Morphophysiological Changes in Staphylococcus aureus Biofilms Treated with Plasma-Activated Hydrogen Peroxide Solution
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Formation of Biofilms
2.2. Preparation of PAH and Sample Treatments
2.3. Determination of Total Amount of Biofilms
2.4. Biofilm Morphology Image
2.5. Laser Scanning Confocal Microscopy (LSCM)
2.6. The Metabolic Capacity of Biofilms
2.7. ROS in S. aureus Biofilms
2.8. Bacterial DNA Isolation and Analysis
2.9. Statistical Analysis
3. Results and Discussion
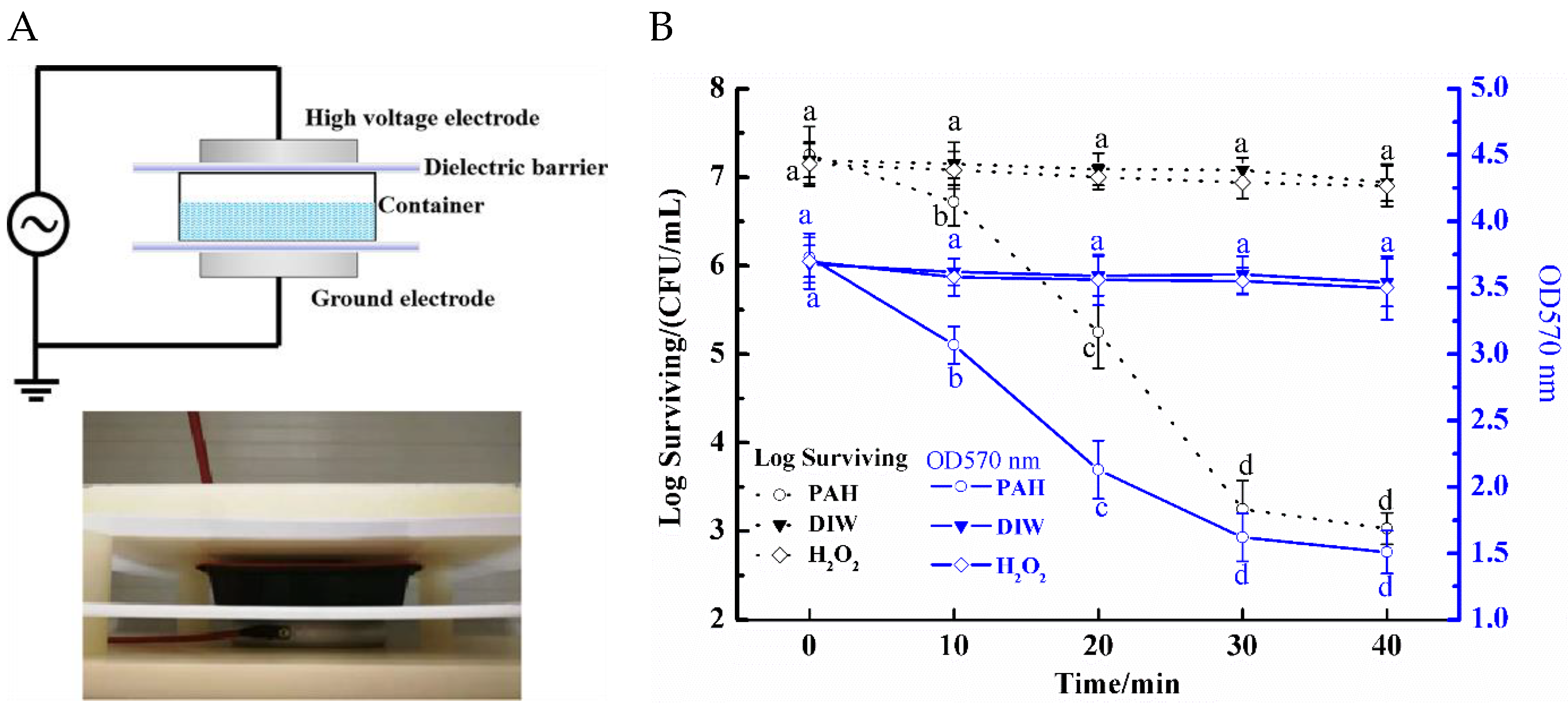
3.1. Anti-Biofilm Efficiency of PAH
3.2. Changes in the Appearance of S. aureus Biofilms
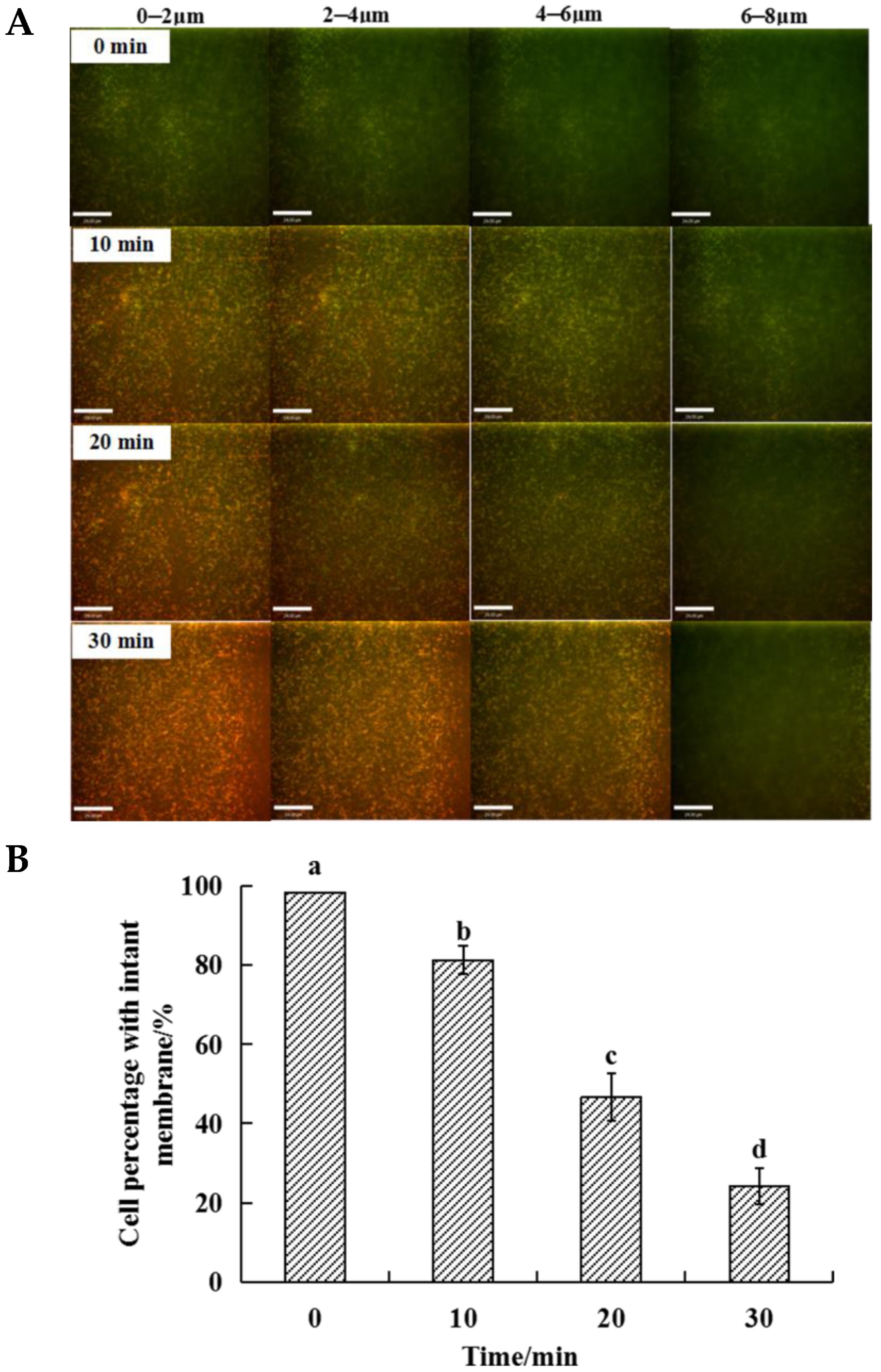
3.3. Changes in the Thickness of S. aureus Biofilms
3.4. Changes in Metabolic Capacity
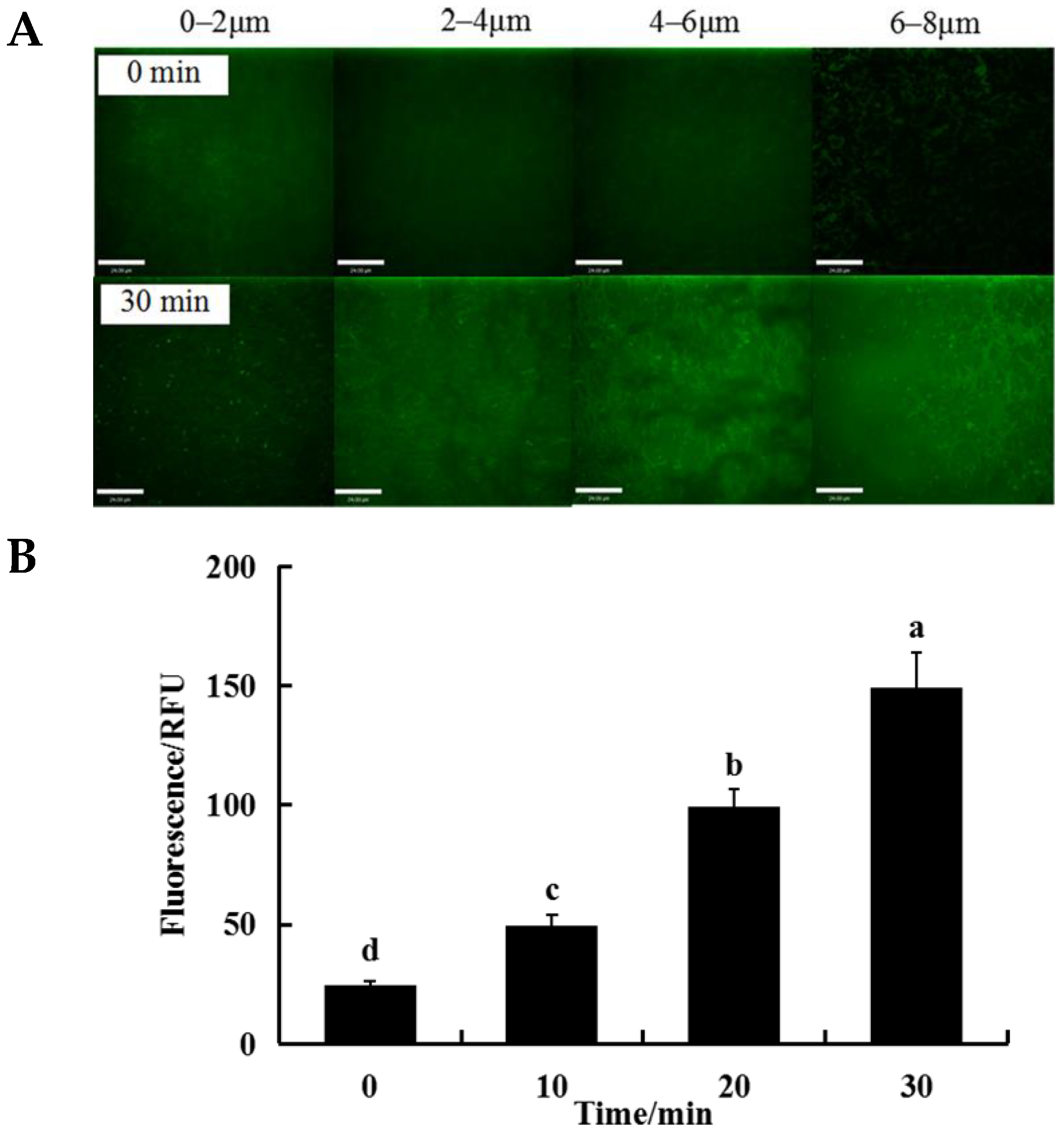
3.5. Changes in ROS Levels of Bacteria Cells within the Biofilm
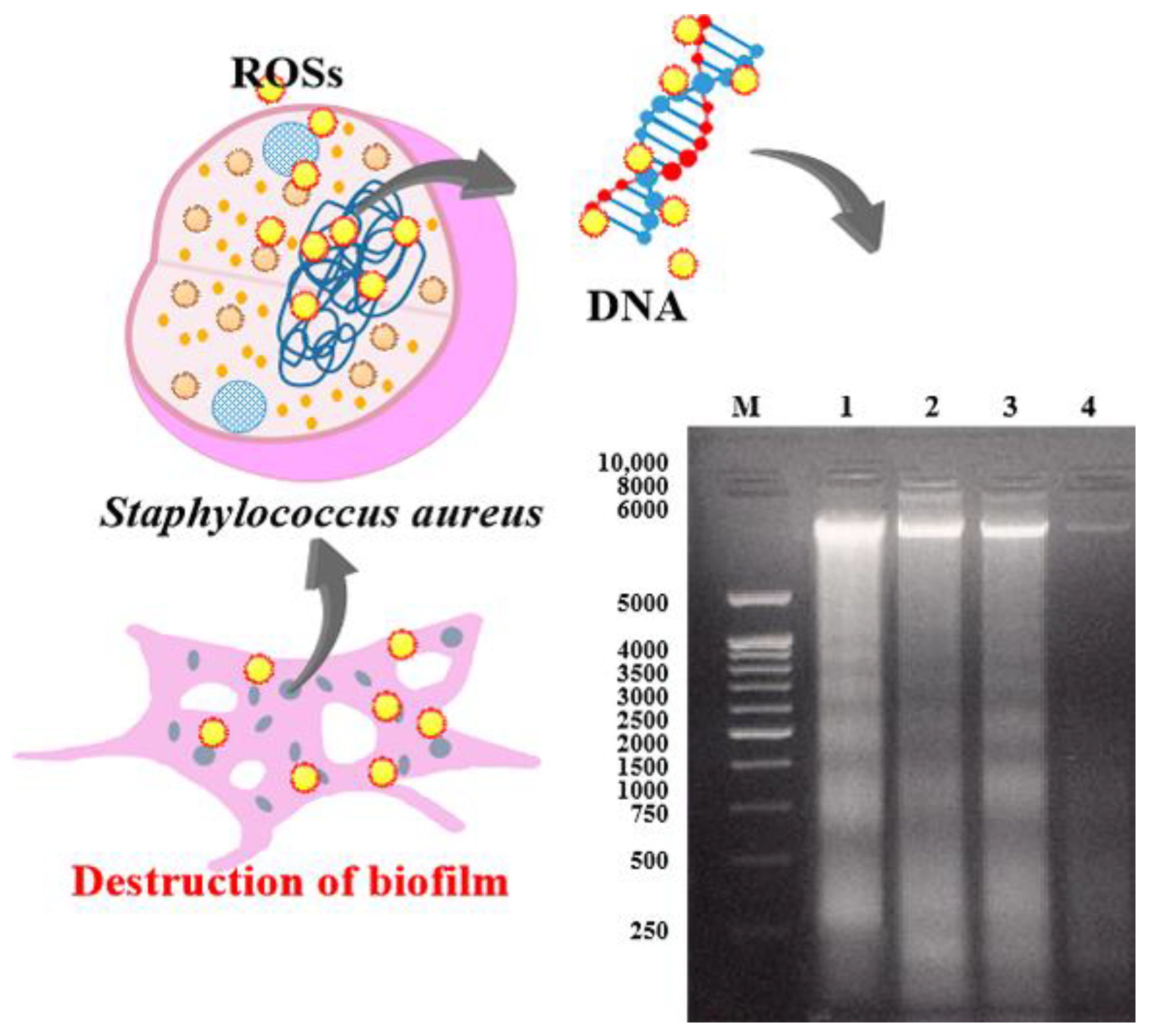
3.6. Changes in DNA Integrity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiong, Z.; Du, T.; Lu, X.; Cao, Y.; Pan, Y. How deep can plasma penetrate into a biofilm? Appl. Phys. Lett. 2011, 98, 221503. [Google Scholar] [CrossRef]
- Ziuzina, D.; Boehm, D.; Patil, S.; Cullen, P.J.; Bourke, P. Cold plasma inactivation of bacterial biofilms and reduction of quorum sensing regulated virulence factors. PLoS ONE 2015, 10, 0138209. [Google Scholar] [CrossRef]
- Byun, K.H.; Han, S.H.; Yoon, J.w.; Park, S.H.; Ha, S.D. Efficacy of chlorine-based disinfectants (sodium hypochlorite and chlorine dioxide) on Salmonella Enteritidis planktonic cells, biofilms on food contact surfaces and chicken skin. Food Control 2021, 123, 107838. [Google Scholar] [CrossRef]
- Ellebracht, J.W.; King, D.A.; Castillo, A.; Lucia, L.M.; Acuff, G.R.; Harris, K.B.; Savell, J.W. Evaluation of peroxyacetic acid as a potential pre-grinding treatment for control of Escherichia coli O157:H7 and Salmonella Typhimurium on beef trimmings. Meat Sci. 2005, 70, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Haney, E.F.; Trimble, M.J.; Cheng, J.T.; Valle, Q.; Hancock, R.E.W. Critical assessment of methods to quantify biofilm growth and evaluate antibiofilm activity of host defence peptides. Biomolecules 2018, 8, 29. [Google Scholar] [CrossRef] [Green Version]
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, F.J.; Qu, F.; Ling, Y.; Mao, P.Y.; Xia, P.Y.; Chen, H.P.; Zhou, D.S. Biofilm-associated infections: Antibiotic resistance and novel therapeutic strategies. Future Microbiol. 2013, 8, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Homoe, P.; Bjarnsholt, T.; Wessman, M.; Sorensen, H.C.F.; Johansen, H.K. Morphological evidence of biofilm formation in Greenlanders with chronic suppurative otitis media. Eur. Arch. Oto-Rhino-L 2009, 266, 1533–1538. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, C.; Cui, H.; Lin, L. Feasibility of cold plasma for the control of biofilms in food industry. Trends Food Sci. Technol. 2020, 99, 142–151. [Google Scholar] [CrossRef]
- Srey, S.; Jahid, I.K.; Ha, S.D. Biofilm formation in food industries: A food safety concern. Food Control 2013, 31, 572–585. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Keener, K.M. Cold plasma: Background, applications and current trends. Curr. Opin. Food Sci. 2017, 16, 49–52. [Google Scholar] [CrossRef]
- Oehmigen, K.; Winter, J.; Hähnel, M.; Wilke, C.; Brandenburg, R.; Weltmann, K.-D.; von Woedtke, T. Estimation of Possible Mechanisms of Escherichia coli Inactivation by Plasma Treated Sodium Chloride Solution. Plasma Process. Polym. 2011, 8, 904–913. [Google Scholar] [CrossRef]
- Han, L.; Patil, S.; Boehm, D.; Milosavljevic, V.; Cullen, P.J.; Bourke, P. Mechanisms of inactivation by high-voltage atmospheric cold plasma differ for Escherichia coli and Staphylococcus aureus. Appl. Environ. Microbiol. 2015, 82, 450–458. [Google Scholar] [CrossRef] [Green Version]
- Traba, C.; Liang, J.F. Susceptibility of Staphylococcus aureus biofilms to reactive discharge gases. Biofouling 2011, 27, 763–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ercan, U.K.; Wang, H.; Ji, H.; Fridman, G.; Brooks, A.D.; Joshi, S.G. Nonequilibrium plasma-activated antimicrobial solutions are broad-spectrum and retain their efficacies for extended period of time. Plasma Process. Polym. 2013, 10, 544–555. [Google Scholar] [CrossRef]
- Fernandez, A.; Shearer, N.; Wilson, D.R.; Thompson, A. Effect of microbial loading on the efficiency of cold atmospheric gas plasma inactivation of Salmonella enterica serovar Typhimurium. Int. J. Food Microbiol. 2012, 152, 175–180. [Google Scholar] [CrossRef]
- Tian, Y.; Ma, R.; Zhang, Q.; Feng, H.; Liang, Y.; Zhang, J.; Fang, J. Assessment of the physicochemical properties and biological effects of water activated by non-thermal plasma above and beneath the water surface. Plasma Process. Polym. 2015, 12, 439–449. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, Y.; Wang, J.; Yu, W.; Wang, W.; Ma, X. Monitoring of cell viability and proliferation in hydrogel-encapsulated system by resazurin assay. Appl. Biochem. Biotechnol. 2010, 162, 1996–2007. [Google Scholar] [CrossRef]
- Pan, Y.Y.; Zhang, Y.; Cheng, J.H.; Sun, D.W. Inactivation of Listeria monocytogenes at various growth temperatures by ultrasound pretreatment and cold plasma. LWT-Food Sci. Technol. 2020, 118, 108635. [Google Scholar] [CrossRef]
- Lukes, P.; Dolezalova, E.; Sisrova, I.; Clupek, M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: Evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sources Sci. Technol. 2014, 23, 015019. [Google Scholar] [CrossRef]
- Park, J.H.; Kumar, N.; Park, D.H.; Yusupov, M.; Neyts, E.C.; Verlackt, C.C.; Bogaerts, A.; Kang, M.H.; Uhm, H.S.; Choi, E.H.; et al. A comparative study for the inactivation of multidrug resistance bacteria using dielectric barrier discharge and nano-second pulsed plasma. Sci. Rep. 2015, 5, 13849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, J.; Zhuang, H.; Nasiru, M.M.; Muhammad, U.; Zhang, J.; Yan, W. Action of plasma-activated lactic acid on the inactivation of inoculated Salmonella Enteritidis and quality of beef. Innov. Food Sci. Emerg. Technol. 2019, 57, 102196. [Google Scholar] [CrossRef]
- Hertwig, C.; Reineke, K.; Ehlbeck, J.; Knorr, D.; Schluter, O. Decontamination of whole black pepper using different cold atmospheric pressure plasma applications. Food Control 2015, 55, 221–229. [Google Scholar] [CrossRef]
- Duffy, G.; Sheridan, J.J. The effect of temperature, pH and medium in a surface adhesion immunofluorescent technique for detection of Listeria monocytogenes. J. Appl. Microbiol. 1997, 83, 95–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, R.; Zhou, R.; Wang, P.; Xian, Y.; Mai-Prochnow, A.; Lu, X.; Cullen, P.J.; Ostrikov, K.; Bazaka, K. Plasma-activated water: Generation, origin of reactive species and biological applications. J. Phys. D Appl. Phys. 2020, 53, 303001. [Google Scholar] [CrossRef]
- Alkawareek, M.Y.; Algwari, Q.T.; Laverty, G.; Gorman, S.P.; Graham, W.G.; O’Connell, D.; Gilmore, B.F. Eradication of Pseudomonas aeruginosa biofilms by atmospheric pressure non-thermal plasma. PLoS ONE 2012, 7, e44289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devi, Y.; Thirumdas, R.; Sarangapani, C.; Deshmukh, R.R.; Annapure, U.S. Influence of cold plasma on fungal growth and aflatoxins production on groundnuts. Food Control 2017, 77, 187–191. [Google Scholar] [CrossRef]
- Chauvin, J.; Judee, F.; Yousfi, M.; Vicendo, P.; Merbahi, N. Analysis of reactive oxygen and nitrogen species generated in three liquid media by low temperature helium plasma jet. Sci. Rep. 2017, 7, 4562. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Qian, J.; Luo, J.; Huang, M.; Yan, W.; Zhang, J. Morphophysiological Changes in Staphylococcus aureus Biofilms Treated with Plasma-Activated Hydrogen Peroxide Solution. Appl. Sci. 2021, 11, 11597. https://doi.org/10.3390/app112411597
Zhao J, Qian J, Luo J, Huang M, Yan W, Zhang J. Morphophysiological Changes in Staphylococcus aureus Biofilms Treated with Plasma-Activated Hydrogen Peroxide Solution. Applied Sciences. 2021; 11(24):11597. https://doi.org/10.3390/app112411597
Chicago/Turabian StyleZhao, Jianying, Jing Qian, Ji Luo, Mingming Huang, Wenjing Yan, and Jianhao Zhang. 2021. "Morphophysiological Changes in Staphylococcus aureus Biofilms Treated with Plasma-Activated Hydrogen Peroxide Solution" Applied Sciences 11, no. 24: 11597. https://doi.org/10.3390/app112411597






