Accurate Estimation of Bicarbonate and Acetic Acid Concentrations with Wider Ranges in Anaerobic Media Using Classical FOS/TAC Titration Method
Abstract
:1. Introduction
2. Materials and Methods
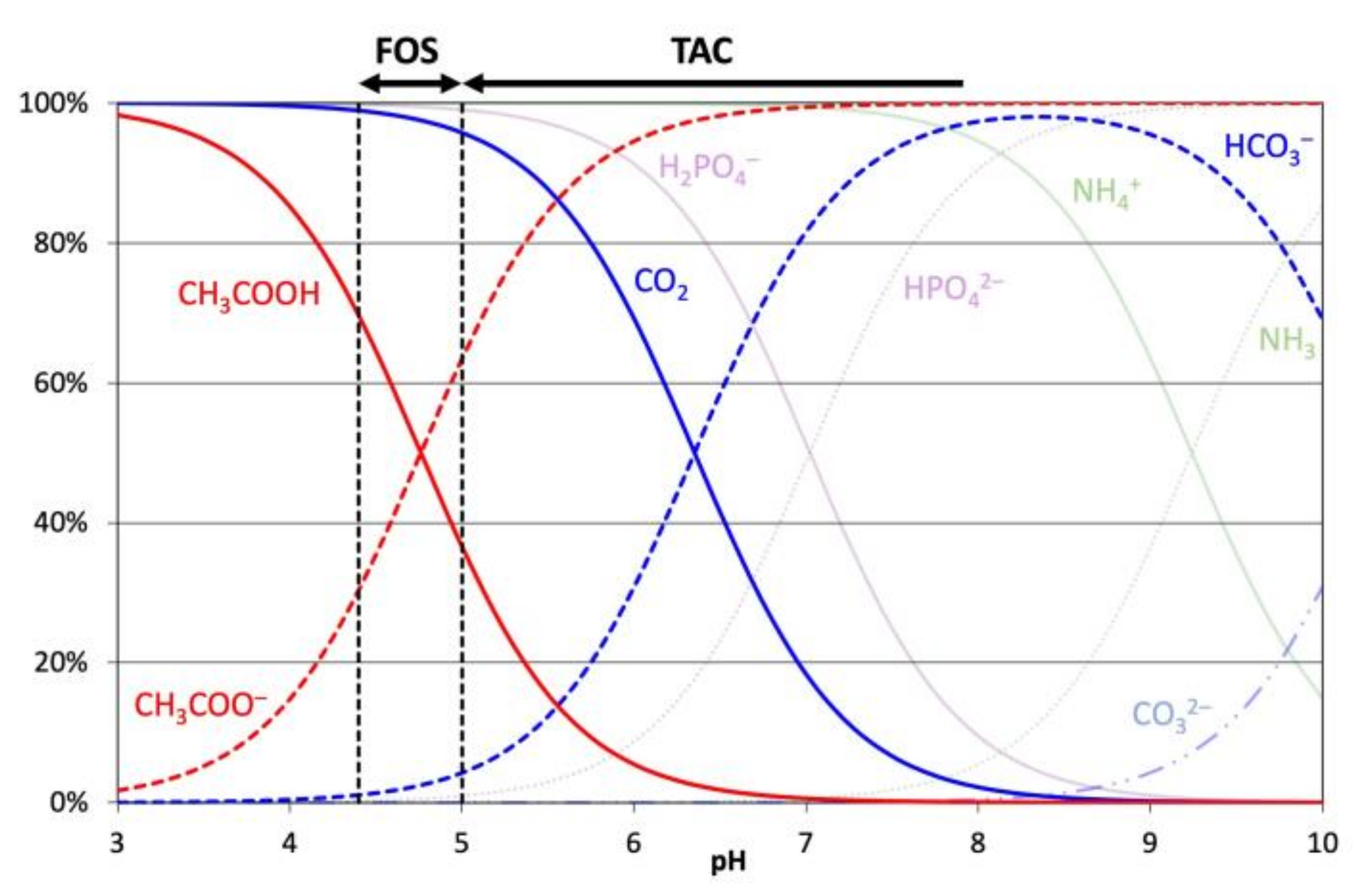
2.1. Nordmann’s FOS/TAC Titration
2.1.1. Presentation of the Method
2.1.2. Preparation of Sodium Bicarbonate and Sodium Acetate Solutions for FOS/TAC Titration
2.1.3. Experimental Realization
2.2. Simulation of FOS/TAC Titration Curve
2.3. Multiple Linear Regression of Simulated Results
2.4. Experimental Validation of the Proposed New Formulas
3. Results and Discussion
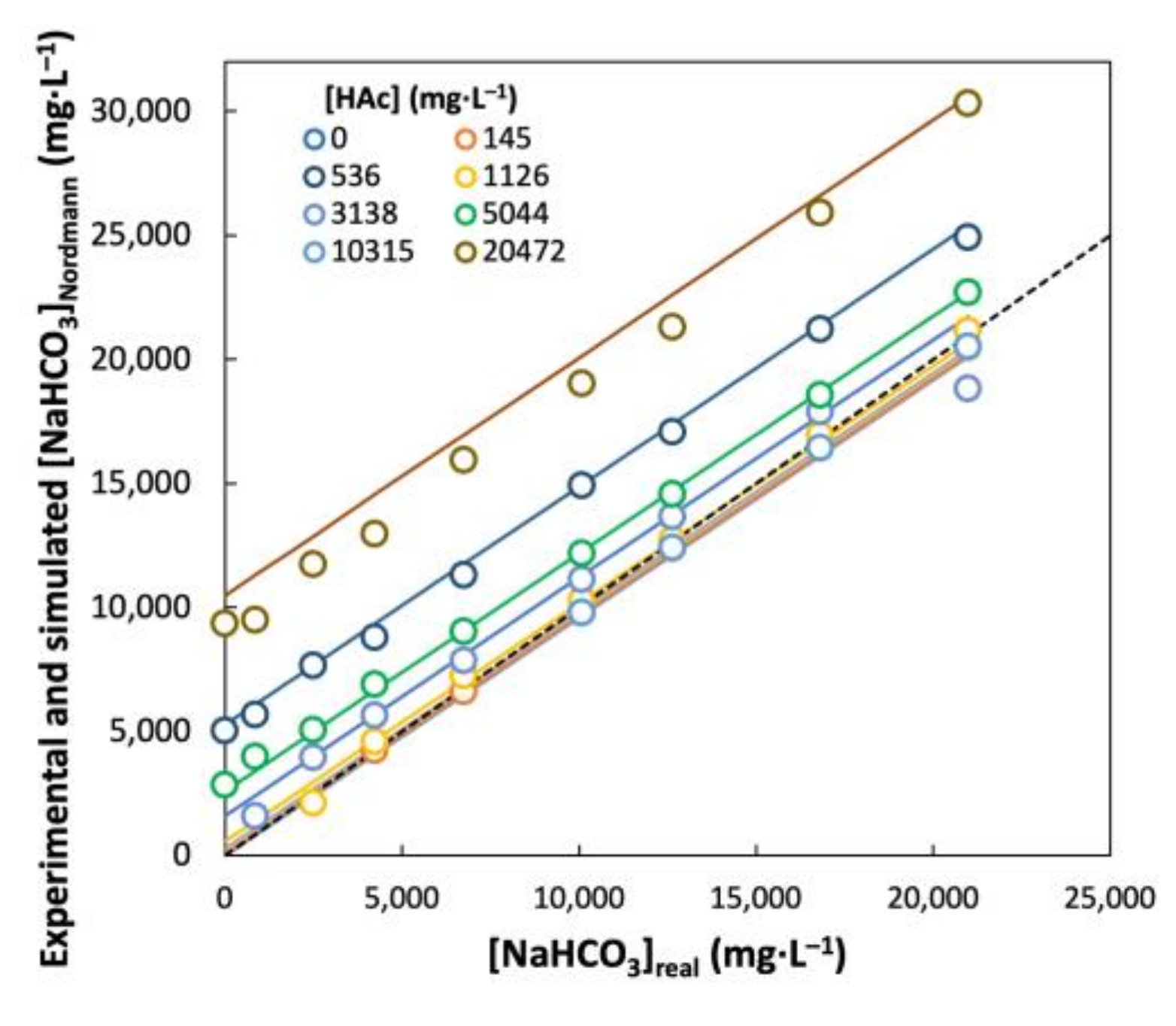
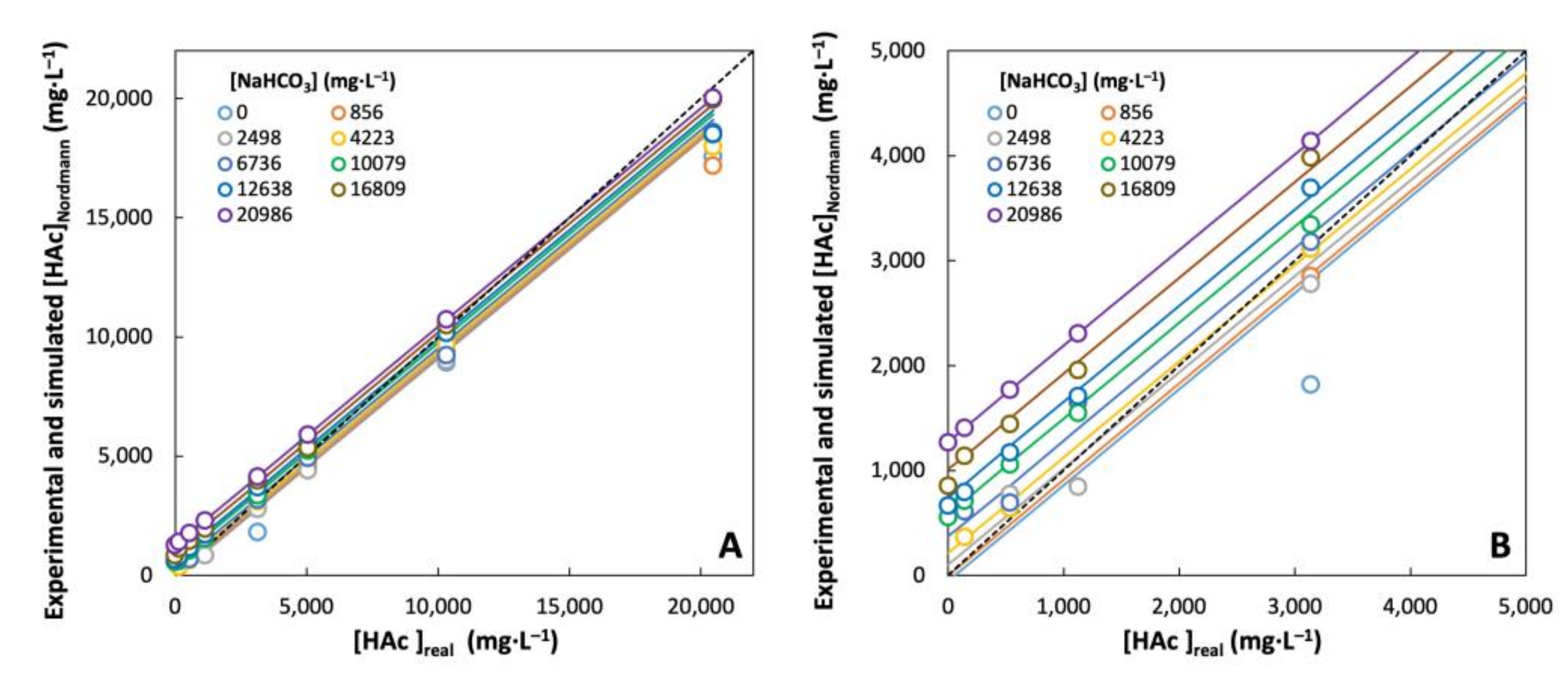
3.1. FOS/TAC Titration of Artificial Solutions with Pure NaHCO3 and NaAc
3.2. Simulation of Titration Curves
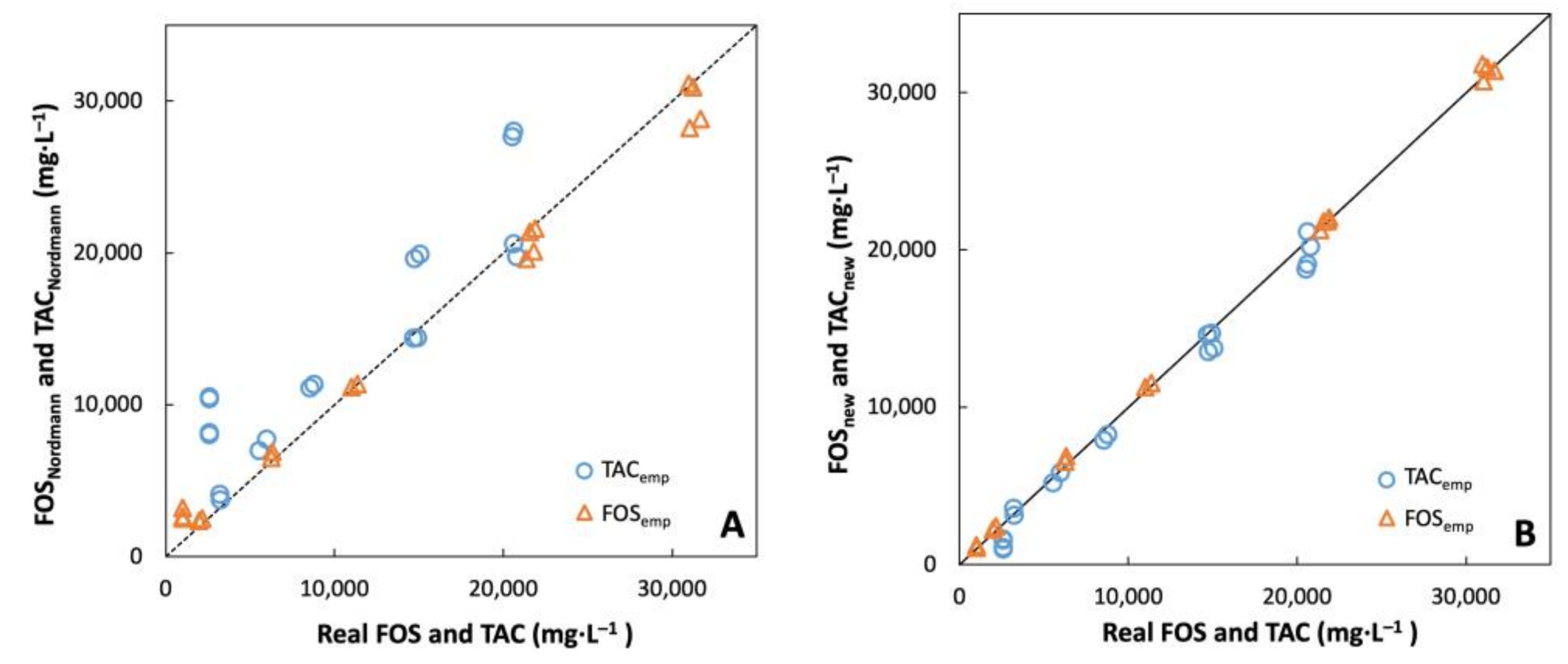
3.3. Proposing New Formulas for FOS and TAC Estimation
3.4. Experimental Validation of the Proposed Formulas
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amodeo, C.; Hattou, S.; Buffière, P.; Benbelkacem, H. Temperature Phased Anaerobic Digestion (TPAD) of Organic Fraction of Municipal Solid Waste (OFMSW) and Digested Sludge (DS): Effect of Different Hydrolysis Conditions. Waste Manag. 2021, 126, 21–29. [Google Scholar] [CrossRef] [PubMed]
- DiLallo, R.; Albertson, O.E. Volatile Acids by Direct Titration. J. Water Pollut. Control. Fed. 1961, 33, 356–365. [Google Scholar]
- Lili, M.; Biró, G.; Sulyok, E.; Petis, M.; Borbély, J.; Tamás, J. Novel Approach on the Basis of FOS/TAC Method. In Proceedings of the Analele Universităţii din Oradea, Fascicula Protecţia Mediului, Oradea, Romania, 4–5 November 2011; Volume 17, pp. 713–718. [Google Scholar]
- Møller, H.B.; Ward, A.J. Modeling Volatile Fatty Acid Concentration in Livestock Manure–Based Anaerobic Digesters by Simple Titration. Environ. Eng. Sci. 2011, 28, 507–513. [Google Scholar] [CrossRef]
- Wu, C.; Huang, Q.; Yu, M.; Ren, Y.; Wang, Q.; Sakai, K. Effects of Digestate Recirculation on a Two-Stage Anaerobic Digestion System, Particularly Focusing on Metabolite Correlation Analysis. Bioresour. Technol. 2018, 251, 40–48. [Google Scholar] [CrossRef]
- Nordmann, W. Die Überwachung Der Schlammfaulung. KA-Inf. Betr. Beil. Korresp. Abwasser 1977, 3, 77. [Google Scholar]
- Voß, E.; Weichgrebe, D.; Rosenwinkel, K. FOS/TAC–Deduction, Methods, Application and Significance. In Proceedings of the Internationale Wis-Senschaftstagung, Biogas Science, Erding, Germany, 2–4 December 2009. [Google Scholar]
- McGhee, T.J. A Method for Approximation of the Volatile Acid Concentrations in Anaerobic Digesters. Water Sew. Works 1968, 165, 162–166. [Google Scholar]
- Jenkins, S.R.; Morgan, J.M.; Sawyer, C.L. Measuring Anaerobic Sludge Digestion and Growth by a Simple Alkalimetric Titration. J. Water Pollut. Control. Fed. 1983, 55, 448–453. [Google Scholar]
- Ripley, L.E.; Boyle, W.C.; Converse, J.C. Improved Alkalimetric Monitoring for Anaerobic Digestion of High-Strength Wastes. J. Water Pollut. Control. Fed. 1986, 58, 406–411. [Google Scholar]
- Kapp, H. Schlammfaulung Mit Hohem Feststoffgehalt. Stuttgarter Berichte Zur Siedlungswasserwirtschaft; Kommissionsverlag Oldenbourg: München, Germany, 1984; p. 300. [Google Scholar]
- Moosbrugger, R.E.; Wentzel, M.C.; Ekama, G.A.; Marais, G.V.R. A 5 PH Point Titration Method for Determining the Carbonate and SCFA Weak Acid/Bases in Anaerobic Systems. Water Sci. Technol. 1993, 28, 237–245. [Google Scholar] [CrossRef]
- Lahav, O.; Shlafman, E.; Morgan, B.E.; Loewenthal, R.E.; Tarre, S. Accurate On-Site Volatile Fatty Acids (VFA) Measurement in Anaerobic Digestion—Verification of a New Titrative Method. In Proceedings of the VII Latin American Workshop and Symposium on Anaerobic Digestion, Merida, Mexico, 22–25 October 2002; pp. 118–125. [Google Scholar]
- Lahav, O.; Morgan, B.E.; Loewenthal, R.E. Rapid, Simple, and Accurate Method for Measurement of VFA and Carbonate Alkalinity in Anaerobic Reactors. Environ. Sci. Technol. 2002, 36, 2736–2741. [Google Scholar] [CrossRef]
- Buchauer, K. A comparison of two simple titration procedures to determine volatile fatty acids in influents to waste-water and sludge treatment processes. Water SA 1998, 24, 49–56. [Google Scholar]
- Jobling Purser, B.J.; Thai, S.-M.; Fritz, T.; Esteves, S.R.; Dinsdale, R.M.; Guwy, A.J. An Improved Titration Model Reducing over Estimation of Total Volatile Fatty Acids in Anaerobic Digestion of Energy Crop, Animal Slurry and Food Waste. Water Res. 2014, 61, 162–170. [Google Scholar] [CrossRef]
- Lahav, O.; Morgan, B. Titration Methodologies for Monitoring of Anaerobic Digestion in Developing Countries? A Review. J. Chem. Technol. Biotechnol. 2004, 79, 1331–1341. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Wu, S.; Dong, R. Monitoring Volatile Fatty Acids and Carbonate Alkalinity in Anaerobic Digestion: Titration Methodologies. Chem. Eng. Technol. 2016, 39, 599–610. [Google Scholar] [CrossRef]
- Vannecke, T.P.W.; Lampens, D.R.A.; Ekama, G.A.; Volcke, E.I.P. Evaluation of the 5 and 8 PH Point Titration Methods for Monitoring Anaerobic Digesters Treating Solid Waste. Environ. Technol. 2015, 36, 861–869. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.E.; Donlan, R.J. Direct Method for Differentiating Bicarbonate and Acetate in Digester Control. In Abstracts of Papers of the American Chemical Society; American Chemical Society 1155 16TH ST, NW: Washington, DC, USA, 1977; Volume 173, p. 121. [Google Scholar]
- Pauss, A.; Roza, A.; Ledrut, M.-J.; Naveau, H.; Nyns, E.-J. Bicarbonate Determination in Complex Acid-base Solutions by a Back-titration Method. Environ. Technol. 1990, 11, 469–476. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 15th ed.; American Public Health Association: Washington, DC, USA, 1980. [Google Scholar]
- Nativ, P.; Gräber, Y.; Aviezer, Y.; Lahav, O. A Simple and Accurate Approach for Determining the VFA Concentration in Anaerobic Digestion Liquors, Relying on Two Titration Points and an External Inorganic Carbon Analysis. ChemEngineering 2021, 5, 15. [Google Scholar] [CrossRef]
- Mu, Z.-X.; He, C.-S.; Jiang, J.-K.; Zhang, J.; Yang, H.-Y.; Mu, Y. A Modified Two-Point Titration Method for the Determination of Volatile Fatty Acids in Anaerobic Systems. Chemosphere 2018, 204, 251–256. [Google Scholar] [CrossRef]
- Münch, E.V.; Greenfield, P.F. Estimating VFA Concentrations in Prefermenters by Measuring PH. Water Res. 1998, 32, 2431–2441. [Google Scholar] [CrossRef]
- Charnier, C.; Latrille, E.; Lardon, L.; Miroux, J.; Steyer, J.P. Combining PH and Electrical Conductivity Measurements to Improve Titrimetric Methods to Determine Ammonia Nitrogen, Volatile Fatty Acids and Inorganic Carbon Concentrations. Water Res. 2016, 95, 268–279. [Google Scholar] [CrossRef]
- Sun, H.; Guo, J.; Wu, S.; Liu, F.; Dong, R. Development and Validation of a Simplified Titration Method for Monitoring Volatile Fatty Acids in Anaerobic Digestion. Waste Manag. 2017, 67, 43–50. [Google Scholar] [CrossRef]
- Chatterjee, B.; Radhakrishnan, L.; Mazumder, D. New Approach for Determination of Volatile Fatty Acid in Anaerobic Digester Sample. Environ. Eng. Sci. 2018, 35, 333–351. [Google Scholar] [CrossRef]
- Zan, F.; Zeng, Q.; Chi, K.; Hao, T.; Ekama, G.A. A Novel Approach for Rapidly Measuring Volatile Fatty Acids in Anaerobic Process. Water Res. 2020, 182, 115960. [Google Scholar] [CrossRef] [PubMed]

| Total TAC (mg CaCO3·L−1) | |||||||
|---|---|---|---|---|---|---|---|
| X | X + 596 | X + 2979 | X + 5958 | X + 11,916 | X + 17,874 | ||
| Total FOS (mg HAc·L−1) | Y | ✓ | ✓ | ✓ | |||
| Y + 1000 | ✓ | ||||||
| Y + 5000 | ✓ | ||||||
| Y + 10,000 | ✓ | ||||||
| Y + 20,000 | ✓ | ✓ | |||||
| Y + 30,000 | ✓ | ✓ | |||||
| MAPE | [NaHCO3]Nordmann | [NaHCO3]new | [NaHCO3]corr |
|---|---|---|---|
| [NaHCO3]real | 1346% | 0.33% | 0.33% |
| MAPE | [HAc]Nordmann | [HAc]new | [HAc]corr |
| [HAc]real | 159% | 0.37% | 0.41% |
| Total TAC (mg CaCO3·L−1) | |||||||
|---|---|---|---|---|---|---|---|
| X a | X + 596 | X + 2979 | X + 5958 | X + 11,916 | X + 17,874 | ||
| Total FOS (mg HAc·L−1) | Y b | NA c NA | 3%/153% 1%/14% | 3%/215% 3%/13% | |||
| Y + 1000 | 22%/18% d 8%/13% e | ||||||
| Y + 5000 | 28%/7% 5%/7% | ||||||
| Y + 10,000 | 30%/1% 7%/2% | ||||||
| Y + 20,000 | 211%/8% 40%/1% | 33%/1% 9%/1% | |||||
| Y + 30,000 | 302%/9% 61%/1% | 35%/1% 8%/2% | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; André, L.; Mercier-Huat, M.; Grosmaître, J.-M.; Pauss, A.; Ribeiro, T. Accurate Estimation of Bicarbonate and Acetic Acid Concentrations with Wider Ranges in Anaerobic Media Using Classical FOS/TAC Titration Method. Appl. Sci. 2021, 11, 11843. https://doi.org/10.3390/app112411843
Liu X, André L, Mercier-Huat M, Grosmaître J-M, Pauss A, Ribeiro T. Accurate Estimation of Bicarbonate and Acetic Acid Concentrations with Wider Ranges in Anaerobic Media Using Classical FOS/TAC Titration Method. Applied Sciences. 2021; 11(24):11843. https://doi.org/10.3390/app112411843
Chicago/Turabian StyleLiu, Xiaojun, Laura André, Maël Mercier-Huat, Jean-Marie Grosmaître, André Pauss, and Thierry Ribeiro. 2021. "Accurate Estimation of Bicarbonate and Acetic Acid Concentrations with Wider Ranges in Anaerobic Media Using Classical FOS/TAC Titration Method" Applied Sciences 11, no. 24: 11843. https://doi.org/10.3390/app112411843
APA StyleLiu, X., André, L., Mercier-Huat, M., Grosmaître, J.-M., Pauss, A., & Ribeiro, T. (2021). Accurate Estimation of Bicarbonate and Acetic Acid Concentrations with Wider Ranges in Anaerobic Media Using Classical FOS/TAC Titration Method. Applied Sciences, 11(24), 11843. https://doi.org/10.3390/app112411843









