Microstructure and Corrosion Performance of Aluminium Matrix Composites Reinforced with Refractory High-Entropy Alloy Particulates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication
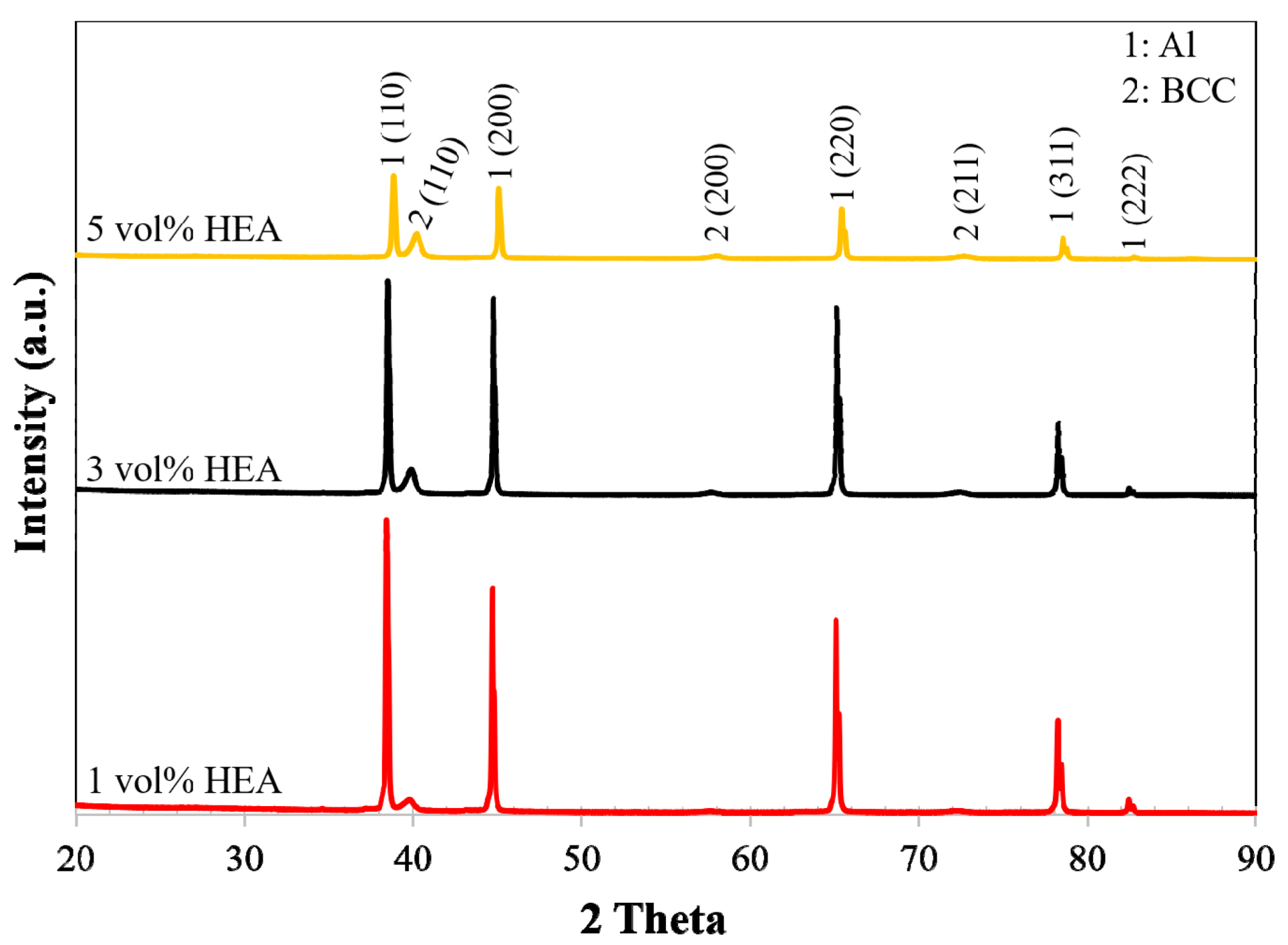
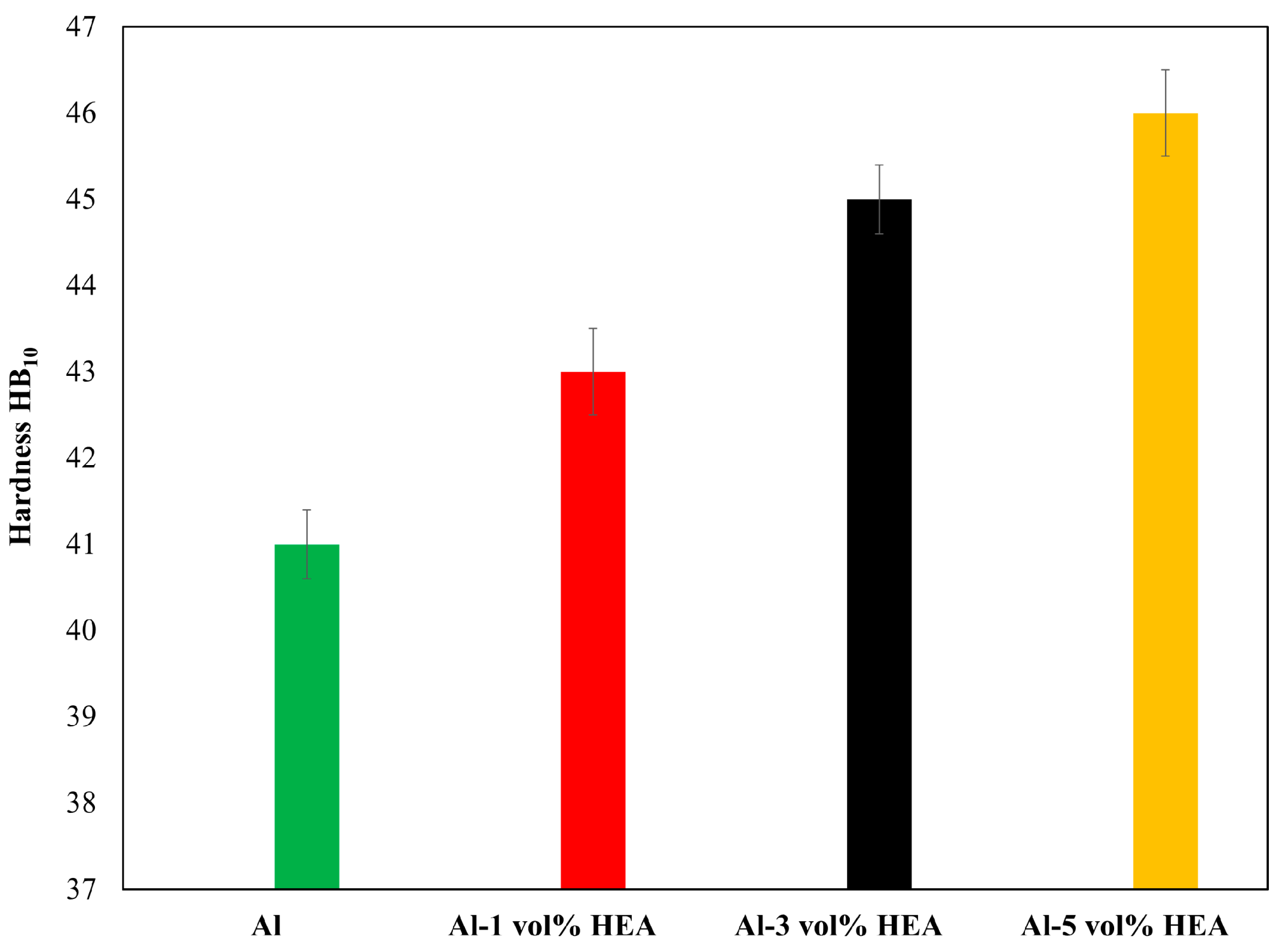
2.2. X-ray Diffraction and Hardness Testing
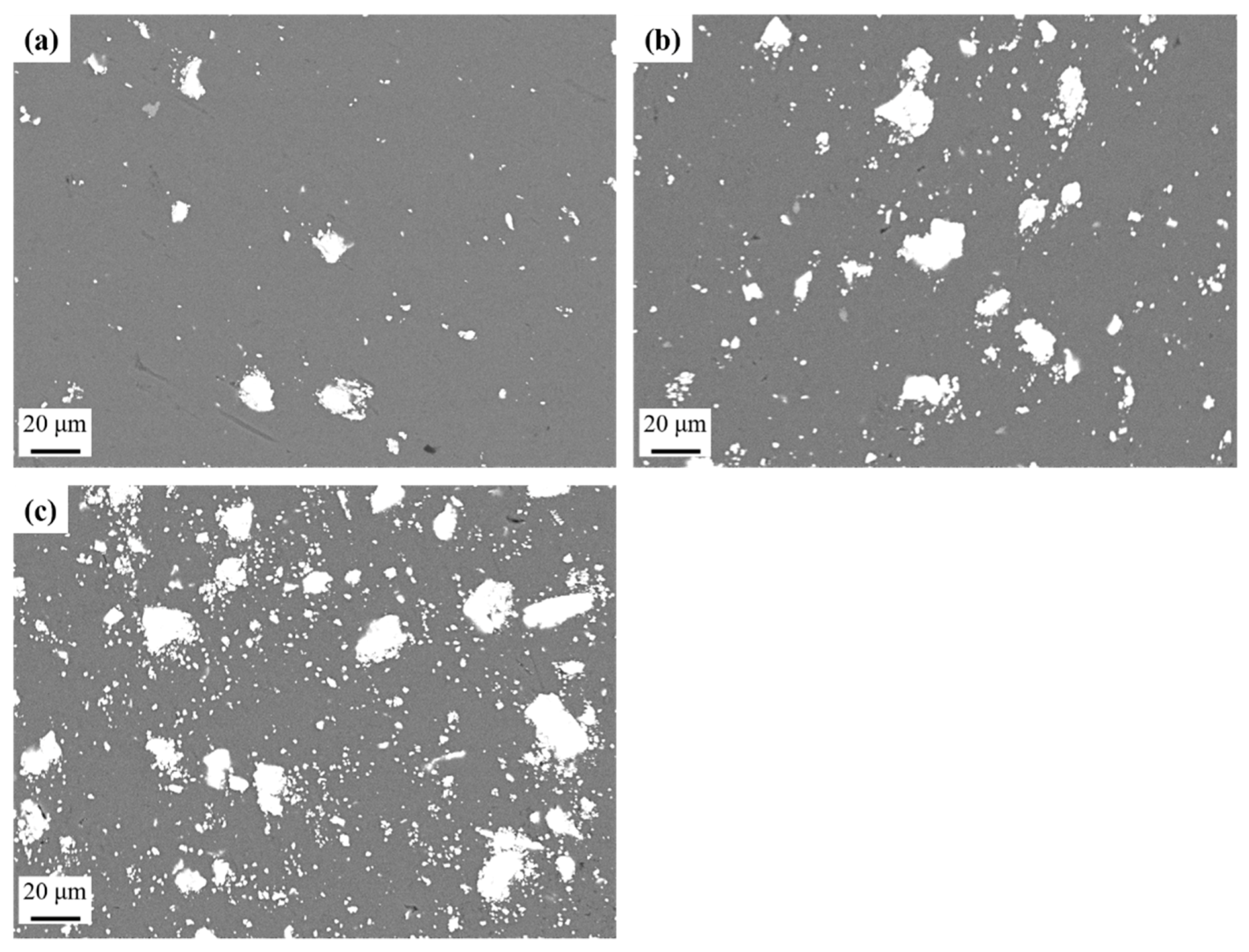
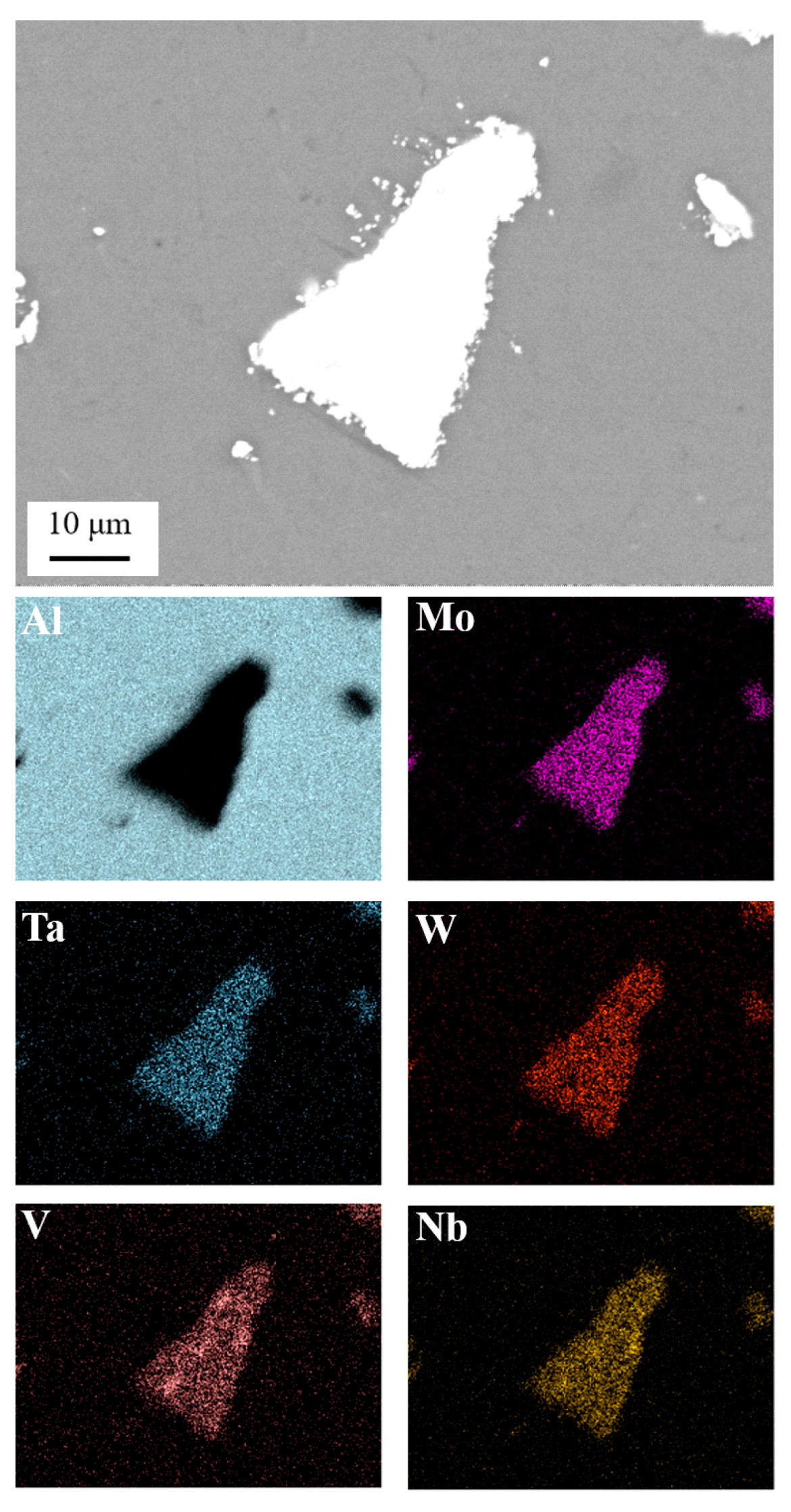
2.3. Scanning Electron Microscopy
2.4. Corrosion Testing
3. Results and Discussion
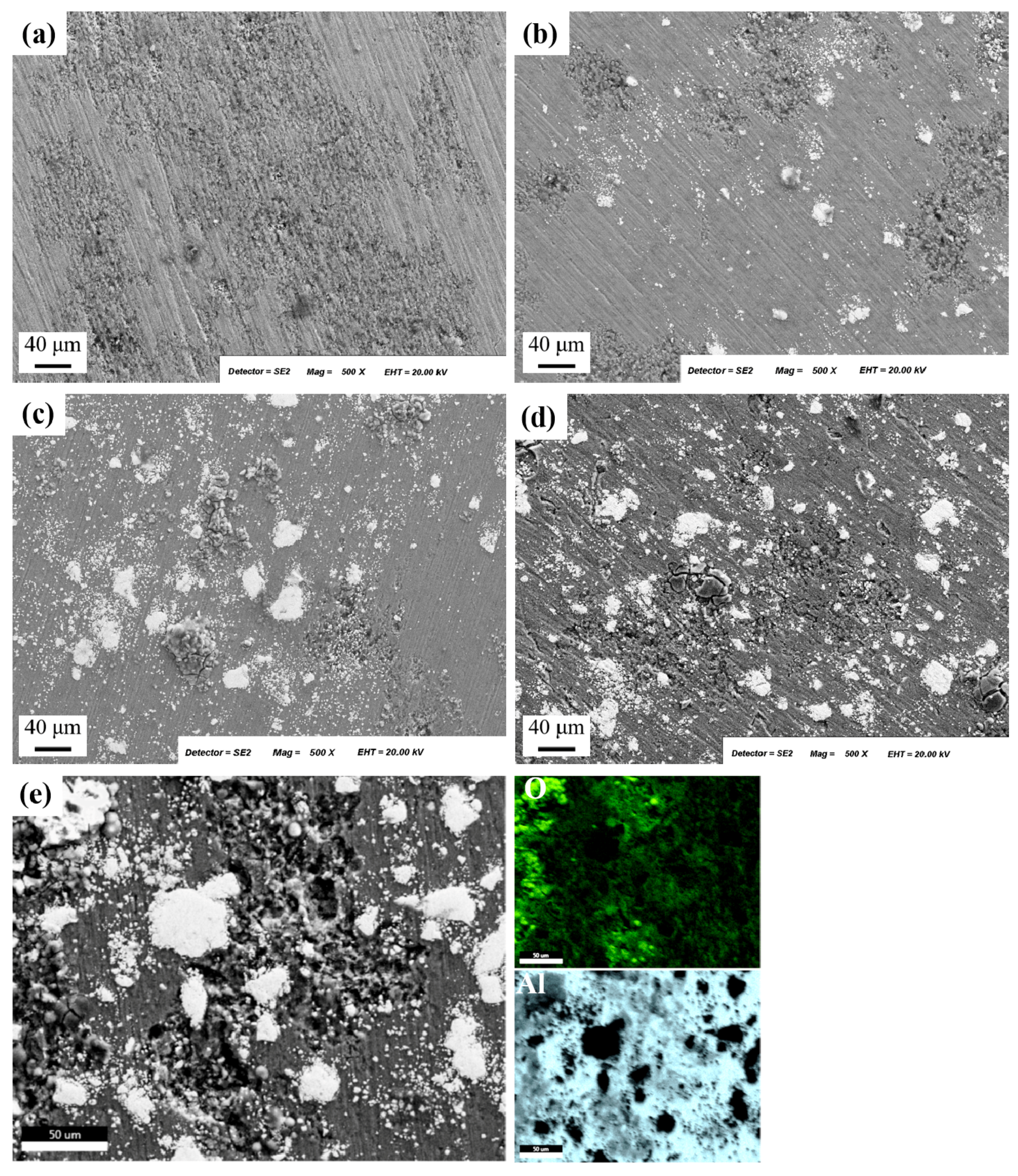
3.1. Microstructural Analysis-Hardness
3.2. Corrosion Performance
4. Conclusions
- Novel aluminium matrix composites reinforced by MoTaNbVW refractory high-entropy alloy particulates have been successfully produced by powder metallurgy.
- The microstructure of the produced AMCs appears to be homogenous, with low porosity, few defects, and good distribution of the reinforcing phase in the Al matrix.
- No secondary intermetallic phases have been formed while the interface between matrix and reinforcement showed good bonding without any signs of reactivity.
- Increasing the volume of the reinforcing phase leads to increased hardness values.
- Al-HEA composites exhibited typical polarization behaviour for Al and Al alloys in NaCl solutions. All examined materials were susceptible to localised forms of corrosion in 3.5% NaCl solution, while aluminium matrix on the reinforcement/matrix interface has been subject to selective dissolution. The corrosion behaviour of the produced composites appears to be mainly controlled by the corrosion of the matrix.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Surappa, M.K. Aluminium matrix composites: Challenges and opportunities. Sadhana 2003, 28, 319–334. [Google Scholar] [CrossRef]
- Wlodarczyk-Fligier, A.; Dobrzanski, L.A.; Adamiak, M. Influence of heat treatment on properties and corrosion resistance of Al-composite. J. Achiev. Mater. Manuf. Eng. 2007, 21, 55–58. [Google Scholar]
- Mattern, A.; Huchler, B.; Staudenecker, D.; Oberacker, R.; Nagel, A.; Hofmann, M.J. Preparation of interpenetrating ceramic-metal composites. J. Eur. Ceram. Soc. 2004, 24, 3399–3408. [Google Scholar] [CrossRef]
- Prabakaran, R.K.; Sait, A.N.; Sentilkumar, V. Synthesis and Characteristization of High Entropy Alloy (CrMnFeNiCu) Reinforced AA6061 Aluminium Matrix Composite. J. Mech. Mechan. Eng. 2017, 21, 415–424. [Google Scholar]
- Miracle, D.B.; Donaldson, S.L. Handbook ASM; ASM International: Materials Park, OH, USA, 2001; pp. 27–34. [Google Scholar]
- El-Aziz, K.A.; Saber, D.; Sallam, H.E.-D.M. Wear and Corrosion Behavior of Al-Si Matrix Composite Reinforced with Alumina. J. Bio. Tribo. Corros. 2015, 1, 5. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.C.; Chan, S.L.I. Tensile properties of nanometric Al2O3 particulate-reinforced aluminum matrix composites. Mater. Chem. Phys. 2004, 85, 438–443. [Google Scholar] [CrossRef]
- Mazahery, A.; Ostadshabani, M. Investigation on mechanical properties of nano-Al2O3-reinforced aluminum matrix composites. J. Comp. Mat. 2011, 45, 2579–2586. [Google Scholar] [CrossRef]
- Hesabi, Z.R.; Simchi, A.; Reihani, S.M.S. Structural evolution during mechanical milling of nanometric and micrometric Al2O3 reinforced Al matrix composites. Mater. Sci. Eng. A 2006, 428, 159–168. [Google Scholar] [CrossRef]
- Gurrappa, I.; Bhanu Prasad, V.V. Corrosion characteristics of aluminium based metal matrix composites. Mater. Sci. Tech. 2006, 22, 115–122. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Chen, Z.H.; Wu, H.L.; Wang, H.M. The Corrosion Behaviour of the Aluminum Alloy 7075/SiCp Metal Matrix Composite Prepared by Spray Deposition. Mater. Corros. 2007, 58, 280–284. [Google Scholar] [CrossRef]
- Candan, S.; Bilgic, E. Corrosion Behavior of Al-60 vol.% SiCp Composites in NaCl Solution. Mater. Lett. 2004, 58, 2787–2790. [Google Scholar] [CrossRef]
- Zakaria, H.M. Microstructural and corrosion behavior of Al/SiC metal matrix composites. Ain Shams Eng. J. 2014, 5, 831–838. [Google Scholar] [CrossRef]
- Lekatou, A.G.; Poulia, A.; Mavros, H.; Karantzalis, A.E. Thermal Treatment, Sliding Wear and Saline Corrosion of Al In Situ Reinforced with Mg2Si and Ex Situ Reinforced with TiC Particles. J. Mater. Eng. Perform. 2018, 27, 5030–5039. [Google Scholar] [CrossRef]
- Lekatou, A.; Karantzalis, A.E.; Evangelou, A.; Gousia, V.; Kaptay, G.; Gácsi, Z.; Baumli, P.; Simon, A. Aluminium reinforced by WC and TiC nanoparticles (ex-situ) and aluminide particles (in-situ): Microstructure, wear and corrosion behaviour. Mater. Des. 2015, 65, 1121–1135. [Google Scholar] [CrossRef]
- Dikici, B.; Bedir, F.; Gavgali, M. The effect of high TiC particle content on the tensile cracking and corrosion behavior of Al–5Cu matrix composites. J. Comp. Mater. 2020, 54, 1681–1690. [Google Scholar] [CrossRef]
- Han, Y.M.; Chen, X.G. Electrochemical Behavior of Al-B4C Metal Matrix Composites in NaCl Solution. Materials 2015, 32, 6455–6470. [Google Scholar] [CrossRef] [Green Version]
- Kalaiselvan, K.; Murugan, N.; Parameswaran, S. Production and characterization of AA6061–B4C stir cast composite. Mater. Des. 2011, 32, 4004–4009. [Google Scholar] [CrossRef]
- Topcu, I.; Gulsoy, H.O.; Kadioglu, N.; Gulluoglu, A.N. Processing and mechanical properties of B4C reinforced Al matrix composites. J. Alloy Compd. 2009, 482, 516–521. [Google Scholar] [CrossRef]
- Nie, C.; Gu, J.; Liu, J.; Zhang, D. nvestigation on microstructures and interface character of B4C particles reinforced 2024Al matrix composites fabricated by mechanical alloying. J. Alloy Compd. 2008, 454, 118–122. [Google Scholar] [CrossRef]
- Nunes, P.C.R.; Ramanathan, L.V. Corrosion Behavior of Alumina-Aluminum and Silicon Carbide-Aluminum Metal-Matrix Composites. Corrosion 1995, 51, 610–617. [Google Scholar] [CrossRef]
- Liu, Z.S.; Wu, B.T.; Gu, M.Y. Effect of AlN Particles on the Corrosion Behavior of Al/AlNp Composites. J. Mater. Sci. 2007, 42, 5736–5741. [Google Scholar] [CrossRef]
- Nath, D.; Namboodhiri, T.K.G. Some corrosion characteristics of aluminium-mica particulate composites. Corros. Sci. 1989, 23, 1215–1221, 1223–1229. [Google Scholar] [CrossRef]
- Hihara, L.H.; Latanision, R.M. Galvanic Corrosion of Aluminum-Matrix Composites. Corrosion 1992, 48, 546–552. [Google Scholar] [CrossRef]
- Trowsdale, A.G.; Noble, B.; Harris, S.J.; Gibbins, I.S.R.; Thompson, G.E.; Wood, G.C. The influence of silicon carbide reinforcement on the pitting behaviour of aluminium. Corros. Sci. 1996, 38, 177–191. [Google Scholar] [CrossRef]
- De Salazar, J.M.G.; Urena, A.; Manzanedo, S.; Barrena, M.I. Corrosion behaviour of AA6061 and AA7005 reinforced with Al2O3 particles in aerated 3.5% chloride solutions: Potentiodynamic measurements and microstructure evaluation. Corros. Sci. 1988, 41, 529–545. [Google Scholar] [CrossRef]
- Paciej, R.C.; Agarwala, V.S. Influence of Processing Variables on the Corrosion Susceptibility of Metal-Matrix Composites. Corrosion 1988, 44, 680–684. [Google Scholar] [CrossRef]
- Lekatou, A.G.; Gkikas, N.; Gousia, V.; Lentzaris, K.; Karantzalis, A.E. Effects of In Situ Intermetallics on the Microstructural Array and Saline Corrosion Performance of Cast Al/WCp Composites. J. Mater. Eng. Perform. 2018, 27, 5164–5176. [Google Scholar] [CrossRef]
- Ananda Murthy, H.C.; Bheema Raju, V.; Shivakumara, C. Effect of TiN Particulate Reinforcement on Corrosive Behaviour of Aluminium 6061 Composites in Chloride Medium. Bull. Mater. Sci. 2013, 36, 1057–1066. [Google Scholar] [CrossRef] [Green Version]
- Seah, K.H.W.; Krishna, M.; Vijayalakshmi, V.T.; Uchil, J. Corrosion Behaviour of Garnet Particulate Reinforced LM13 Al Alloy MMCs. Corros. Sci. 2002, 44, 917–925. [Google Scholar] [CrossRef]
- Griffiths, A.J.; Turnbull, A. An investigation of the electrochemical polarization behaviour of 6061 aluminum metal matrix composites. Corros. Sci. 1994, 34, 23–35. [Google Scholar] [CrossRef]
- Hu, J.; Chu, W.Y.; Fei, W.D.; Zhao, L.C. Effect of Interfacial Reaction on Corrosion Behavior of Alumina Borate Whisker Reinforced 6061Al Composite. Mater. Sci. Eng. A 2004, 374, 153–159. [Google Scholar] [CrossRef]
- Kiourtsidis, G.E.; Skolianos, S.M. Corrosion Behavior of Squeeze-Cast Silicon Carbide-2024 Composites in Aerated 3.5 wt.% Sodium Chloride. Mater. Sci. Eng. A 1998, 248, 165–172. [Google Scholar] [CrossRef]
- Albiter, A.; Contreras, A.; Salazar, M.; Gonzalez-Rodriguez, J.G. Corrosion Behaviour of Aluminium Metal Matrix Composites Reinforced with TiC Processed by Pressureless Melt Infiltration. App. Electr. 2006, 36, 303–308. [Google Scholar] [CrossRef]
- Kiourtsidis, G.E.; Skolianos, S.M. Pitting Corrosion of Artificially Aged T6 AA2024/SiCp Composites in 3.5 wt.% NaCl Aqueous Solution. Corros. Sci. 2007, 49, 2711–2725. [Google Scholar] [CrossRef]
- Alaneme, K.K.; Bodunrin, M.O. Corrosion Behavior of Alumina Reinforced Aluminum (6063) Metal Matrix Composites. J. Min. Mat. Char. Eng. 2011, 10, 1153–1165. [Google Scholar]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, T.S.; Chin, T.S.; Shun, T.T. Nanostructured high entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mat. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Yeh, J. Recent progress in high-entropy alloys. Ann. Chim. Sci. Mat. 2006, 31, 633–648. [Google Scholar] [CrossRef]
- Karthik, G.M.; Panikar, S.; Janaki Ram, G.D.; Kottada, R.S. Additive manufacturing of an aluminum matrix composite reinforced with nanocrystalline high-entropy alloy particles. Mater. Sci. Eng. A 2017, 679, 193–203. [Google Scholar] [CrossRef]
- Yang, X.; Dong, P.; Yan, Z.; Cheng, B.; Zhai, X.; Chen, H.; Zhang, H.; Wang, W. AlCoCrFeNi high-entropy alloy particle reinforced 5083Al matrix composites with fine grain structure fabricated by submerged friction stir processing. J. Alloy. Compd. 2020, 836, 155411. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Li, Z.; Wang, X.; Fan, X.; Liu, J. Formation of transition layer and its effect on mechanical properties of AlCoCrFeNi high-entropy alloy/Al composites. J. Alloy. Compd. 2019, 780, 558–564. [Google Scholar] [CrossRef]
- Tan, Z.; Wang, L.; Xue, Y.; Zhang, P.; Cao, T.; Cheng, X. High entropy alloy particle reinforced Al-based amorphous alloy composite with ultrahigh strength prepared by spark plasma sintering. Mater. Des. 2016, 109, 219–226. [Google Scholar] [CrossRef]
- Lu, T.; Scudino, S.; Chen, W.; Wang, P.; Li, D.; Mao, M.; Kang, L.; Liu, Y.; Fu, Z. The influence of nanocrystalline CoNiFeAl0.4Ti0.6Cr0.5 high-entropy alloy particles addition on microstructure and mechanical properties of SiCp/ 7075Al composites. Mater. Sci. Eng. A 2018, 726, 126–136. [Google Scholar] [CrossRef]
- Li, Q.; Bao, X.; Zhao, S.; Zhu, Y.; Lan, Y.; Feng, X.; Zhang, Q. The Influence of AlFeNiCrCoTi High-Entropy Alloy on Microstructure, Mechanical Properties and Tribological Behaviors of Aluminum Matrix Composites. Int. J. Met. 2020, 14, 1–11. [Google Scholar] [CrossRef]
- Kumar, K.P.; Krishna, G.M.; Rao, J.B.; Bhargava, N. Fabrication and characterization of 2024 aluminium—High entropy alloy composites. J. Alloy Comp. 2015, 640, 421–427. [Google Scholar] [CrossRef]
- Yuan, Z.; Tian, W.; Li, F.; Fu, Q.; Wang, X.; Qian, W.; An, W. Effect of heat treatment on the interface of high-entropy alloy particles reinforced aluminum matrix composites. J. Alloy. Comp. 2020, 822, 153658. [Google Scholar] [CrossRef]
- Wang, N.; Wu, B.; Wu, W.; Li, J.; Ge, C.; Dong, Y.; Zhang, L.; Wang, Y. Microstructure and properties of aluminium-high entropy alloy composites fabricated by mechanical alloying and spark plasma sintering. Mater. Today Commun. 2020, 25, 101366. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Poulia, A.; Georgatis, E.; Lekatou, A.; Karantzalis, A.E. Microstructure and wear behavior of a refractory high entropy alloy. Int. J. Refract. Met. Hard Mater. 2016, 57, 50–63. [Google Scholar] [CrossRef]
- Poulia, A.; Georgatis, E.; Lekatou, A.; Karantzalis, A.E. Dry sliding wear response of MoTaWNbV high entropy alloy. Adv. Eng. Mater. 2017, 19, 1600535. [Google Scholar] [CrossRef]
- Lekatou, A.; Sfikas, A.K.; Karantzalis, A.E.; Sioulas, D. Microstructure and corrosion performance of Al-32% Co alloys. Corros. Sci. 2012, 63, 193–209. [Google Scholar] [CrossRef]
- Lekatou, A.; Sfikas, A.K.; Petsa, C.; Karantzalis, A.E. Al-Co Alloys Prepared by Vacuum Arc Melting: Correlating Microstructure Evolution and Aqueous Corrosion Behavior with Co Content. Metals 2016, 6, 46. [Google Scholar] [CrossRef] [Green Version]
- Lekatou, A.; Sfikas, A.K.; Karantzalis, A.E. The influence of the fabrication route on the microstructure and surface degradation properties of Al reinforced by Al9Co2. Mater. Chem. Phys. 2017, 200, 33–49. [Google Scholar] [CrossRef]
- Poulia, A. Development and Characterization of Refractory High Entropy Alloys-Evaluation of Their Mechanical Properties and Surface Degradation Behavior. Ph.D. Thesis, University of Ioannina, Ioannina, Greece, 2018. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ananiadis, E.; Argyris, K.T.; Matikas, T.E.; Sfikas, A.K.; Karantzalis, A.E. Microstructure and Corrosion Performance of Aluminium Matrix Composites Reinforced with Refractory High-Entropy Alloy Particulates. Appl. Sci. 2021, 11, 1300. https://doi.org/10.3390/app11031300
Ananiadis E, Argyris KT, Matikas TE, Sfikas AK, Karantzalis AE. Microstructure and Corrosion Performance of Aluminium Matrix Composites Reinforced with Refractory High-Entropy Alloy Particulates. Applied Sciences. 2021; 11(3):1300. https://doi.org/10.3390/app11031300
Chicago/Turabian StyleAnaniadis, Elias, Konstantinos T. Argyris, Theodore E. Matikas, Athanasios K. Sfikas, and Alexandros E. Karantzalis. 2021. "Microstructure and Corrosion Performance of Aluminium Matrix Composites Reinforced with Refractory High-Entropy Alloy Particulates" Applied Sciences 11, no. 3: 1300. https://doi.org/10.3390/app11031300
APA StyleAnaniadis, E., Argyris, K. T., Matikas, T. E., Sfikas, A. K., & Karantzalis, A. E. (2021). Microstructure and Corrosion Performance of Aluminium Matrix Composites Reinforced with Refractory High-Entropy Alloy Particulates. Applied Sciences, 11(3), 1300. https://doi.org/10.3390/app11031300






