1. Introduction
In November 2019, a new disease was diagnosed in Wuhan City, Hubei Province, China that gave rise to severe pneumonia with flu-like symptoms. The disease was called COVID-19 due to the fact that it was caused by the SARS-CoV-2 coronavirus [
1,
2]. The coronavirus led to a pandemic, with approximately 242 million cases and almost 5 million deaths reported worldwide [
1].
The SARS-CoV-2 coronavirus is transmitted mainly by airborne droplets through close physical contact [
1,
2,
3]. The WHO called for the application of preventive measures, such as disinfection, distancing, and the appropriate use of personal protective equipment (PPE) [
1,
4].
Recently, a large amount has been written regarding the manufacturing of masks, helmets, adapters, swabs, and respiratory by means of AM technology [
5,
6,
7,
8,
9]. It has been found that AM methods can be a suitable tool for the further development and production of PPE, and that in the event of supply shortages, additive manufacturing can be rapidly applied. Still, more research is required regarding design procedures, suitable materials, sterilization, etc.
At the start of the pandemic, the personal protective equipment, especially mask filters, used by medical staff in emergency wards, was virtually unavailable in Poland and around the world [
10,
11].
The available publications offer examples on the use of additive technologies. However, these are mainly examples of producing a single adapter for the full-face snorkel mask, with the FDM/FFF technology. In our work, we wanted to focus on presenting the method developed for on-demand additive manufacturing of spare parts and consumables for medical equipment, as well as personal protective equipment in a dispersed environment during crisis situations. This includes the case study of producing an adapter that allows the connection of a filter to the Honeywell North Pano CL3 full-face mask. The use of the methodology enables the selection of the best-suited AM technology and material for the application. The case includes the demand made by a hospital, upon which a prototype was made with the fused deposition modelling technology from the ABS material, and finally, a series of 150 adapters was produced using the selective laser melting (SLS) technology from the PA12 material. The adapters were used to equip all of the employees of a local COVID ward. This made it possible to multiply the number of masks available. Particularly, in a crisis, it helped maintain the continuity of medical staff work. In addition, the article presents the results of research into the cytotoxicity of the samples tested against HaCaT, L929, A549 cell lines, and antibacterial properties through the assessment of the surface colonisation capacity by microorganisms in tryptic soy broth (TSB) and in artificial saliva (AS).
2. Materials and Methods
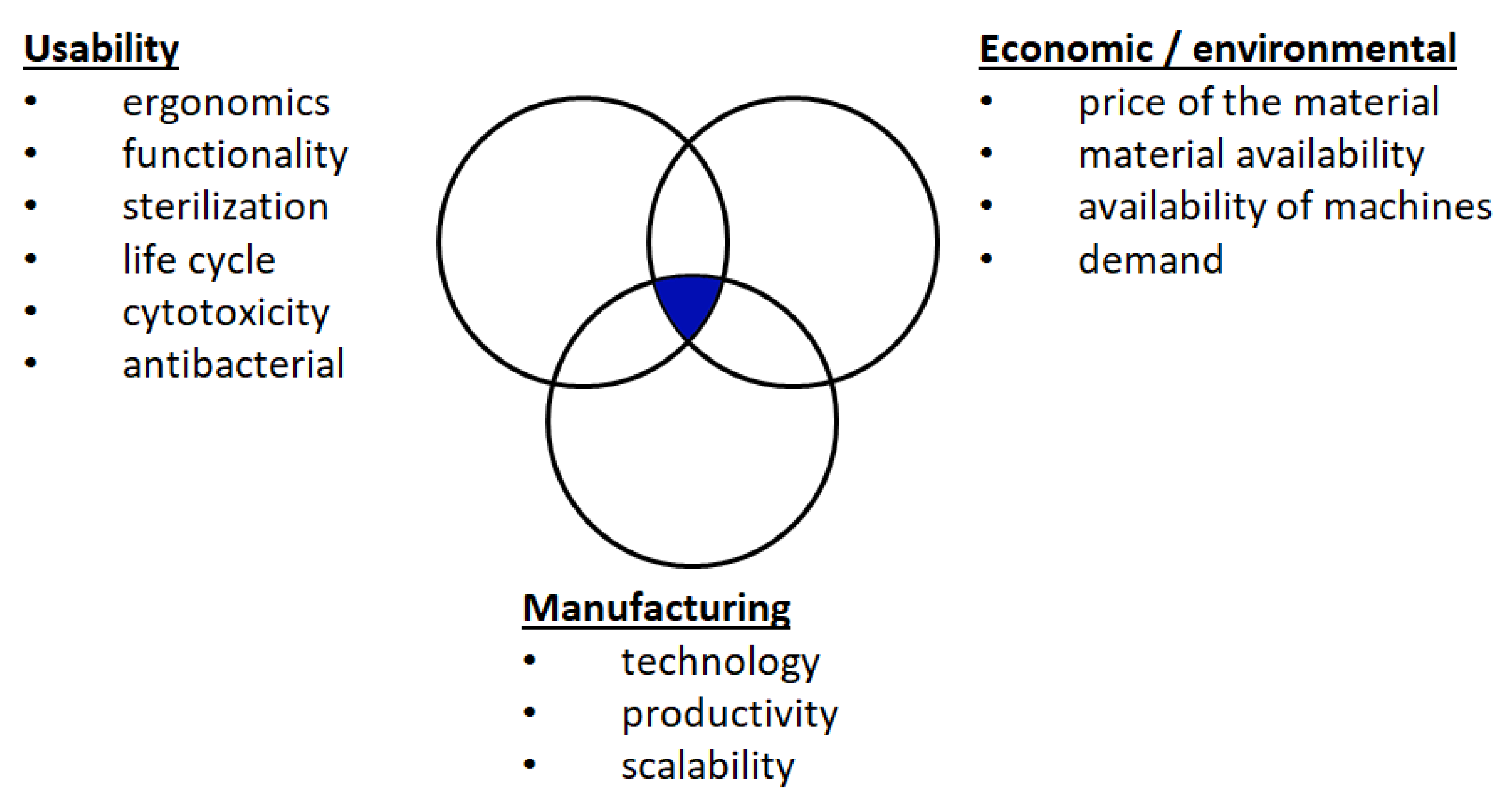
To create the AM Virtual Storage or a digital parts repository, a method was developed for the adaption of medical equipment to additive manufacturing in the event of its inaccessibility as a result of a crisis. At each stage of the method, it is important to define the characteristics of the part, the proposed manufacturing method, and materials in the following areas (
Figure 1):
Usability;
Manufacturing;
Economic/environmental.
Regarding the operational criteria, the shape of a part is defined by specifying its function and ergonomics, including its mass. The medical equipment must meet the requirements of cytotoxicity and antibacterial properties. The product needs to be identified for its usability—one-time or repeated–and sterility.
Economic and environmental criteria include the current price and availability of materials of which the part will be produced. In addition, it is crucial to determine the availability of the means of production under given conditions, and the machine costs of manufacturing. Moreover, the current and future demand should be determined.
Manufacturing criteria include the ability of a given manufacturing method to shape the part with a given roughness, porosity, and strength. These factors constitute the input data for the design for additive manufacturing (DfAM). Production capacity, which is understood as the efficiency of a single machine, should be taken into account. The last aspect is the scalability of the method, understood as the potential to increase production efficiency by increasing the number of machines or dispersing production among many market entities, such as service bureaus.
The method described here offers a solution that meets the requirements in all of the three aforementioned areas. The qualitative characteristics of individual additive manufacturing methods (
Table 1) [
12] may be helpful for the initial evaluation.
In the developed method (
Figure 2), the first step is to evaluate the part in terms of its suitability for additive manufacturing in relation to other manufacturing methods. The parts, for which additive manufacturing is not justified, are eliminated since there are other manufacturing methods that are capable of producing them faster or in a less costly way. The next step is to select the AM method and material.
The selection of a method and material is described as a subprocess, in which we use the knowledge already gathered in the database or generate new knowledge by conducting research in three areas. These steps are listed in order of importance for medical equipment.
The first step is to determine whether the part is in contact with the human body and whether the material will meet the cytotoxicity requirements. To determine whether these requirements are met, it is proposed to test the cytotoxicity of samples according to ISO 10993-5: 2009 [
13] and ISO/IEC 17025: 2005 [
14] against the HaCaT keratinocyte line, L929 fibroblasts, and pulmonary alveoli lines A549. The test should be carried out in eight replications.
The second step is to determine whether the material must exhibit antibacterial properties. For this purpose, it is recommended to test it with standard microbiological techniques using quantitative inoculation, the crystal violet method, and the method based on the assessment of the metabolic activity of cells. It is proposed to assess the microbial colonisation capacity/potential antimicrobial activity in the standard Tryptic soya broth medium (System I) and artificial saliva (System II) for Pseudomonas aeruginosa, Staphylococcus aureus, and Clostridium difficile. The measurement should be performed 8 h after settlement.
The third stage is the requirement related to the strength properties of the incrementally processed material. As part of this criterion, attention should be paid to the possibility and impact of the selected sterilization methods on the properties of the product whether sterilization is necessary.
If the requirements in the above areas are met, in the last stage, the efficiency of the selected production method should be compared with the specific demand for a given product. In the event of a crisis, the selection of materials and methods must be carried out in accordance with the current availability of machines and materials.
In the next step, the part should be designed or redesigned using DfAM. A prototype or a series of parts should be verified by medical personnel. At this stage, you may need to iterate the process through design improvements. Positive verification enables the transition to the stage of designing the technological process for serial production. The resulting technological data are saved in the verified parts of the database in the AM Virtual Storage web application, which is specifically prepared for this purpose. The use of a web application enables the commencement of distributed production using the resources of entities registered on the platform.
3. Results and Discussion
3.1. Adapter Characteristics
Using the described method, actions were taken in response to the lack of filters for full-face masks in the COVID-19 ward of the local medical facility. The goal was to deliver a prototype of an adapter within 24 h to connect the Honeywell North Pano CL3 mask to the widely available Covidien DAR filter (
Figure 3), rather than the dedicated Honeywell 1788155 filter, whose availability was very limited at the time. The next stage was to deliver the adapters to all of the medical staff working in the ward within 72 h after prototype approval.
A secondary motivating factor for the use of the Covidien DAR filter was the medical staff complaints against the dedicated filter, including its considerable weight (395 g) and size limiting the view, all of which made the whole day’s duty more strenuous. Of note, the weight of the Covidien DAR filter is 28 g and it does not obstruct the user’s view due to its considerably smaller dimensions.
The characteristics of the product were determined on the basis of an interview conducted with a team of doctors. These are described in
Table 2 (operational criteria). By the same token, the demand was determined at the level of 300 units.
3.2. Selection of Material and Technology
After analysing the defined areas, criteria (
Figure 1), and requirements (
Table 2), it had transpired that the adapter meets the conditions for its production with the use of additive manufacturing. Therefore, the material and technology selection stage began. Regarding the economic and environmental criteria, the current availability and price of the materials were analysed. The choice of material that was used to produce the adapter was largely dependent on the availability of 3D printing devices in the laboratory. On this basis, the following technologies and materials were found to be available:
The results of biological tests carried out for the selected materials in the subsequent stages of the subprocess are presented below. In the FDM method, the ABS material was used in the form of a wire under the trade name ABS-M30 manufactured by Stratasys. In the SLS method, the samples were made of PA12 powder, available under the trade name PA2200 manufactured by EOS [
15]. The materials were applied as delivered, with no pretreatment. In
Figure 4, the results of the cytotoxicity tests of the materials are shown.
Samples with a cytotoxicity below 30% are considered noncytotoxic, according to the standard. The “minus” sign next to the numerical value means that the extract from the incubation of the samples in the culture medium stimulated the test cells to grow beyond the cell growth level in the control system (untreated extract). The chart below shows that both the ABS material processed in the FDM and the PA12 material processed in the SLS are not cytotoxic for the tested cell lines. Of note, the PA12 material is characterised by a much lower level of cytotoxicity (−11.5%) for Keratinocyte HaCaT compared to the ABS material (−1.1%). Regarding the remaining cell lines, the PA12 material is also characterised by a lower level of cytotoxicity compared to the ABS material, but these differences are not very significant. For Fibroblast L929, the values are −14.5% for PA12 and −9.5% for ABS. For epithelial lung cells A459, which have the highest importance in this application, these values are −13% for PA12 and −11.5% for ABS, respectively.
The assessment of the surface colonisation capacity by the microorganisms/potential antimicrobial activity in the Tryptic soya broth (system I) and in the artificial saliva (system II) are presented in
Figure 5a,b, respectively. The figure shows the % of the number of live cells on the surface of the test samples compared to the number of cells grown on the control surfaces in the test system.
The ABS for all of the analysed microbial strains shows low potential antimicrobial activity, both in the Tryptic soya broth (TSB) microbial medium and in artificial saliva (AS), as evidenced by the high percentage of the number of live cells on the surface of the tested samples compared to the number of cells grown on the control surfaces in research systems. For S. aureus, P. aeruginosa, and C. difficile, it was 98.4%, 93.1%, 91.5% in the Tryptic soya broth (TSB) microbial medium, and 98.5%, 87.2%, 87.4%, respectively in artificial saliva.
On the other hand, the PA12 material was characterised by a much higher potential antimicrobial activity compared to ABS. The survival of the analysed strains of S. aureus, P. aeruginosa, and C. difficile was found to be at the level of 98.5%, 87.2%, 87.4% in the microbiological medium of Tryptic soya broth, and 54.4%, 88%, 61.7%, respectively in artificial saliva.
The above studies have shown that the selected materials are characterised by low cytotoxicity, which is particularly important when the manufactured elements come into direct contact with humans. In this case, it cannot be concluded that the selected materials are antibacterial. In particular, the ABS material has very low potential antimicrobial activity. Regarding the PA12 material, this activity is noticeable for the
S. aureus and
C. difficile strains, while for the
P. aeruginosa strain, this activity is at a level similar to ABS. This fully indicates the need to sterilize the adapters, which can be done with different techniques [
16]. Sterilisation of ABS products produced with FDM technology can be performed with ethylene oxide (EtO) or Gamma radiation [
17,
18]. On the other hand, sterilization of PA12 products produced with SLS technology can be performed by the autoclave (121 °C) and ethylene oxide or steam formaldehyde at 60–80 °C [
16,
17].
Taking into consideration that the adapters were manufactured using the process parameters recommended by the manufacturer of the material/device, it was assumed that the mechanical properties were in line with the material sheet. Furthermore, the mechanical properties of ABS material processed in FDM [
19] and PA12 material [
15] processed in SLS have been described in the literature [
20,
21].
The description of the mechanical properties contained in the above-mentioned material data sheets and articles leads to the conclusion that the above materials are suitable for the production of the adapter. The mechanical properties of PA12, however, are higher than those of ABS [
15,
19]. Furthermore, the products made of PA12 material after the SLS process are characterised by closed porosity at the level of about 5% [
20], while in the case of ABS products, after the FDM process, the products are characterised by open porosity. It is important, particularly in the case of the manufactured adapter, where it is necessary to maintain an air-tight connection with the mask filter.
Based on the conducted analysis (
Table 3), previous research, and literature review, it was confirmed that it is possible to use FDM and SLS technologies to produce a prototype and small series for the medical industry.
3.3. Product Design with the Use of DfAM and Rapid Prototyping
As indicated by the usability requirements, when designing the adapter geometry, it was necessary to take into account the geometric features of the full-face mask, the DAR filter, and the criteria presented in
Table 2. After the measurements, it was found that the full-face mask has an internal thread RD 40 with a length of 20 mm, while the DAR filter has an outlet with a diameter of Ø 22 mm and a length of 15 mm.
After receiving the mask and the DAR filter from the hospital, a prototype adapter was designed and manufactured. Despite the advantage of the SLS method in the described application, at the prototyping stage, individual pieces of the product are produced, therefore, for economic reasons, the FDM method was applied using the Stratasys F170 machine with GrabCAD Print software. Adapter prototypes were printed using a Ø 0.33 mm nozzle and a layer height of 0.33 mm. The filling density was set to 100%. The correctness of the prototype was tested by mounting it to the supplied mask and filter, thus ensuring that the minimum operational requirements were met. The adapter prototype was delivered to the hospital 22 h after the need emerged (
Figure 6).
After the adapter tests had been carried out, information was obtained that there were difficulties with screwing in/unscrewing the adapter from the mask. This indicates that the adapter cannot be fully screwed into the mask, which can in turn result in an increased distance between the mask and filter inlet (
Figure 7).
To facilitate the screwing in/unscrewing of the adapter from the mask, it was decided to add gripping elements. Two design variants were proposed with two and eight grips, and two other designs with two and eight gripping tongues. After further tests, it was found that version 3 of the adapter is easier to screw in/unscrew. Sadly, the elements that facilitate the screwing in/unscrewing of the adapter in version 3 had sharp edges, which tore the disposable gloves. The sharp edges resulted directly from the limitation of the FDM technology and appeared at the point of contact of the model with the platform and at the seam.
The authors of [
22] presented a whole range of different PPEs produced with the use of FDM technology to fight against COVID-19. Moreover, the authors of [
23] presented a model of an adapter for the full-face snorkel mask, which was manufactured with the FDM technology. However, the above publications did not consider a systematic approach that takes into account risk inducing factors, such as material cytotoxicity and antimicrobial activity properties. Due to the limitations of FDM technology, the produced parts are more susceptible to leakage. This is an important factor in the protection against COVID-19. Therefore, the SLS technology was selected for the production of the batch of adapters, since this technology ensures that the manufactured elements will be solid and characterised by low porosity [
20].
3.4. Batch Production
All of the comments from the hospital on the functionality of the prototypes were analysed, as shown above (
Table 4). It was decided to introduce final modifications and start the production of a series of adapters in version 4 using the SLS technology. This technology was selected as the target production method corresponding to the indicated demand. The same approach can also produce air-tight elements and the problem of sharp edges is eliminated.
The EOS Formiga P110 device and Magics 24.1 software (Materialize) were used. Since it was not possible to meet the demand in one construction process, due to the working area of the device, the products were made in two batches of 150 pieces each. With this number, the filling density of the working area was 16.39%, which does not generate the risk of local heat accumulation (
Figure 8).
The adapters were produced with a layer height of 0.1 mm. The designed manufacturing technology included 27 h of build time and time related to preparatory and finishing activities, giving a total time of 55 h per batch (
Figure 9).
The production of the second batch followed the same pattern, and started on the day following the completion of the delivery. The second production batch allowed hospital workers to have two adapters per mask. The final adapter, version 4, is presented in
Figure 10.
4. Conclusions
This article describes the production of a fully functional full-face mask adapter. Additionally, it confirms the effectiveness of the procedure of the additive manufacturing method selection for producing medical consumables, spare parts or PPE. The proposed methodology offers the possibility of the immediate production of additive spare parts, alternative medical equipment, and personal protective equipment in a dispersed environment in crisis situations.
The described method contributed to the implementation of an internet platform, in which medical personnel can report, in emergency situations, the demand for the required equipment, that can be quickly produced with the use of AM, using a distributed production environment.
The designed full-face mask filter adapter made a more efficient use of the available resources, in a crisis moment of shortage of dedicated filters, caused by the interrupted supply chain during the COVID-19 pandemic.
Standard materials, such as ABS in FDM and PA12 in SLS, are not cytotoxic. They do not show significant antibacterial properties either, thus sterilisation is necessary.
The FDM technology has proven to be a perfect solution at the rapid prototyping stage of custom products used in healthcare. The drawbacks of this technology resulted in sharp edges of the product, which damaged disposable gloves. The economic aspect of manufacturing has yet to be considered. As for the case described above, the cost of FDM technology would be significantly higher than the cost of producing a series of 150 items by means of SLS technology.
In this paper, we speculate that the solution presented does not provide better protection than the dedicated mask filters. Still, in the event of a shortage of dedicated filters caused by interrupted supply chains, the presented solution can be applied to multiply the available number of masks. In the crisis caused by COVID-19, it ensured the continuity of healthcare provision.
The method presented in this paper was used to evaluate and prepare a mask filter adapter to be additionally manufactured in compliance with minimal medical requirements, which increased the number of available full-face masks in Poland’s Specialist Hospitals during COVID-19 while the supply chains were broken.