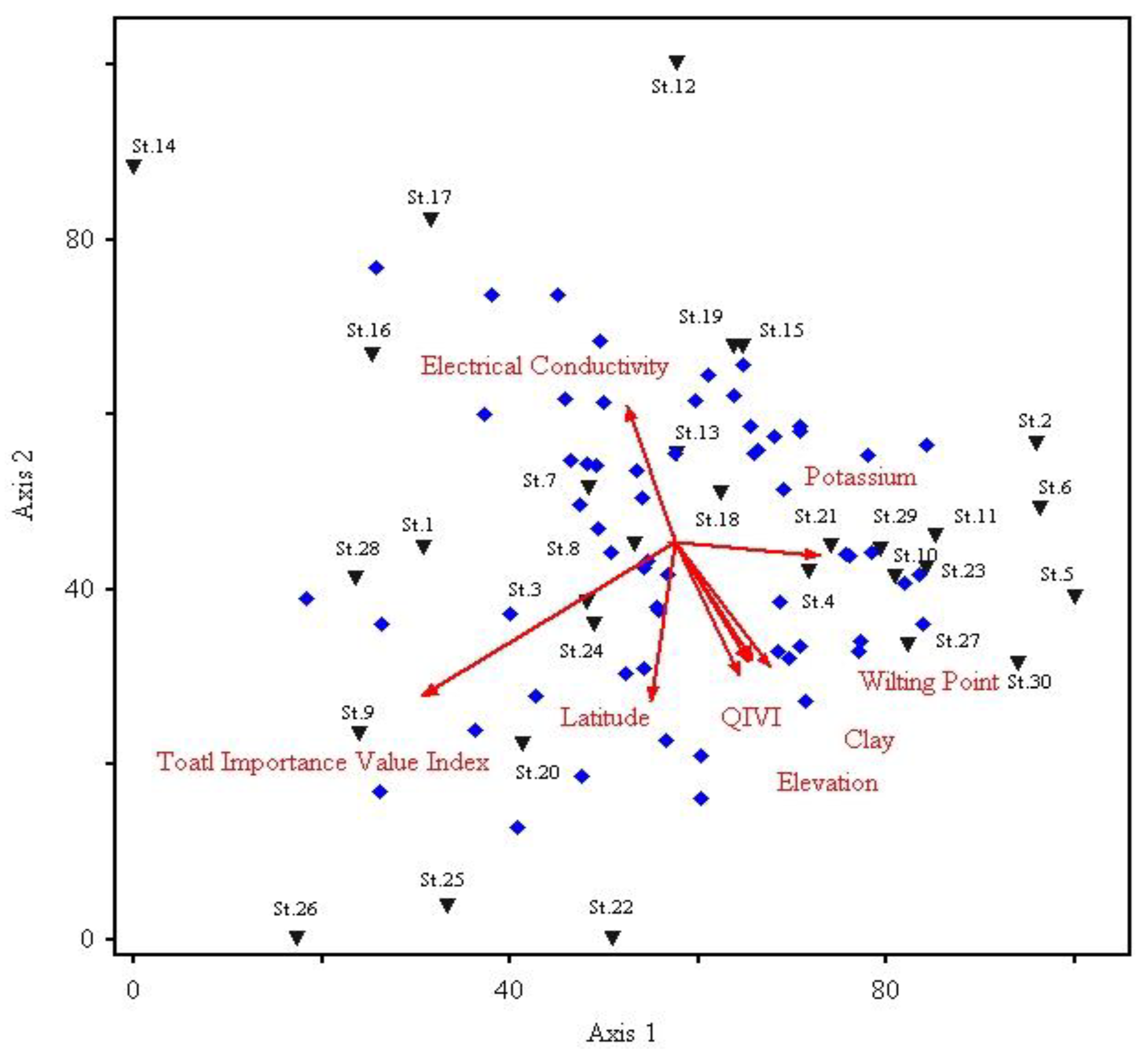
3.5. Influence of Physiographic and Edaphic Variables on the Understory Species Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Musarella, C.M.; Mendoza-Fernández, A.J.; Mota, J.F.; Alessandrini, J.F.M.A.; Bacchetta, G.; Brullo, G.B.S.; Caldarella, O.; Ciaschetti, O.C.G.; Conti, F.; Di Martino, F.C.L.; et al. Checklist of gypsophilous vascular flora in Italy. Phyto Keys 2018, 103, 61–82. [Google Scholar] [CrossRef] [PubMed]
- Perrino, E.V.; Signorile, G. Checklist of the vascular flora from the Monopoli coast (Apulia). Ital. Bot. 2009, 41, 263–279. [Google Scholar]
- Shah, M.; Farrukh HSyed NMSImran AHumaira, W. Life Form And Floristic Characteristics Along Altitudinal Gradient Of Humid Temperate Forests Located In Remote Area Of Pakistan. Glob. J. Biodivers. Sci. Manag. 2013, 3, 276–281. [Google Scholar]
- Rafaqat, M. An Annotated Checklist of Amphibians and Reptiles of Margalla Hills National Park, Pakistan. Pak. J. Zool. 2011, 43, 1041–1048. [Google Scholar]
- Shimwell, D.W. Festuco-Brometea Br.-Bl. & R. Tx. 1943 in the British isles: The Phytogeography and Phytosociology of Limestone Grasslands: Part I (A) General Introduction;(B) Xerobromion in England. Vegetatio 1971, 23, 1–27. [Google Scholar]
- Raunkiaer, C. The Life Forms of Plants and Statistical Plant Geography; Being the Collected Papers of C. Raunkiaer. In The Life Forms of Plants and Statistical Plant Geography; Being the Collected Papers of C. Raunkiaer; Clarendon Press: Oxford, UK, 1934. [Google Scholar]
- Oosting, H.J.; Hess, D.W. Microclimate and a relic stand of Tsuga canadensis in the lower piedmont of North Carolina. Ecology 1956, 37, 28–39. [Google Scholar] [CrossRef]
- Bailey, I.W.; Sinnott, E.W. A botanical index of Cretaceous and Tertiary climates. Science 1915, 41, 831–834. [Google Scholar] [CrossRef]
- Cain, S.A.; De Oliveira Castro, G.M. Manual of Vegetation Analysis; Haper and Row: New York, NY, USA, 1959. [Google Scholar]
- Augusto, L.; Dupouey, J.L.; Ranger, J. Effects of tree species on understory vegetation and environmental conditions in temperate forests. Ann. For. Sci. 2003, 60, 823–831. [Google Scholar] [CrossRef]
- Gracia, M.; Montané, F.; Piqué, J.; Retana, J. Overstory structure and topographic gradients determining diversity and abundance of understory shrub species in temperate forests in central Pyrenees (NE Spain). For. Ecol. Manag. 2007, 242, 391–397. [Google Scholar] [CrossRef]
- Gilliam, F.S. The ecological significance of the herbaceous layer in temperate forest ecosystems. Bioscience 2007, 57, 845–858. [Google Scholar] [CrossRef]
- Messier, C.; Parent, S.; Bergeron, Y. Effects of overstory and understory vegetation on the understory light environment in mixed boreal forests. J. Veg. Sci. 1998, 9, 511–520. [Google Scholar] [CrossRef]
- Nilsson, M.C.; Wardle, D.A. Understory vegetation as a forest ecosystem driver: Evidence from the northern Swedish boreal forest. Front. Ecol. Environ. 2005, 3, 421–428. [Google Scholar] [CrossRef]
- Bartels, S.F.; Chen, H.Y.H. Is understory plant species diversity driven by resource quantity or resource heterogeneity? Ecology 2010, 91, 1931–1938. [Google Scholar] [CrossRef] [PubMed]
- Dauber, J.; Hirsch, M.; Simmering, D.; Waldhardt, R.; Otte, A.; Wolters, V. Landscape structure as an indicator of biodiversity: Matrix effects on species richness. Agric. Ecosyst. Environ. 2003, 98, 321–329. [Google Scholar] [CrossRef]
- Duguid, M.C.; Frey, B.R.; Ellum, D.S.; Kelty, M.; Ashton, M.S. The influence of ground disturbance and gap position on understory plant diversity in upland forests of southern New England. For. Ecol. Manag. 2013, 303, 148–159. [Google Scholar] [CrossRef]
- Koncz, G.; Papp, M.; Török, P.; Kotroczó, Z.; Krakomperger, Z.; Matus, G.; Tóthmérész, B. The role of seed bank in the dynamics of understorey in an oak forest in Hungary. Acta Biol. Hung. 2010, 61, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Barbier, S.; Gosselin, F.; Balandier, P. Influence of tree species on understory vegetation diversity and mechanisms involved—A critical review for temperate and boreal forests. For. Ecol. Manag. 2008, 254, 1–15. [Google Scholar] [CrossRef]
- Yu, M.; Sun, O.J. Effects of forest patch type and site on herb-layer vegetation in a temperate forest ecosystem. For. Ecol. Manag. 2013, 300, 14–20. [Google Scholar] [CrossRef]
- Roberts, M.R.; Zhu, L. Early response of the herbaceous layer to harvesting in a mixed coniferous–deciduous forest in New Brunswick, Canada. For. Ecol. Manag. 2002, 155, 17–31. [Google Scholar] [CrossRef]
- Hart, S.A.; Chen, H.Y.H. Fire, logging, and overstory affect understory abundance, diversity, and composition in boreal forest. Ecol. Monogr. 2008, 78, 123–140. [Google Scholar] [CrossRef]
- Ellsworth, J.W.; Harrington, R.A.; Fownes, J.H. Seedling emergence, growth, and allocation of Oriental bittersweet: Effects of seed input, seed bank, and forest floor litter. For. Ecol. Manag. 2004, 190, 255–264. [Google Scholar] [CrossRef]
- Chávez, V.; Macdonald, S.E. The influence of canopy patch mosaics on understory plant community composition in boreal mixedwood forest. For. Ecol. Manag. 2010, 259, 1067–1075. [Google Scholar] [CrossRef]
- Koorem, K.; Moora, M. Positive association between understory species richness and a dominant shrub species (Corylus avellana) in a boreonemoral spruce forest. For. Ecol. Manag. 2010, 260, 1407–1413. [Google Scholar] [CrossRef]
- Warren, R.J. Mechanisms driving understory evergreen herb distributions across slope aspects: As derived from landscape position. Plant Ecol. 2008, 198, 297–308. [Google Scholar] [CrossRef]
- Ali, S.I.; Qaiser, M. A phytogeographical analysis of the phanerogams of Pakistan and Kashmir. Proceedings of the Royal Society of Edinburgh. Sect. B Biol. Sci. 1986, 89, 89–101. [Google Scholar] [CrossRef]
- Ilahi, I.; Suleman, M. Species composition and relative abundance of mosquitoes in Swat, Pakistan. Int. J. Innov. Appl. Stud. 2013, 2, 454–463. [Google Scholar]
- Beg, A.R.; Mirza, H.K. Some more plant communities and the future of dry oak forest zone in Swat valley. Pak. J. For. 1984, 34, 25–35. [Google Scholar]
- Rahman, I.U.; Ijaz, F.; Iqbal, Z.; Afzal, A.; Ali, N.; Afzal, M.; Khan, M.A.; Muhammad, S.; Qadir, G.; Asif, M. A novel survey of the ethno medicinal knowledge of dental problems in Manoor Valley (Northern Himalaya), Pakistan. J. Ethnopharmacol. 2016, 194, 877–894. [Google Scholar] [CrossRef]
- Qian, H.; Klinka, K.; Økland, R.H.; Krestov, P.; Kayahara, G.J. Understorey vegetation in boreal Picea mariana and Populus tremuloides stands in British Columbia. J. Veg. Sci. 2003, 14, 173–184. [Google Scholar] [CrossRef]
- Wulf, M.; Naaf, T. Herb layer response to broadleaf tree species with different leaf litter quality and canopy structure in temperate forests. J. Veg. Sci. 2009, 20, 517–526. [Google Scholar] [CrossRef]
- Nasir, E.; Ali, S.I. Flora of West Pakistan Department of Botany; University of Karachi: Karachi, Pakistan, 2007; Volume 1971, pp. 112–115. [Google Scholar]
- Ali, S.I. Significance of flora with special reference to Pakistan. Pak. J. Bot. 2008, 40, 967–971. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis. Part 3. Chemical Method; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Summer, M.E., Eds.; Soil Science Society of America and American Society of Agronomy: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar] [CrossRef]
- Yeomans, J.C.; Bremner, J.M. Carbon and nitrogen analysis of soils by automated combustion techniques. Commun. Soil Sci. Plant Anal. 1991, 22, 843–850. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, G.; Ni, J. Effects of topographical and edaphic factors on the distribution of plant communities in two subtropical karst forests, southwestern China. J. Mt. Sci. 2013, 10, 95–104. [Google Scholar] [CrossRef]
- Agriculture Chemistry Council, Soil Science Society of China. General Analysis Methods of Soil Agriculture Chemistry; Science Press: Beijing, China, 1983. (In Chinese) [Google Scholar]
- Day, P.R. Particle Fractionation and Particle-Size Analysis; No. methods of soil analysis; American Society of Agronomy Madison, Soil Science Society of America: Madison, WI, USA, 1965; pp. 545–567. [Google Scholar]
- Orlóci, L. An agglomerative method for classification of plant communities. J. Ecol. 1967, 55, 193–206. [Google Scholar] [CrossRef]
- Jongman, E.; Jongman, S.R.R. Data Analysis in Community and Landscape Ecology; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Braak, C.J.F.T. The analysis of vegetation-environment relationships by canonical correspondence analysis. Vegetation 1987, 69, 69–77. [Google Scholar] [CrossRef]
- Skinner, W.R.; Jefferies, R.L.; Carleton, T.J.; Abraham, R.R.D.K. Prediction of reproductive success and failure in lesser snow geese based on early season climatic variables. Glob. Chang. Biol. 1998, 4, 3–16. [Google Scholar] [CrossRef]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Shehata, H.F.S. Ecology and nutritive status of Sisymbrium irio L. in the Nile delta. Egypt. J. Exp. Biol. 2014, 10, 127–142. [Google Scholar]
- Khattak, N.S.; Nouroz, F.; Rahman, I.U.; Noreen, S. Ethno veterinary uses of medicinal plants of district Karak. Pak. J. Ethnopharmacol. 2015, 171, 273–279. [Google Scholar] [CrossRef]
- Shah, G.M.; Khan, M.A. Common medicinal folk recipes of siran valley, Mansehra, Pakistan. Ethnobot. Leafl. 2006, 10, 49–62. [Google Scholar]
- Ullah, R.; Iqbal, Z.H.Z.; Hussain, J.; Khan, F.U.; Khan, N.; Muhammad, Z.; Ayaz, S.; Ahmad, S.; Rehman, N.U.; Hussain, I. Traditional uses of medicinal plants in Darra Adam Khel NWFP Pakistan. J. Med. Plants Res. 2010, 4, 1815–1821. [Google Scholar]
- Meher-Homji, V.M. Life-Forms and Biological Spectra as Epharmonic Criteria of Aridity and Humidity in the Tropics; The Bangalore Press: Mysore, India, 1964. [Google Scholar]
- Amjad, M.S.; Qaeem, M.F.; Ahmad, I.; Khan, S.U.; Chaudhari, S.K.; Zahid Malik, N.; Shaheen, H.; Khan, A.M. Descriptive study of plant resources in the context of the ethnomedicinal relevance of indigenous flora: A case study from Toli Peer National Park, Azad Jammu and Kashmir, Pakistan. PLoS ONE 2017, 12, e0171896. [Google Scholar] [CrossRef]
- Haq, F.; Ahmad, H.; Iqbal, Z. Vegetation description and phytoclimatic gradients of subtropical forests of Nandiar Khuwar catchment District Battagram. Pak. J. Bot. 2015, 47, 1399–1405. [Google Scholar]
- Khan, M.; Hussain, F.; Musharaf, S. Floristic composition and biological characteristics of the vegetation of Sheikh Maltoon Town District Mardan, Pakistan. Annu. Res. Rev. Biol. 2013, 3, 31–41. [Google Scholar]
- Shimwell, D.W. The Description and Classification of Vegetation Sedgwick and Jackson; Sidgwick & Jackson: London, UK, 1971; p. 322. [Google Scholar]
- Nasir, Z.A.; Sultan, S. Floristic, biological and leaf size spectra of weeds in gram, lentil, mustard and wheat fields of district Chakwal, Pakistan. Pak. J. Biol. Sci. 2002, 5, 758–762. [Google Scholar]
- Barik, K.L.; Misra, B.N. Biological spectrum of grassland ecosystem of South Orissa. Ecoprint 1998, 5, 73–77. [Google Scholar]
- Badshah, L.; Hussain, F.; Sher, Z. Floristic inventory, ecological characteristics and biological spectrum of rangeland, District Tank, Pakistan. Pak. J. Bot. 2013, 45, 1159–1168. [Google Scholar]
- Sher, Z.; Hussain, F.; Badshah, L. Biodiversity and ecological characterization of the flora of Gadoon rangeland, district Swabi, Khyber Pukhtunkhwa, Pakistan. Iran. J. Bot. 2014, 20, 96–108. [Google Scholar]
- Tareen, R.B.; Qadir, S.A. Phytosociology of the hills of Quetta district. Pak. J. Bot. 1991, 23, 90–114. [Google Scholar]
- Batalha, M.A.; Martins, F.R. Floristic, frequency, and vegetation life-form spectra of a cerrado site. Braz. J. Biol. 2004, 64, 201–209. [Google Scholar] [CrossRef][Green Version]
- Tareen, R.B.; Qadir, S. Harnai, Sinjawi to Duki regions of Pakistan. Pak. Bot. Soc. 1993, 25, 83–92. [Google Scholar]
- Thomsen, R.P.; Svenning, J.; Balslev, H. Overstorey control of understorey species composition in a near-natural temperate broadleaved forest in Denmark. Plant Ecol. 2005, 181, 113–126. [Google Scholar] [CrossRef]
- Ali, K.; Begum, H.A. A comparative assessment of climate change effect on some of the important tree species of Hindu-Kush Himalayas, using predictive modelling techniques. Int. J. Adv. Res. 2015, 3, 1230–1240. [Google Scholar]
- Qian, H.; Klinka, K.; Sivak, B. Diversity of the understory vascular vegetation in 40 year-old and old-growth forest stands on Vancouver Island, British Columbia. J. Veg. Sci. 1997, 8, 773–780. [Google Scholar] [CrossRef]
- Triantafyllidis, V.; Zotos, A.; Kosma, C.; Kokkotos, E. Effect of land-use types on edaphic properties and plant species diversity in Mediterranean agroecosystem. Saudi J. Biol. Sci. 2020, 27, 3676–3690. [Google Scholar] [CrossRef]
- Rahman, I.U.; Khan, N.; Ali, K. Classification and ordination of understory vegetation using multivariate techniques in the Pinus wallichiana forests of Swat Valley, northern Pakistan. Sci. Nat. 2017, 104, 24. [Google Scholar] [CrossRef] [PubMed]
- Nazaryuk, V.M.; Klenova, M.I.; Kalimullina, F.R. Ecoagrochemical approaches to the problem of nitrate pollution in agroecosystems. Russ. J. Ecol. 2002, 33, 392–397. [Google Scholar] [CrossRef]
- Colombo, C.; Palumbo, G.; Sellitto, V.M.; Di Iorio, E.; Castrignanò, A.; Stelluti, M. The effects of land use and landscape on soil nitrate availability in Southern Italy (Molise region). Geoderma 2015, 239, 1–12. [Google Scholar] [CrossRef]
- Saxena, R.S.; Gupta, B.; Saxena, K.K.; Singh, R.C.; Prasad, D.N. Study of anti-inflammatory activity in the leaves of Nyctanthes arbor tristis Linn. An Indian Medicinal Plant. J. Ethnopharmacol. 1987, 11, 319–330. [Google Scholar] [CrossRef]
- Macdonald, S.E.; Fenniak, T.E. Understory plant communities of boreal mixedwood forests in western Canada: Natural patterns and response to variable-retention harvesting. For. Ecol. Manag. 2007, 242, 34–48. [Google Scholar] [CrossRef]
- Small, C.J.; McCarthy, B.C. Spatial and temporal variability of herbaceous vegetation in an eastern deciduous forest. Plant Ecol. 2003, 164, 37–48. [Google Scholar] [CrossRef]
| Taxon | Family | Habit | Chorotype | Life-Form | Leaf Size |
|---|---|---|---|---|---|
| Achyranthes aspera L. | Amaranthaceae | Herb | Plurireg | Th | N |
| Adiantum venustum D. Don | Pteridaceae | Herb | Cosm | G | N |
| Ajuga bracteosa Wall. ex Benth. | Lamiaceae | Herb | Unireg | H | Mi |
| Amaranthus viridis L. | Amaranthaceae | Herb | Cosm | Th | Mi |
| Arabidopsis thaliana (L.) Heynh. | Brassicaceae | Herb | Cosm | Th | L |
| Arenaria serpyllifolia L. | Caryophyllaceae | Herb | Cosm | Th | N |
| Artemisia vulgaris L. | Asteraceae | Herb | Plurireg | Th | N |
| Asplenium trichomanes L. | Aspleniaceae | Herb | Bireg | H | N |
| Astragalus grahamianus Benth. | Fabaceae | Herb | Unireg | Ch | L |
| Atropa acuminata Royle ex Lindl. | Solanaceae | Herb | Plurireg | G | Mi |
| Berberis lycium Royle | Berberidaceae | Shrub | Unireg | NPh | L |
| Bidens cernua L. | Asteraceae | Herb | Bireg | H | Mi |
| Buddleja asiatica Lour. | Scrophulariaceae | Shrub | Cosm | NPh | Mi |
| Calamintha vulgaris (L.) H. Karst. | Lamiaceae | Herb | Plurireg | Th | N |
| Chenopodium album L. | Chenopodiaceae | Herb | Cosm | H | N |
| Convolvulus arvensis L. | Convolvulaceae | Herb | Cosm | H | N |
| Erigeron canadensis L. | Asteraceae | Herb | Cosm | Th | L |
| Cynodon dactylon (L.) Pers. | Poaceae | Herb | Plurireg | H | L |
| Daphne mucronata Royle | Thymelaeaceae | Shrub | Bireg | NPh | N |
| Dioscorea deltoidea Wall. ex Griseb. | Dioscoreaceae | Herb | Unireg | H | Mi |
| Dodonaea viscosa Jacq. | Sapindaceae | Shrub | Cosm | NPh | Mi |
| Dryopteris stewartii Fraser-Jenk. | Dryopteridaceae | Herb | Bireg | G | Me |
| Elaeagnus angustifolia L. | Elaeagnaceae | Shrub | Bireg | NPh | Mi |
| Festuca gigantea (L.) Vill. | Poaceae | Herb | Bireg | G | L |
| Fragaria indica Andrews | Rosaceae | Herb | Plurireg | H | N |
| Galium asperifolium Wall. | Rubiaceae | Herb | Unireg | Th | L |
| Hibiscus syriacus L. | Malvaceae | Shrub | Plurireg | NPh | Mi |
| Indigofera gerardiana Graham ex Baker | Fabaceae | Herb | Unireg | NPh | N |
| Indigofera heterantha Wall. ex Brandis | Fabaceae | Herb | Unireg | NPh | L |
| Isodon rugosus (Wall. ex Benth.) Codd. | Lamiaceae | Herb | Unireg | NPh | Mi |
| Justicia adathoda L. | Lamiaceae | Herb | Plurireg | NPh | Me |
| Lamium album L. | Lamiaceae | Herb | Unireg | Th | Mi |
| Lespedeza juncea (L. f.) Pers. | Fabaceae | Herb | Unireg | H | N |
| Lotus corniculatus L. | Fabaceae | Herb | Bireg | H | Mi |
| Malva neglecta Wallr. | Malvaceae | Herb | Unireg | H | Mi |
| Medicago lupulina L. | Fabaceae | Herb | Plurireg | Ch | N |
| Myrsine africana L. | Primulaceae | Shrub | Bireg | NPh | L |
| Origanum vulgare L. | Lamiaceae | Herb | Unireg | Th | N |
| Otostegia limbata (Benth.) Boiss. | Lamiaceae | Herb | Unireg | NPh | L |
| Oxytropis mollis Royle ex Benth. | Fabaceae | Herb | Unireg | H | L |
| Plantago lanceolata L. | Plantiganaceae | Herb | Plurireg | H | Mi |
| Plantago major L. | Plantaginaceae | Herb | Plurireg | H | Mi |
| Polygonatum verticillatum (L.) All. | Asparagaceae | Herb | Plurireg | Th | N |
| Pteris cretica L. | Pteridaceae | Herb | Plurireg | G | Mi |
| Ranunculus laetus Salisb. | Ranunculaceae | Herb | Unireg | H | Me |
| Rosa webbiana Wall. ex Royle | Rosaceae | Herb | Plurireg | NPh | N |
| Rumex denticulatus K. Koch. | Polygonaceae | Herb | Bireg | H | Me |
| Rumex nepalensis Spreng. | Polygonaceae | Herb | Bireg | H | Me |
| Sarcococca saligna (D.Don) Müll.Arg. | Buxaceae | Shrub | Bireg | NPh | Mi |
| Smilax lanceolata L. | Smilacaceae | Herb | Bireg | L | Mi |
| Solidago virgaurea L. | Asteraceae | Herb | Unireg | H | Mi |
| Tagetes minuta L. | Asteraceae | Herb | Bireg | Th | Mi |
| Taraxacumofficinale (L.) Weber ex F.H.Wigg. | Asteraceae | Herb | Cosm | H | Mi |
| Trifolium repens L. | Fabaceae | Herb | Cosm | G | N |
| Viburnum grandiflorum Wall. ex DC. | Viburnaceae | Shrub | Unireg | NPh | Ma |
| Violaserpens Wall. ex Ging. | Violaceae | Shrub | Plurireg | G | Mi |
| Youngia japonica (L.) DC | Asteraceae | Herb | Cosm | Th | Mi |
| Zanthoxylum armatum DC. | Rutaceae | Tree | Cosm | MPh | Mi |
| Species Name | Code | Cluster I | Cluster II | Cluster III |
|---|---|---|---|---|
| M ± SE | M ± SE | M ± SE | ||
| Achyranthes aspera L. | As | 2.41 ± 2.41 | - | - |
| Adiantum venustum D. Don | Av | - | - | 0.77 ± 0.77 |
| Ajuga bracteosa Wall. ex Benth. | Ab | 3.87 ± 2.65 | - | - |
| Amaranthus viridis L. | Am | 5.10 ± 3.23 | - | 2.41 ± 1.44 |
| Arabidopsis thaliana (L.) Heynh. | At | - | - | 1.16 ± 0.79 |
| Arenaria serpyllifolia L. | Am | - | - | 0.53 ± 0.53 |
| Artimisia vulgaris L. | Av | 7.2 ± 4.71 | - | - |
| Asplenium trichomanes L. | As | 9.14 ± 4.71 | 4.23 ± 4.23 | 6.6 ± 2.6 |
| Astragalus grahamianus Benth. | Ag | - | - | 0.53 ± 0.53 |
| Atropa acuminata Royle ex Lindl. | Aa | 3.4 ± 3.46 | - | 2.42 ± 2.43 |
| Berberis lycium Royle | B | 9.86 ± 5.49 | 7.66 ± 4.91 | 7.82 ± 3.54 |
| Bidens cernua L. | Bc | - | 1.71 ± 1.71 | - |
| Buddleja asiatica Lour. | Ba | 0.93 ± 0.93 | - | 1.69 ± 1.17 |
| Calamintha vulgaris (L.) H. Karst. | Cv | - | 11.5 ± 4.11 | 3.46 ± 1.66 |
| Chenopodium album L. | Ca | - | 4.4 ± 4.42 | - |
| Convolvulus arvensis L. | Ca | 2.5 ± 2.52 | 4.2 ± 2.74 | 6.87 ± 1.76 |
| Erigeron canadensis L. | Co | 0.85 ± 0.85 | - | - |
| Cynodon dactylon (L.) Pers. | Cd | 7 ± 5 | 6.17 ± 6.17 | 4.88 ± 3.32 |
| Daphne mucronata Royle | Dm | 3.83 ± 3.83 | - | 1.94 ± 1.34 |
| Dioscorea deltoidea Wall. ex Griseb. | Dd | 1.83 ± 1.83 | - | - |
| Dodonaea viscosa Jacq. | Do | 1.42 ± 1.42 | - | - |
| Dryopteris stewartii Fraser-Jenk. | Ds | - | 9.86 ± 9.86 | - |
| Elaeagnus angustifolia L. | Ea | 2.3 ± 2.3 | - | - |
| Festuca gigantea (L.) Vill. | Fg | - | - | 0.46 ± 0.46 |
| Fragaria indica Andrews | Fi | - | 2.71 ± 2.71 | 5.53 ± 2.22 |
| Galium asperifolium Wall. | Ga | 4.36 ± 4.36 | - | 1.34 ± 1.34 |
| Hibiscus syriacus L. | Hs | - | - | 0.6 ± 0.62 |
| Indigofera gerardiana Graham ex Baker | Ig | - | - | 3.65 ± 2.86 |
| Indigofera heterantha Wall. ex Brandis | Ih | 8.93 ± 8.93 | - | - |
| Isodon rugosus (Wall. ex Benth.) Codd | Ir | - | 8.2 ± 8.2 | - |
| justica adathoda L. | Ja | - | - | 1.96 ± 1.45 |
| Lamium album L. | La | - | - | 0.53 ± 0.53 |
| Lespedeza juncea (L. f.) Pers. | Lj | 5.02 ± 5.02 | - | 2.91 ± 2.91 |
| Lotus corniculatus L. | Lc | 2.5 ± 2.5 | - | 2.52 ± 1.57 |
| Malva neglecta Wallr. | Mn | - | - | 1.06 ± 1.06 |
| Medicago lupulina L. | Ml | - | - | 1.21 ± 1.21 |
| Myrsine africana L. | Ma | 4.5 ± 3.7 | 1.85 ± 1.85 | 5.96 ± 2.53 |
| Origanum vulgare L. | Ov | - | 2.6 ± 2.68 | - |
| Otostegia limbata (Benth.) Boiss | Ol | - | - | 1.60 ± 1.13 |
| Oxytropis mollis Royle ex Benth. | Om | 3.63 ± 3.63 | 1.71 ± 1.71 | 3.36 ± 1.97 |
| Plantago lanceolata L. | Pl | 2.3 ± 2.3 | 9.34 ± 6.15 | 6.05 ± 3.29 |
| Plantago major L. | Pm | - | 5.9 ± 4 | 0.46 ± 0.46 |
| Polygonatum verticillatum (L.) All. | Pv | - | 1.71 ± 1.71 | - |
| Pteris cretica L. | Pc | - | - | 1.67 ± 1.18 |
| Ranunculus laetus Salisb. | Rl | - | - | 0.93 ± 0.93 |
| Rosa webbiana Wall. ex Royle | Rw | - | - | - |
| Rumex denticulatus K. Koch | Ru | - | - | 4.49 ± 2.57 |
| Rumex nepalensis Spreng. | Rn | - | 3.57 ± 3.57 | 0.53 ± 0.53 |
| Sarcococca saligna (D.Don) Müll.Arg. | Ss | - | - | 0.53 ± 0.53 |
| Smilax lanceolata L. | Sl | 1 ± 12 | - | 3.08 ± 1.79 |
| Solidago virgaurea L. | Sv | - | - | 0.53 ± 0.53 |
| Tagetes minuta L. | Tg | 5.02 ± 5.02 | - | 1.21 ± 1.21 |
| Taraxacumofficinale (L.) Weber ex F.H.Wigg. | To | - | - | 0.6 ± 0.61 |
| Trifolium repens L. | Tr | - | - | 1.36 ± 0.95 |
| Viburnum grandiflorum Wall. ex DC. | Vg | - | - | 2.28 ± 2.28 |
| Violaserpens Wall. ex Ging. | Vs | - | - | 1.35 ± 0.94 |
| Youngia japonica (L.) DC. | Yj | - | 2.7 ± 2.72 | - |
| Zanthoxylum armatum DC. | Za | - | - | 1.06 ± 1.06 |
| Indexes | Group I | Group II | Group III | F | p |
|---|---|---|---|---|---|
| Mean ± SE | Mean ± SE | Mean ± SE | |||
| Total number of Species (S) | 5.87 ± 0.66 b | 4 ± 0.37 a | 10.32 ± 1.5 b | 4.85 | 0.015 |
| Shannon–Wiener Index (H) | 1.84 ± 0.19 a | 1.3 ± 0.11 a | 2.74 ± 0.20 b | 13.93 | 6.97 × 10−5 |
| Pielous Index (J) | 0.97 ± 0.01 | 0.95 ± 0.01 | 0.93 ± 0.01 | 2.09 | 0.14 |
| Margalef Index (M) | 2.9 ± 0.33 a | 1.4 ± 0.21 a | 3.95 ± 0.42 b | 9.48 | 0.00076 |
| Simpson Index (1/D) | 7.13 ± 1.10 a | 3.68 ± 0.42 b | 6 ± 0.61 a | 3.61 | 0.04 |
| Beta diversity (B = S/a) | 6.41 ± 1.27 a | 3.68 ± 0.43 a | 9.85 ± 0.54 b | 16.45 | 2.13 × 10−5 |
| Axis | Axis 1 | Axis 2 | Axis 3 |
|---|---|---|---|
| Eigenvalue | 33.27 | 25.12 | 23.96 |
| Variance in species data | |||
| % of variance explained | 12.42 | 9.41 | 9 |
| Cumulative % explained | 12.41 | 21.81 | 30.82 |
| Pearson Correlation Response-Pred. * | 1.00 | 0.98 | 1 |
| Kendall Correlation Response-Pred. | 0.96 | 0.81 | 0.99 |
| Environmental Variables | Code | Group I | Group II | Group III | F-Value | p-Value |
|---|---|---|---|---|---|---|
| Mean ± SE | Mean ± SE | Mean ± SE | ||||
| Latitude (°) | Lat | 35.0 ± 0.12 a | 34.9694 ± 0.19 b | 34.58 ± 0.050 b | 5.57 | 0.009 |
| Longitude (°) | Long | 72.28 ± 0.03 | 72.29 ± 0.023 | 72.25 ± 0.016 | 1.72 | 0.19 |
| Elevation (m) | Elev | 2017.75 ± 205.23 a | 2289.5 ± 240.22 b | 1659.4 ± 64.48 a | 4.87 | 0.015 |
| Slope (°) | Slope | 41.8 ± 1.81 | 41.14 ± 2.55 | 38.66 ± 1.14 | 1.15 | 0.33 |
| Clay (%) | CLY | 16.37 ± 1.17 a | 17.5 ± 2.2 a | 12.11 ± 1.02 b | 4.65 | 0.01 |
| Silt (%) | SLT | 24.44 ± 2.20 | 26 ± 3.6 | 29.03 ± 2.74 | 0.66 | 0.52 |
| Sand (%) | SND | 59.18 ± 2.66 | 56.38 ± 3.42 | 58.85 ± 3.08 | 0.17 | 0.84 |
| pH (1:5) | pH | 6.4 ± 0.12 | 6.6 ± 0.17 | 6.26 ± 0.13 | 1.25 | 0.30 |
| Organic matter (%) | OM | 2.21 ± 0.78 | 4.62 ± 0.76 | 2.61 ± 0.54 | 2.92 | 0.07 |
| Lime (%) | L | 3.16 ± 0.52 | 4.06 ± 0.61 | 3.61 ± 0.38 | 0.66 | 0.52 |
| Nitrogen (mg/Kg) | N | 0.12 ± 0.04 | 0.18 ± 0.03 | 0.16 ± 0.03 | 0.503 | 0.60 |
| Phosphorus (mg/Kg) | P | 8.24 ± 0.63 | 7.44 ± 1.12 | 12.94 ± 4.46 | 0.61 | 0.54 |
| Potassium (mg/Kg) | K | 146.25 ± 27.07 | 233.42 ± 37.52 | 186.33 ± 26.33 | 1.55 | 0.22 |
| Wilting point (mL/gm) | WP | 0.114 ± 0.42 | 0.11 ± 0.09 | 0.09 ± 0.004 | 4.36 | 0.02 |
| Field capacity (mL/gm) | FC | 0.22 ± 0.61 | 0.23 ± 0.09 | 0.21 ± 0.07 | 1.31 | 0.28 |
| Bulk density (g/cm3) | BD | 1.4 ± 0.11 a | 1.46 ± 0.02 a | 1.52 ± 0.01 ab | 3.43 | 0.04 |
| Saturation Point (0 kPa) | SP | 0.44 ± 0.52 a | 0.44 ± 0.008 a | 0.42 ± 0.06 ab | 3.41 | 0.04 |
| Electrical Conductivity (mS/m) | EC | 12.41 ± 1.63 a | 12.8 ± 0.03 a | 23.46 ± 2.80 b | 5.44 | 0.01 |
| Available water (%) | AW | 0.10 ± 0.38 | 0.11 ± 0.005 | 0.11 ± 0.04 | 0.46 | 0.63 |
| Temperature minimum (°C) | Tpmi | 14.30 ± 1.92 | 11.88 ± 0.37 | 11.89 ± 0.17 | 2.13 | 0.14 |
| Temperature maximum (°F) | Tpma | 28.98 ± 8.75 | 27.89 ± 5.33 | 36.46 ± 5.75 | 2.11 | 0.14 |
| Precipitation (mm) | Preci | 49.84 ± 1.21 | 49.16 ± 1.86 | 51.84 ± 0.97 | 1.32 | 0.28 |
| Relative humidity (%) | RH | 49.84 ± 1.22 | 49.16 ± 1.87 | 51.84 ± 0.98 | 1.32 | 0.28 |
| Water Holding capacity (%) | WHC | 52.12 ± 1.82 | 54.82 ± 1.98 | 53.47 ± 2.89 | 0.18 | 0.83 |
| S. No | Variables | Correlation | Biplot Scores | ||||
|---|---|---|---|---|---|---|---|
| Axis 1 | Axis 2 | Axis 3 | Axis 1 | Axis 2 | Axis 3 | ||
| 1 | Lat | −0.06 | −0.49 ** | −0.02 | −0.55 | −3.94 | −0.155 |
| 2 | Long | −0.19 | 0.18 | −0.22 | −1.81 | 1.48 | −1.74 |
| 3 | E | 0.23 | −0.39 * | 0.06 | 2.18 | −3.09 | 0.518 |
| 4 | ASP | 0.08 | −0.09 | 0.16 | 0.74 | −0.74 | 1.25 |
| 5 | CLY | 0.19 | −0.36 * | −0.19 | 1.77 | −2.92 | −1.48 |
| 6 | SLT | −0.20 | 0.29 | 0.38 * | −1.83 | 2.28 | 2.95 |
| 7 | SND | 0.09 | −0.09 | −0.26 | 0.85 | −0.72 | −2.03 |
| 8 | pH | 0.22 | −0.22 | −0.21 | 1.97 | −1.72 | −1.63 |
| 9 | OM | 0.09 | 0.16 | −0.003 | 0.88 | 1.26 | −0.02 |
| 10 | L | 0.21 | 0.17 | −0.39 ** | 1.96 | 1.32 | −3.05 |
| 11 | N | 0.22 | 0.09 | −0.02 | 1.82 | 0.75 | −0.18 |
| 12 | P | 0.007 | −0.13 | 0.20 | 0.07 | −1.06 | 1.56 |
| 13 | K | 0.36 | −0.04 | −0.12 | 3.31 | −0.31 | −0.96 |
| 14 | WP | 0.18 | −0.37 | −0.18 | 1.66 | −2.96 | −1.39 |
| 15 | FC | 0.03 | −0.13 | 0.09 | 0.27 | −1.05 | 0.72 |
| 16 | BD | −0.10 | 0.30 | 0.05 | −0.93 | 2.37 | 0.42 |
| 17 | SP | 0.104 | −0.31 | −0.05 | 0.95 | −2.38 | −0.37 |
| 18 | EC | −0.121 | 0.43 ** | 0.13 | −1.11 | 3.41 | 1.004 |
| 19 | AW | −0.176 | 0.25 | 0.38 | −1.60 | 1.97 | 2.92 |
| 20 | TIVI | −0.63 ** | −0.47 ** | 0.02 | −5.83 | −3.79 | 0.17 |
| 21 | QIVI | 0.162 | −0.41 ** | 0.13 | 1.48 | −3.28 | 0.97 |
| 22 | BA | −0.025 | −0.11 | 0.22 | −0.23 | −0.88 | 1.55 |
| 23 | D/ha | 0.112 | −0.22 | −0.17 | 1.02 | −1.78 | −1.28 |
| 24 | TpMi | −0.246 | −0.09 | −0.04 | −2.24 | −0.77 | −0.34 |
| 25 | TpMa | −0.185 | 0.01 | 0.32 | −1.68 | 0.11 | 2.48 |
| 26 | Preci | 0.259 | −0.09 | 0.03 | 2.36 | −0.71 | 0.24 |
| 27 | RH | 0.102 | −0.05 | −0.04 | 0.935 | −0.37 | −0.35 |
| 28 | WHC | −0.273 | 0.005 | 0.079 | −2.49 | 0.04 | 0.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, A.; Khan, N.; Ali, K.; Ullah, R.; Khan, M.E.H.; Jones, D.A.; Rahman, I.U. Plant Species Classification and Diversity of the Understory Vegetation in Oak Forests of Swat, Pakistan. Appl. Sci. 2021, 11, 11372. https://doi.org/10.3390/app112311372
Rahman A, Khan N, Ali K, Ullah R, Khan MEH, Jones DA, Rahman IU. Plant Species Classification and Diversity of the Understory Vegetation in Oak Forests of Swat, Pakistan. Applied Sciences. 2021; 11(23):11372. https://doi.org/10.3390/app112311372
Chicago/Turabian StyleRahman, Ataur, Nasrullah Khan, Kishwar Ali, Rafi Ullah, Muhammad Ezaz Hasan Khan, David Aaron Jones, and Inayat Ur Rahman. 2021. "Plant Species Classification and Diversity of the Understory Vegetation in Oak Forests of Swat, Pakistan" Applied Sciences 11, no. 23: 11372. https://doi.org/10.3390/app112311372
APA StyleRahman, A., Khan, N., Ali, K., Ullah, R., Khan, M. E. H., Jones, D. A., & Rahman, I. U. (2021). Plant Species Classification and Diversity of the Understory Vegetation in Oak Forests of Swat, Pakistan. Applied Sciences, 11(23), 11372. https://doi.org/10.3390/app112311372








