Platonin, a Cyanine Photosensitizing Dye, Ameliorates Inflammatory Responses in Vascular Smooth Muscle Cells by Modulating Inflammatory Transcription Factors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Cultivation
2.3. Content of MCP-1 Analysis
2.4. Western Blotting Assay
2.5. Transfection and Luciferase Assays
2.6. Oil Red O Staining
2.7. Statistical Analysis
3. Results
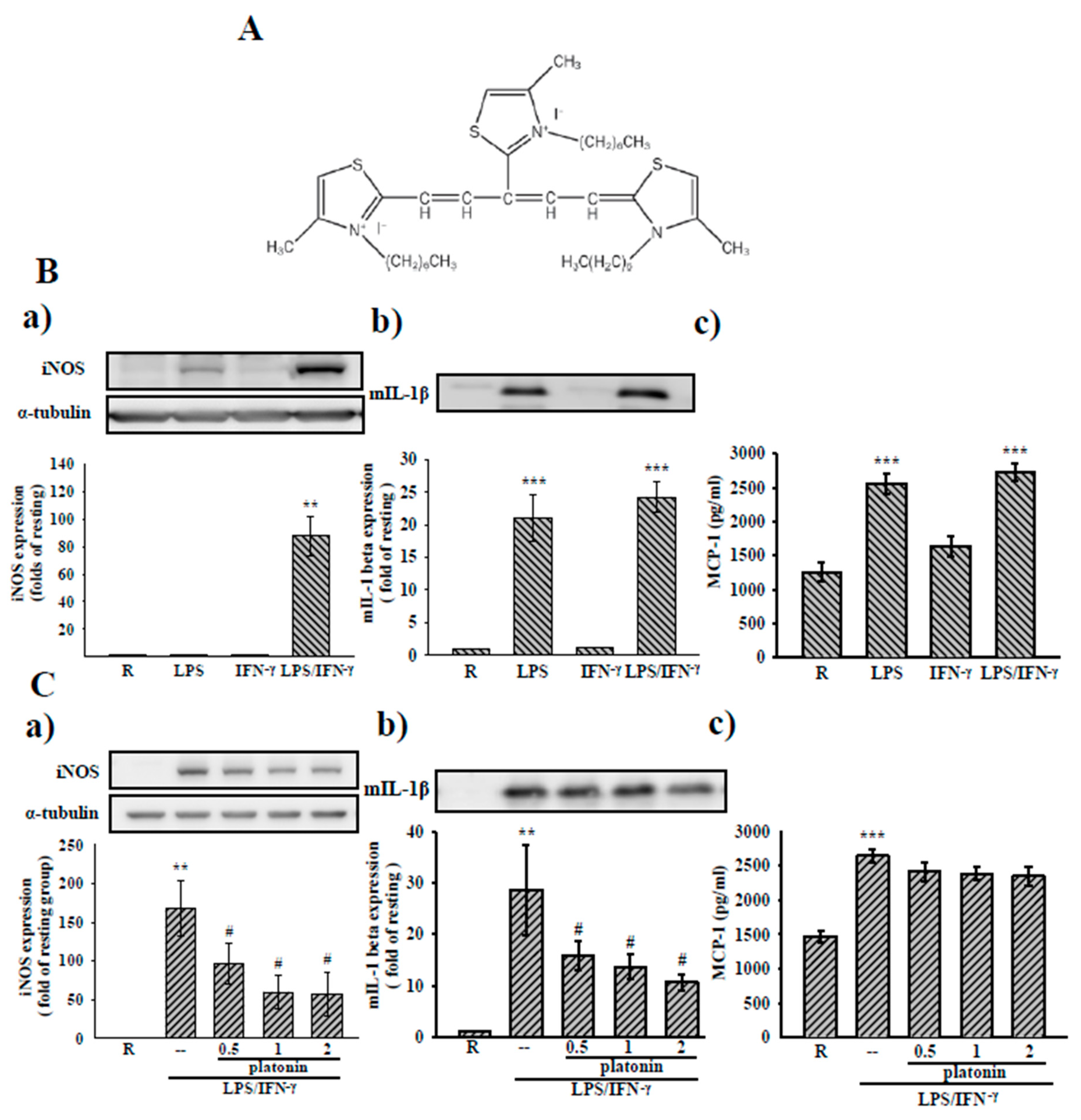
3.1. Effects of Platonin on LPS/IFN-γ-Induced Expression of Inflammatory Mediators in VSMCs
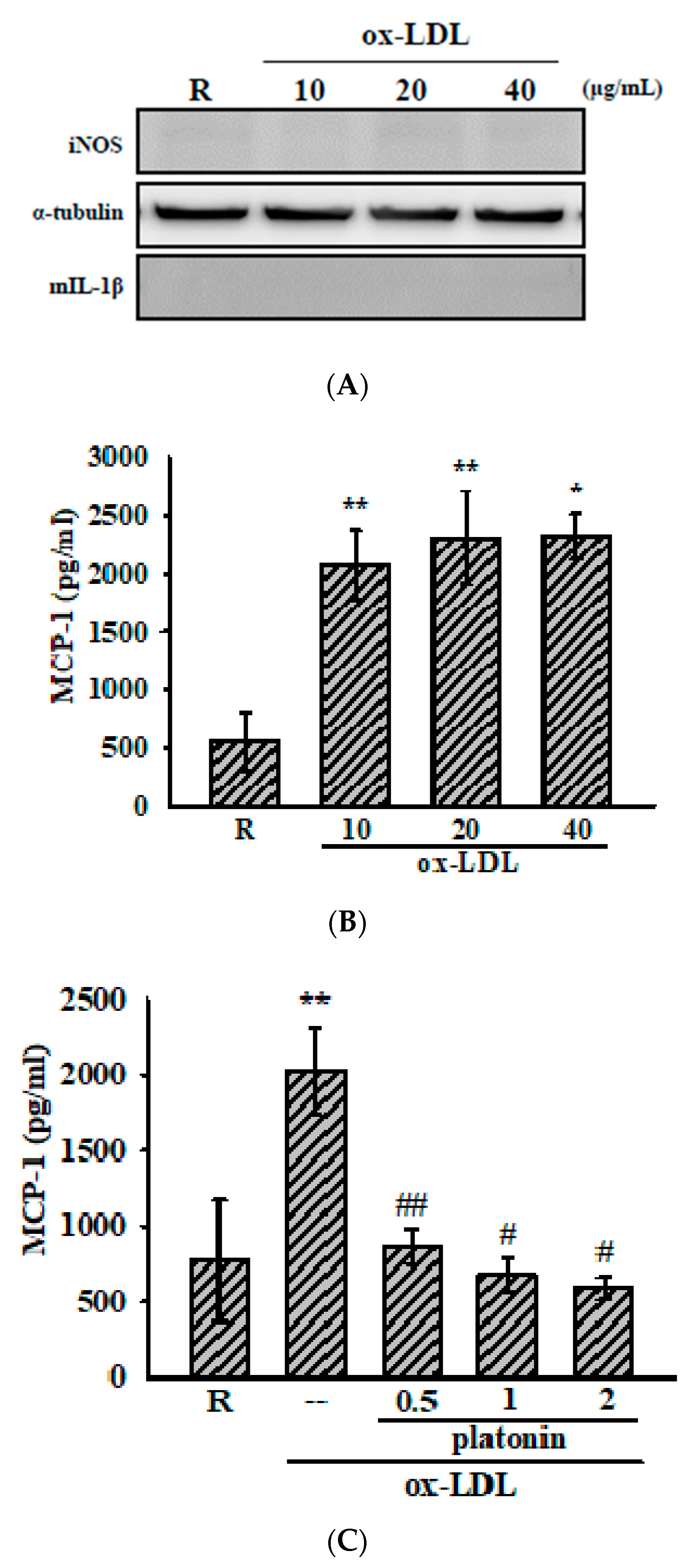
3.2. Platonin Attenuates Ox-LDL-Stimulated MCP-1 Production in VSMCs
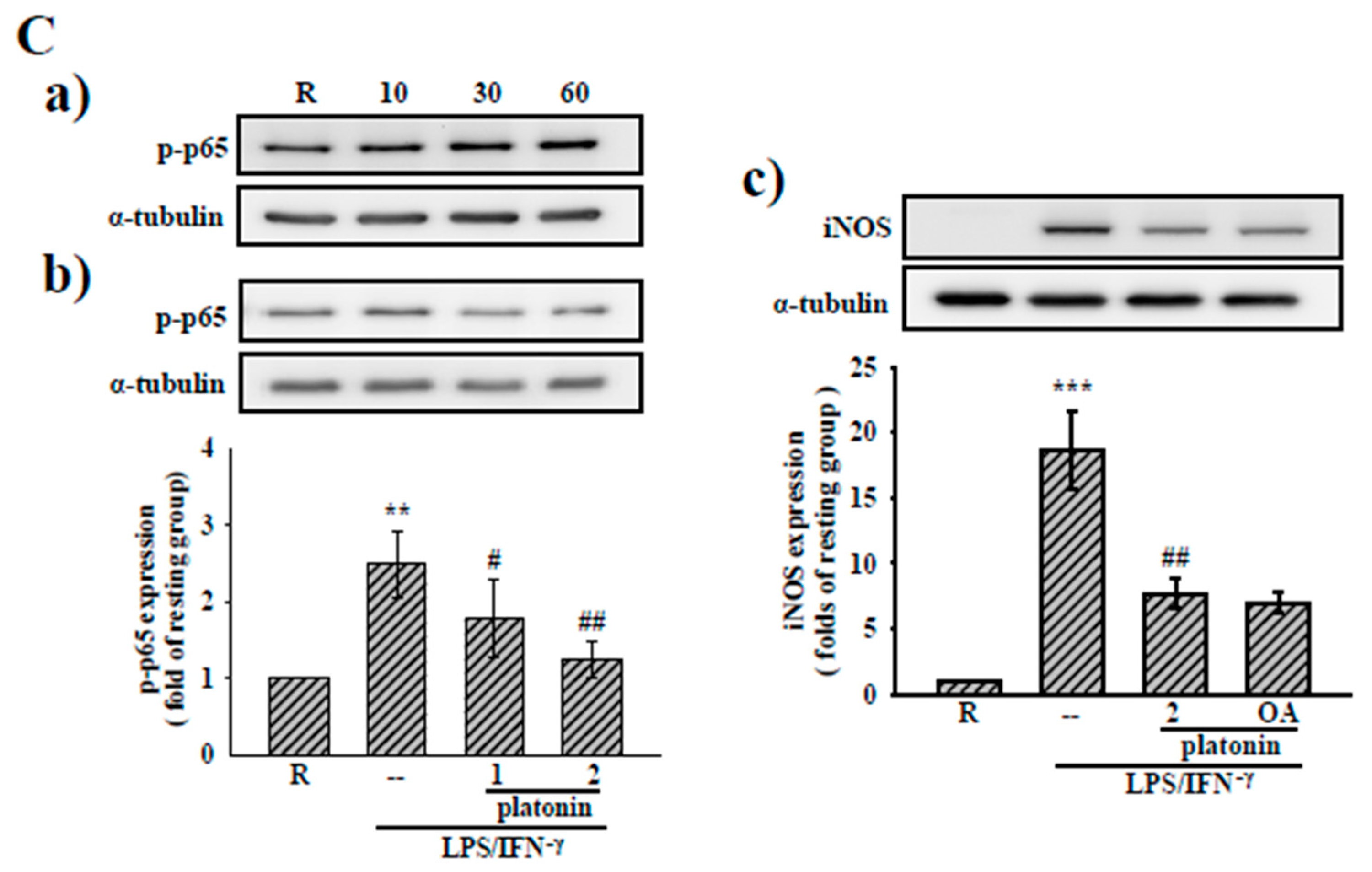
3.3. Effects of Platonin on NF-κB Activation in LPS/IFN-γ-Induced VSMCs
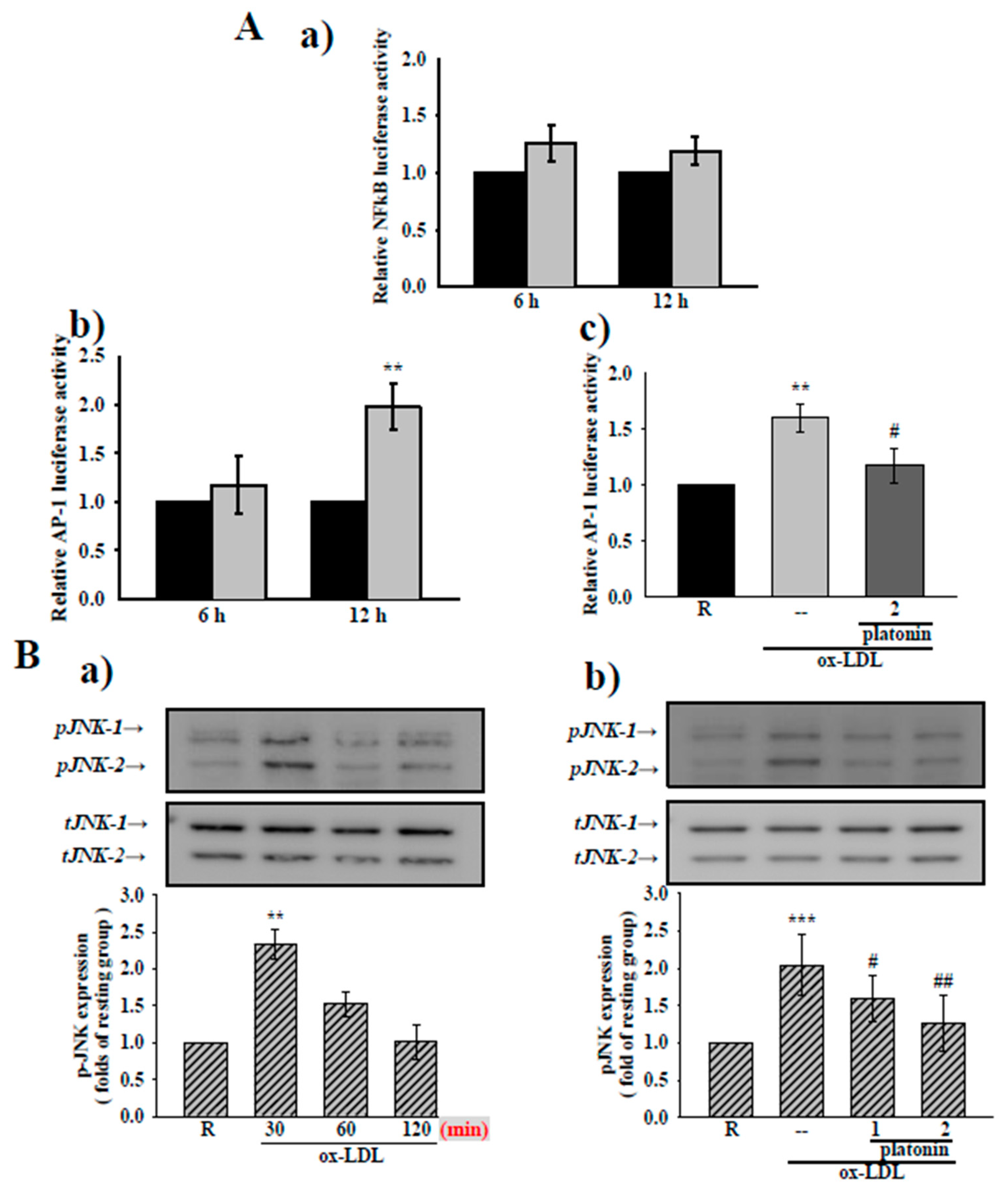
3.4. Platonin Suppresses AP-1 Activation and JNK1/2 Phosphorylation in Ox-LDL-Treated VSMCs
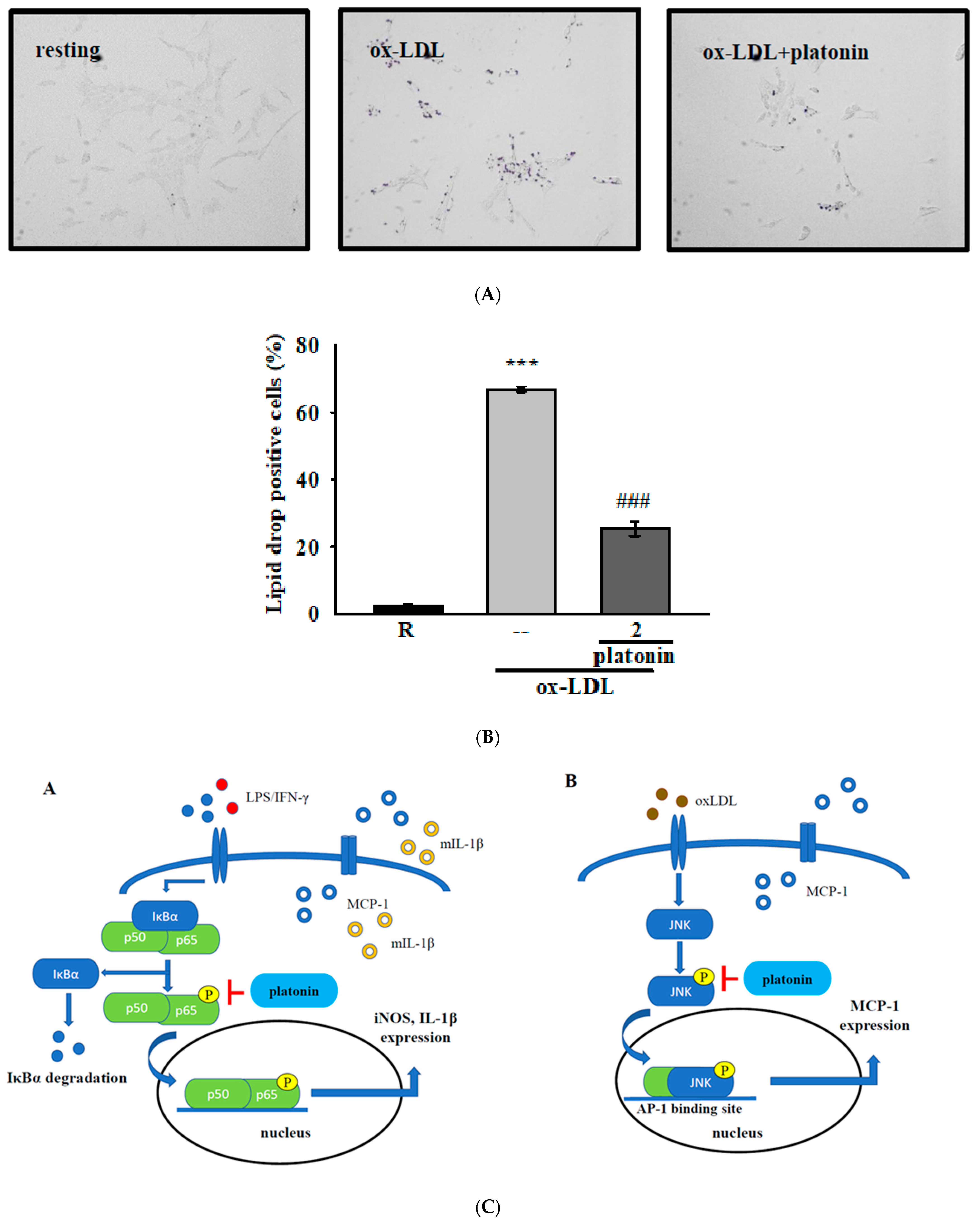
3.5. Treatment with Platonin Attenuates Lipid Accumulation in ox-LDL-Stimulated VSMCs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geovanini, G.R.; Libby, P. Atherosclerosis and inflammation: Overview and updates. Clin. Sci. 2018, 132, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Bäck, M.; Yurdagul, A., Jr.; Tabas, I.; Öörni, K.; Kovanen, P.T. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019, 16, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M. CD36, a scavenger receptor implicated in atherosclerosis. Exp. Mol. Med. 2014, 46, e99. [Google Scholar] [CrossRef] [Green Version]
- Koelwyn, G.J.; Corr, E.M.; Erbay, E.; Moore, K.J. Regulation of macrophage immunometabolism in atherosclerosis. Nat. Immunol. 2018, 19, 526–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, M.R.; Sinha, S.; Owens, G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef]
- Roostalu, U.; Wong, J.K. Arterial smooth muscle dynamics in development and repair. Dev. Biol. 2018, 435, 109–121. [Google Scholar] [CrossRef]
- Allahverdian, S.; Chaabane, C.; Boukais, K.; Francis, G.A.; Bochaton-Piallat, M.L. Smooth muscle cell fate and plasticity in atherosclerosis. Cardiovasc. Res. 2018, 114, 540–550. [Google Scholar] [CrossRef] [Green Version]
- Shankman, L.S.; Gomez, D.; Cherepanova, O.A.; Salmon, M.; Alencar, G.F.; Haskins, R.M.; Swiatlowska, P.; Newman, A.A.; Greene, E.S.; Straub, A.C.; et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat. Med. 2015, 21, 628–637. [Google Scholar] [CrossRef] [Green Version]
- Ramel, D.; Gayral, S.; Sarthou, M.K.; Augé, N.; Nègre-Salvayre, A.; Laffargue, M. Immune and Smooth Muscle Cells Interactions in Atherosclerosis: How to Target a Breaking Bad Dialogue? Front. Pharmacol. 2019, 10, 1276. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Sun, Y.; Zhao, K.; Gao, Y.; Cui, J.; Qi, L.; Huang, L. miR-21/PTEN pathway mediates the cardioprotection of geniposide against oxidized low-density lipoprotein-induced endothelial injury via suppressing oxidative stress and inflammatory response. Int. J. Mol. Med. 2020, 45, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Obermayer, G.; Afonyushkin, T.; Binder, C.J. Oxidized low-density lipoprotein in inflammation-driven thrombosis. J. Thromb. Haemost. 2018, 16, 418–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirillo, A.; Norata, G.D.; Catapano, A.L. LOX-1, OxLDL, and atherosclerosis. Mediat. Inflamm. 2013, 2013, 152786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akagi, M.; Ueda, A.; Teramura, T.; Kanata, S.; Sawamura, T.; Hamanishi, C. Oxidized LDL binding to LOX-1 enhances MCP-1 expression in cultured human articular chondrocytes. Osteoarthr. Cartil. 2009, 17, 271–275. [Google Scholar] [CrossRef] [Green Version]
- Bianconi, V.; Sahebkar, A.; Atkin, S.L.; Pirro, M. The regulation and importance of monocyte chemoattractant protein-1. Curr. Opin. Hematol. 2018, 25, 44–51. [Google Scholar] [CrossRef]
- Motoyoshi, F.; Kondo, N.; Ono, H.; Orii, T. The effect of photosensitive dye platonin on juvenile rheumatoid arthritis. Biotherapy 1991, 3, 241–244. [Google Scholar] [CrossRef]
- Lee, J.J.; Huang, W.T.; Shao, D.Z.; Liao, J.F.; Lin, M.T. Platonin, a cyanine photosensitizing dye, inhibits pyrogen release and results in antipyresis. J. Pharm. Sci. 2003, 93, 376–380. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.C.; Lee, J.J.; Tsai, P.S.; Lu, Y.T.; Huang, C.L.; Huang, C.J. Platonin attenuates LPS-induced CAT-2 and CAT-2B induction in stimulated murine macrophages. Acta Anaesthesiol. Scand. 2006, 50, 604–612. [Google Scholar] [CrossRef]
- Hsiao, G.; Lee, J.J.; Chou, D.S.; Fong, T.H.; Shen, M.Y.; Lin, C.H.; Sheu, J.R. Platonin, a photosensitizing dye, improves circulatory failure and mortality in rat models of endotoxemia. Biol. Pharm. Bull. 2002, 25, 995–999. [Google Scholar] [CrossRef] [Green Version]
- Sheu, J.R.; Chen, Z.C.; Jayakumar, T.; Chou, D.S.; Yen, T.L.; Lee, H.N.; Pan, S.H.; Hsia, C.H.; Yang, C.H.; Hsieh, C.Y. A novel indication of platonin, a therapeutic immunomodulating medicine, on neuroprotection against ischemic stroke in mice. Sci Rep. 2017, 7, 42277. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Hsu, M.J.; Hsiao, G.; Wang, Y.H.; Huang, C.W.; Chen, S.W.; Jayakumar, T.; Chiu, P.T.; Chiu, Y.H.; Sheu, J.R. Andrographolide enhances nuclear factor-kappaB subunit p65 Ser536 dephosphorylation through activation of protein phosphatase 2A in vascular smooth muscle cells. J. Biol. Chem. 2011, 286, 5942–5955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiolino, G.; Rossitto, G.; Caielli, P.; Bisogni, V.; Rossi, G.P.; Calò, L.A. The role of oxidized low-density lipoproteins in atherosclerosis: The myths and the facts. Mediat. Inflamm. 2013, 2013, 714653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Verma, I.M. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Hsiao, G.; Hsu, M.J.; Wang, Y.H.; Sheu, J.R. PMC, a potent hydrophilic α-tocopherol derivative, inhibits NF-κB activation via PP2A but not IκBα-dependent signals in vascular smooth muscle cells. J. Cell. Mol. Med. 2014, 18, 1278–1289. [Google Scholar] [CrossRef]
- Mazière, C.; Mazière, J.C. Activation of transcription factors and gene expression by oxidized low-density lipoprotein. Free Radic. Biol. Med. 2009, 46, 127–137. [Google Scholar] [CrossRef]
- Wu, Z.L.; Wang, Y.C.; Zhou, Q.; Ge, Y.Q.; Lan, Y. Oxidized LDL induces transcription factor activator protein-1 in rat mesangial cells. Cell Biochem. Funct. 2003, 21, 249–256. [Google Scholar] [CrossRef]
- Wang, H.H.; Hsieh, H.L.; Wu, C.Y.; Sun, C.C.; Yang, C.M. Oxidized low-density lipoprotein induces matrix metalloproteinase-9 expression via a p42/p44 and JNK-dependent AP-1 pathway in brain astrocytes. Glia 2009, 57, 24–38. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, L.; Chen, C.; Wang, Q.; Guo, L.; Ma, Q.; Deng, P.; Zhu, G.; Li, B.; Pi, Y.; et al. The critical role of ABCG1 and PPARγ/LXRα signaling in TLR4 mediates inflammatory responses and lipid accumulation in vascular smooth muscle cells. Cell Tissue Res. 2017, 368, 145–157. [Google Scholar] [CrossRef]
- Yin, Y.W.; Liao, S.Q.; Zhang, M.J.; Liu, Y.; Li, B.H.; Zhou, Y.; Chen, L.; Gao, C.Y.; Li, J.C.; Zhang, L.L. TLR4-mediated inflammation promotes foam cell formation of vascular smooth muscle cell by upregulating ACAT1 expression. Cell Death Dis. 2014, 5, e1574. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Uen, Y.H.; Chen, C.C.; Lin, S.C.; Tseng, S.Y.; Wang, Y.H.; Sheu, J.R.; Hsieh, C.Y. Platonin inhibited PDGF-BB-induced proliferation of rat vascular smooth muscle cells via JNK1/2-dependent signaling. Acta Pharm. Sin. 2011, 32, 1337–1344. [Google Scholar] [CrossRef]
- Yeh, C.T.; Kao, M.C.; Chen, C.H.; Huang, C.J. Platonin preserves blood-brain barrier integrity in septic rats. Acta Anaesthesiol Taiwan 2015, 53, 12–15. [Google Scholar] [CrossRef]
- Moriya, J. Critical roles of inflammation in atherosclerosis. J. Cardiol. 2019, 73, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Ramji, D.P.; Davies, T.S. Cytokines in atherosclerosis: Key players in all stages of disease and promising therapeutic targets. Cytokine Growth Factor Rev. 2015, 26, 673–685. [Google Scholar] [CrossRef] [Green Version]
- Bobryshev, Y.V.; Ivanova, E.A.; Chistiakov, D.A.; Nikiforov, N.G.; Orekhov, A.N. Macrophages and Their Role in Atherosclerosis: Pathophysiology and Transcriptome Analysis. Biomed Res. Int. 2016, 2016, 9582430. [Google Scholar] [CrossRef] [Green Version]
- Moore, K.J.; Tabas, I. Macrophages in the pathogenesis of atherosclerosis. Cell 2011, 145, 341–355. [Google Scholar] [CrossRef] [Green Version]
- Moore, K.J.; Sheedy, F.J.; Fisher, E.A. Macrophages in atherosclerosis: A dynamic balance. Nat. Rev. Immunol. 2013, 13, 709–721. [Google Scholar] [CrossRef]
- Gosling, J.; Slaymaker, S.; Gu, L.; Tseng, S.; Zlot, C.H.; Young, S.G.; Rollins, B.J.; Charo., I.F. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J. Clin. Invest. 1999, 103, 773–778. [Google Scholar] [CrossRef] [Green Version]
- Fatkhullina, A.R.; Peshkova, I.O.; Koltsova, E.K. The Role of Cytokines in the Development of Atherosclerosis. Biochemistry 2016, 81, 1358–1370. [Google Scholar] [CrossRef]
- Karin, M. Nuclear factor-kappaB in cancer development and progression. Nature 2006, 441, 431–436. [Google Scholar] [CrossRef]
- Ghosh, S.; Karin, M. Missing pieces in the NF-kappaB puzzle. Cell 2002, 109, S81–S96. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.Y.; Hsu, M.J.; Hsieh, C.Y.; Lee, L.W.; Chen, Z.C.; Sheu, J.R. Andrographolide inhibits nuclear factor-κB activation through JNK-Akt-p65 signaling cascade in tumor necrosis factor-α-stimulated vascular smooth muscle cells. Sci. World J. 2014, 2014, 130381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Mohamed, A.S.; Zhou, S.H. Oxidized low density lipoprotein, stem cells, and atherosclerosis. Lipids Health Dis. 2012, 11, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, M.W.; Chien, C.S.; Hsiao, L.D.; Lin, C.H.; Yang, C.M. OxLDL induces mitogen-activated protein kinase activation mediated via PI3-kinase/Akt in vascular smooth muscle cells. J. Lipid Res. 2003, 44, 1667–1675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garces de Los Fayos Alonso, I.; Liang, H.C.; Turner, S.D.; Lagger, S.; Merkel, O.; Kenner, L. The Role of Activator Protein-1 (AP-1) Family Members in CD30-Positive Lymphomas. Cancers 2018, 10, 93. [Google Scholar] [CrossRef] [Green Version]
- Ley, K.; Miller, Y.I.; Hedrick, C.C. Monocyte and macrophage dynamics during atherogenesis. Arter. Thromb. Vasc. Biol. 2011, 31, 1506–1516. [Google Scholar] [CrossRef] [Green Version]
- Allahverdian, S.; Pannu, P.S.; Francis, G.A. Contribution of monocyte-derived macrophages and smooth muscle cells to arterial foam cell formation. Cardiovasc. Res. 2012, 95, 165–172. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, C.-W.; Yang, C.-H.; Tsai, J.-H.; Hsieh, C.-Y.; Huang, S.-Y. Platonin, a Cyanine Photosensitizing Dye, Ameliorates Inflammatory Responses in Vascular Smooth Muscle Cells by Modulating Inflammatory Transcription Factors. Appl. Sci. 2021, 11, 1130. https://doi.org/10.3390/app11031130
Chiu C-W, Yang C-H, Tsai J-H, Hsieh C-Y, Huang S-Y. Platonin, a Cyanine Photosensitizing Dye, Ameliorates Inflammatory Responses in Vascular Smooth Muscle Cells by Modulating Inflammatory Transcription Factors. Applied Sciences. 2021; 11(3):1130. https://doi.org/10.3390/app11031130
Chicago/Turabian StyleChiu, Chih-Wei, Chih-Hao Yang, Jie-Heng Tsai, Cheng-Ying Hsieh, and Shih-Yi Huang. 2021. "Platonin, a Cyanine Photosensitizing Dye, Ameliorates Inflammatory Responses in Vascular Smooth Muscle Cells by Modulating Inflammatory Transcription Factors" Applied Sciences 11, no. 3: 1130. https://doi.org/10.3390/app11031130




