Underestimation of Dry Matter of Anaerobic Media with High Bicarbonate Concentration
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Sodium Bicarbonate and Acetic Acid Solution
2.2. Volatile Fatty Acids Interference
2.3. Bicarbonate Interference
3. Results and Discussion
3.1. Volatile Fatty Acids Interference
3.2. Bicarbonate Interference
3.2.1. Pure Bicarbonate Solutions
3.2.2. Bicarbonate Thermal Losses in Real AD Media
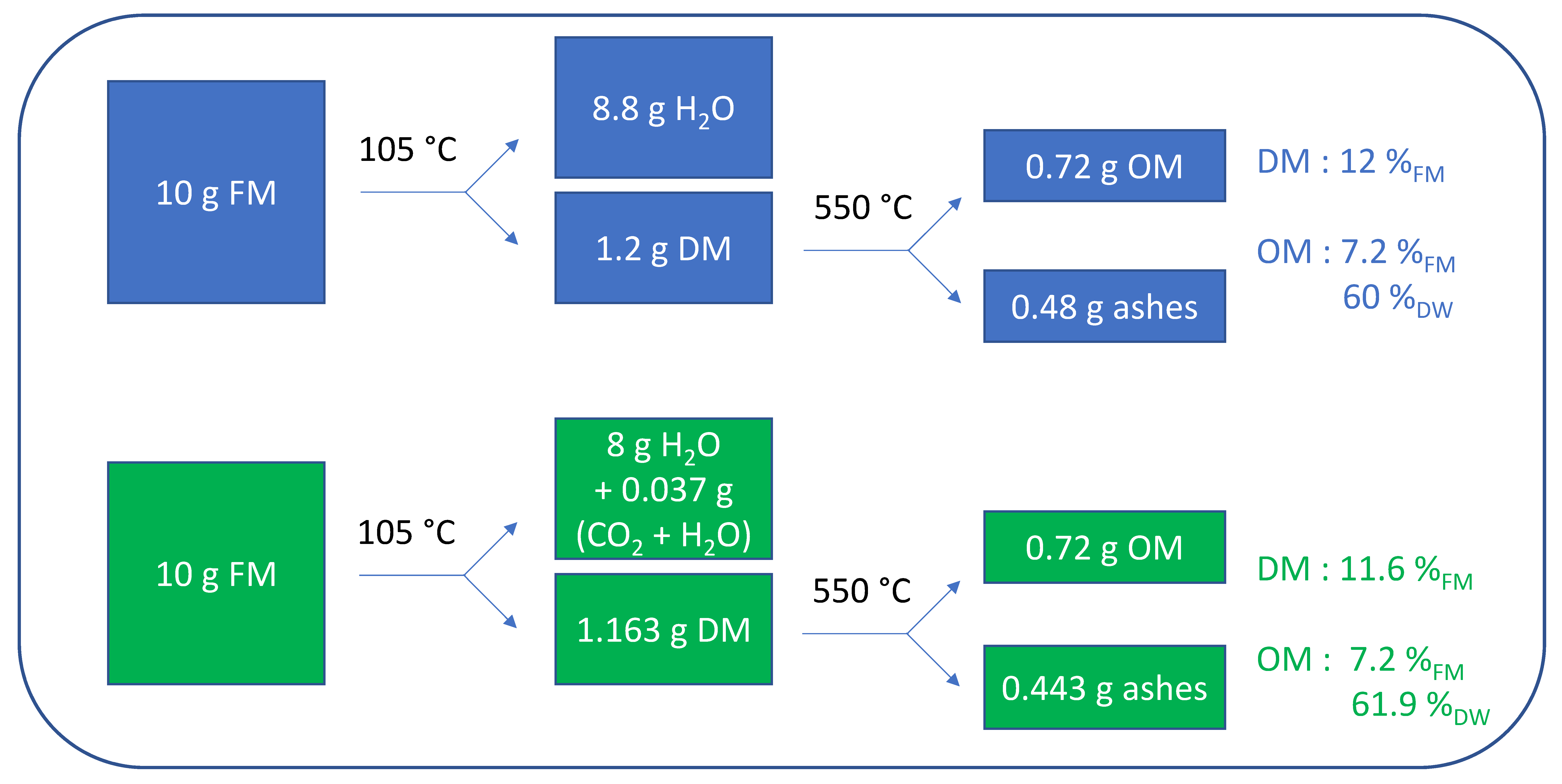
3.2.3. Importance and Effect of the Correction for Accurate DM Estimations
3.2.4. Impact of Thermal Bicarbonate Decomposition on OM Values
3.2.5. Influence of Temperature on the Bicarbonate Decomposition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meegoda, J.N.; Li, B.; Patel, K.; Wang, L.B. A Review of the Processes, Parameters, and Optimization of Anaerobic Digestion. Int. J. Environ. Res. Public Health 2018, 15, 2224. [Google Scholar] [CrossRef] [PubMed]
- NF EN 15934; Boues, Bio-Déchets Traités, Sols et Déchets–Calcul de La Teneur En Matière Sèche Par Détermination Du Résidu Sec Ou de La Teneur En Eau; AFNOR Editions: Paris, France, 2012.
- VDI 4630; Fermentation of Organic Materials–Characterization of the Substrate, Sampling, Collection of Material Data, Fermentation Tests; VDI: Düsseldorf, Germany, 2016.
- ISO 20675:2018; Biogas—Biogas Production, Conditioning, Upgrading and Utilization—Terms, Definitions and Classification Scheme; ISO: Geneva, Switzerland, 2018.
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Bareha, Y.; Affes, R.; Moinard, V.; Buffet, J.; Girault, R. A Simple Mass Balance Tool to Predict Carbon and Nitrogen Fluxes in Anaerobic Digestion Systems. Waste Manag. 2021, 135, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; de Wilde, V.; et al. Towards a Standardization of Biomethane Potential Tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef] [PubMed]
- Svensson, K.; Kjørlaug, O.; Horn, S.J.; Agger, J.W. Comparison of Approaches for Organic Matter Determination in Relation to Expression of Bio-Methane Potentials. Biomass Bioenergy 2017, 100, 31–38. [Google Scholar] [CrossRef]
- Ribeiro, T.; Cresson, R.; Pommier, S.; Preys, S.; André, L.; Béline, F.; Bouchez, T.; Bougrier, C.; Buffière, P.; Cacho, J.; et al. Measurement of Biochemical Methane Potential of Heterogeneous Solid Substrates: Results of a Two-Phase French Inter-Laboratory Study. Water 2020, 12, 2814. [Google Scholar] [CrossRef]
- Hafner, S.D.; Fruteau de Laclos, H.; Koch, K.; Holliger, C. Improving Inter-Laboratory Reproducibility in Measurement of Biochemical Methane Potential (BMP). Water 2020, 12, 1752. [Google Scholar] [CrossRef]
- Weinrich, S.; Schäfer, F.; Bochmann, G.; Liebetrau, J. Value of Batch Tests for Biogas Potential Analysis: Method Comparison and Challenges of Substrate and Efficiency Evaluation of Biogas Plants. In IEA Bioenergy Task 37; IEA Bioenergy: Paris, France, 2018; ISBN 978-1-910154-48-9. [Google Scholar]
- Chauzy, J.; Cretenot, D.; Bausseron, A.; Deleris, S. Anaerobic Digestion Enhanced by Thermal Hydrolysis: First Reference BIOTHELYS® at Saumur, France. Water Pract. Technol. 2008, 3, wpt2008004. [Google Scholar] [CrossRef]
- Banks, C.; Heaven, S.; Zhang, Y.; Baier, U. Food Waste Digestion: Anaerobic Digestion of Food Waste for a Circular Economy. In IEA Bioenergy Task 37; IEA Bioenergy: Paris, France, 2018; ISBN 978-1-910154-57-1. [Google Scholar]
- Derikx, P.J.L.; Willers, H.C.; ten Have, P.J.W. Effect of PH on the Behaviour of Volatile Compounds in Organic Manures during Dry-Matter Determination. Bioresour. Technol. 1994, 49, 41–45. [Google Scholar] [CrossRef]
- Minson, D.J.; Lancaster, R.J. The Effect of Oven Temperature on the Error in Estimating the Dry Matter Content of Silage. N. Z. J. Agric. Res. 1963, 6, 140–146. [Google Scholar] [CrossRef][Green Version]
- Fenner, H.; Barnes, H.D. Improved Method for Determining Dry Matter in Silage. J. Dairy Sci. 1965, 48, 1324–1328. [Google Scholar] [CrossRef]
- McDonald, P.; Dewar, W.A. Determination of Dry Matter and Volatiles in Silage. J. Sci. Food Agric. 1960, 11, 566–570. [Google Scholar] [CrossRef]
- Huida, L.; Vaeaetaeinen, H.; Lampila, M. Comparison of Dry Matter Contents in Grass Silages as Determined by Oven Drying and Gas Chromatographic Water Analysis. Ann. Agric. Fenn. Finl. 1986, 25, 215–230. [Google Scholar]
- Hayward, G.; Pavlicik, V. A Corrected Method for Dry Matter Determination for Use in Anaerobic Digester Control. Biol. Wastes 1990, 34, 101–111. [Google Scholar] [CrossRef]
- Dei, L.; Guarini, G.G.T. The Thermal Decomposition of NaHCO3. J. Therm. Anal. 1997, 50, 773–783. [Google Scholar] [CrossRef]
- Keener, T.C.; Frazier, G.C.; Davis, W.T. Thermal Decomposition of Sodium Bicarbonate. Chem. Eng. Commun. 1985, 33, 93–105. [Google Scholar] [CrossRef]
- Voß, E.; Weichgrebe, D.; Rosenwinkel, K. FOS/TAC–Deduction, Methods, Application and Significance. Internatioanle Winenschaftskonferenz. Biogas Sci. Meets Pract. 2009. Available online: https://scholar.google.com/citations?view_op=view_citation&hl=th&user=g9mqZNoAAAAJ&citation_for_view=g9mqZNoAAAAJ:RYcK_YlVTxYC (accessed on 16 January 2022).
- Liu, X.; André, L.; Mercier-Huat, M.; Grosmaître, J.-M.; Pauss, A.; Ribeiro, T. Accurate Estimation of Bicarbonate and Acetic Acid Concentrations with Wider Ranges in Anaerobic Media Using Classical FOS/TAC Titration Method. Appl. Sci. 2021, 11, 11843. [Google Scholar] [CrossRef]


| Loss at 70 °C | Loss at 105 °C | |
|---|---|---|
| Acetic acid solution (pH 2.53) | ||
| crucible with lid | 99.6 ± 0.1% | 99.2 ± 0.2% |
| crucible without lid | 99.9 ± 0.1% | 99.5 ± 0.1% |
| Sodium acetate solution (pH 9.23) | ||
| crucible with lid | 1.55 ± 0.05% | 1.35 ± 0.05% |
| crucible without lid | 1.05 ± 0.05% | 1.3 ± 0.1% |
| Samples Tested | Loss at 70 °C | Loss at 105 °C |
|---|---|---|
| Sodium bicarbonate solution (10 g/L) | 35.9 ± 0.5% | 35.9 ± 0.1% |
| Mussel shell | 0.9 ± 0.1% | 0.8 ± 0.3% |
| Slipper shell | 0.7 ± 0.05% | 0.7 ± 0.3% |
| Samples Tested | Loss at 105 °C | Loss after Freeze-Drying |
|---|---|---|
| Bicarbonate solution (50 g/L) | 37.4 ± 0.0% | 3.9 ± 0.3% |
| Bicarbonate (50 g/L) solution after freeze-drying | 35.0 ± 0.3% | – |
| Samples Tested | Initial Fresh Mass (g) | DM (g) | Loss at 105 °C |
|---|---|---|---|
| Effluent from the reactor treating blue mussel subproducts | |||
| Sample 1 | 10.01 ± 0.01 | 0.21 ± 0.01 | – |
| Sample 1 plus bicarbonate | 12.55 ± 0.10 | 0.29 ± 0.01 | 37 ± 1% |
| Sample 2 | 50.00 ± 0.01 | 0.95 ± 0.01 | – |
| Sample 2 plus bicarbonate | 51.00 ± 0.01 | 1.63 ± 0.01 | 32 ± 1% |
| Effluent from the reactor treating intermediate energy crops | |||
| Effluent | 50.00 ± 0.01 | 2.39 ± 0.01 | – |
| Effluent plus bicarbonate | 52.00 ± 0.01 | 3.59 ± 0.01 | 39 ± 1% |
| [NaHCO3] (g/L) | Without Correction | With Correction | |
|---|---|---|---|
| Influent, over 20 days feeding | average: 9.38 | 48.8 kgDM | 54.0 kgDM |
| Effluent, over 20 days feeding | average: 18.2 | 38.4 kgDM | 48.8 kgDM |
| DM conversion | – | 21.4% | 10.5% |
| OM conversion | – | 21.7% | 21.7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, T.; André, L.; Liu, X.; Mercier-Huat, M.; Fayolle, J.; Grosmaître, J.-M.; Pauss, A. Underestimation of Dry Matter of Anaerobic Media with High Bicarbonate Concentration. Appl. Sci. 2022, 12, 1105. https://doi.org/10.3390/app12031105
Ribeiro T, André L, Liu X, Mercier-Huat M, Fayolle J, Grosmaître J-M, Pauss A. Underestimation of Dry Matter of Anaerobic Media with High Bicarbonate Concentration. Applied Sciences. 2022; 12(3):1105. https://doi.org/10.3390/app12031105
Chicago/Turabian StyleRibeiro, Thierry, Laura André, Xiaojun Liu, Maël Mercier-Huat, Joseph Fayolle, Jean-Marie Grosmaître, and André Pauss. 2022. "Underestimation of Dry Matter of Anaerobic Media with High Bicarbonate Concentration" Applied Sciences 12, no. 3: 1105. https://doi.org/10.3390/app12031105
APA StyleRibeiro, T., André, L., Liu, X., Mercier-Huat, M., Fayolle, J., Grosmaître, J.-M., & Pauss, A. (2022). Underestimation of Dry Matter of Anaerobic Media with High Bicarbonate Concentration. Applied Sciences, 12(3), 1105. https://doi.org/10.3390/app12031105









