Effect of Pd on the Electrocatalytic Activity of Pt towards Oxidation of Ethanol in Alkaline Solutions
Abstract
1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Synthesis of Nanoparticles and Modification of GCE
3. Results and Discussion
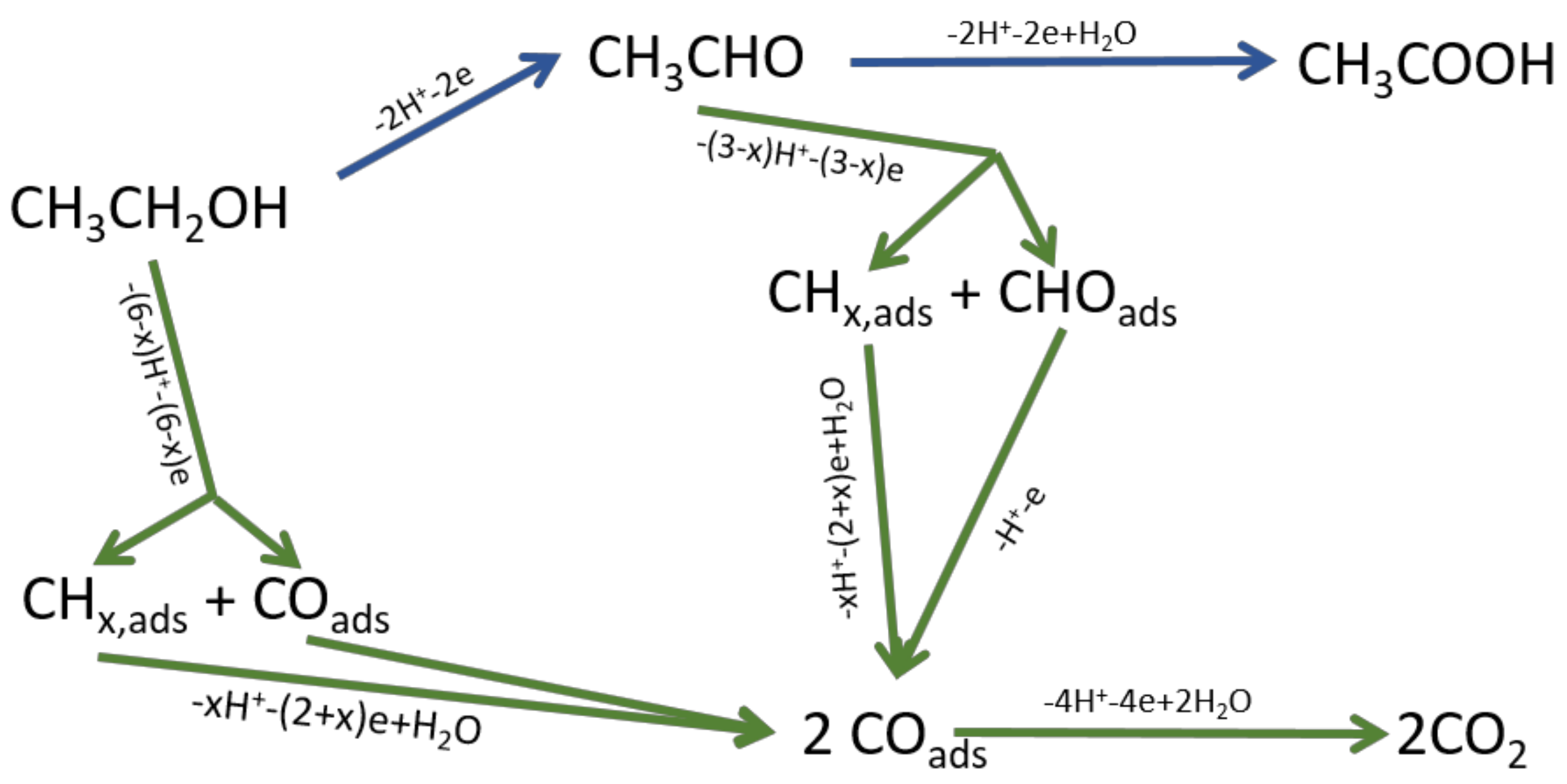
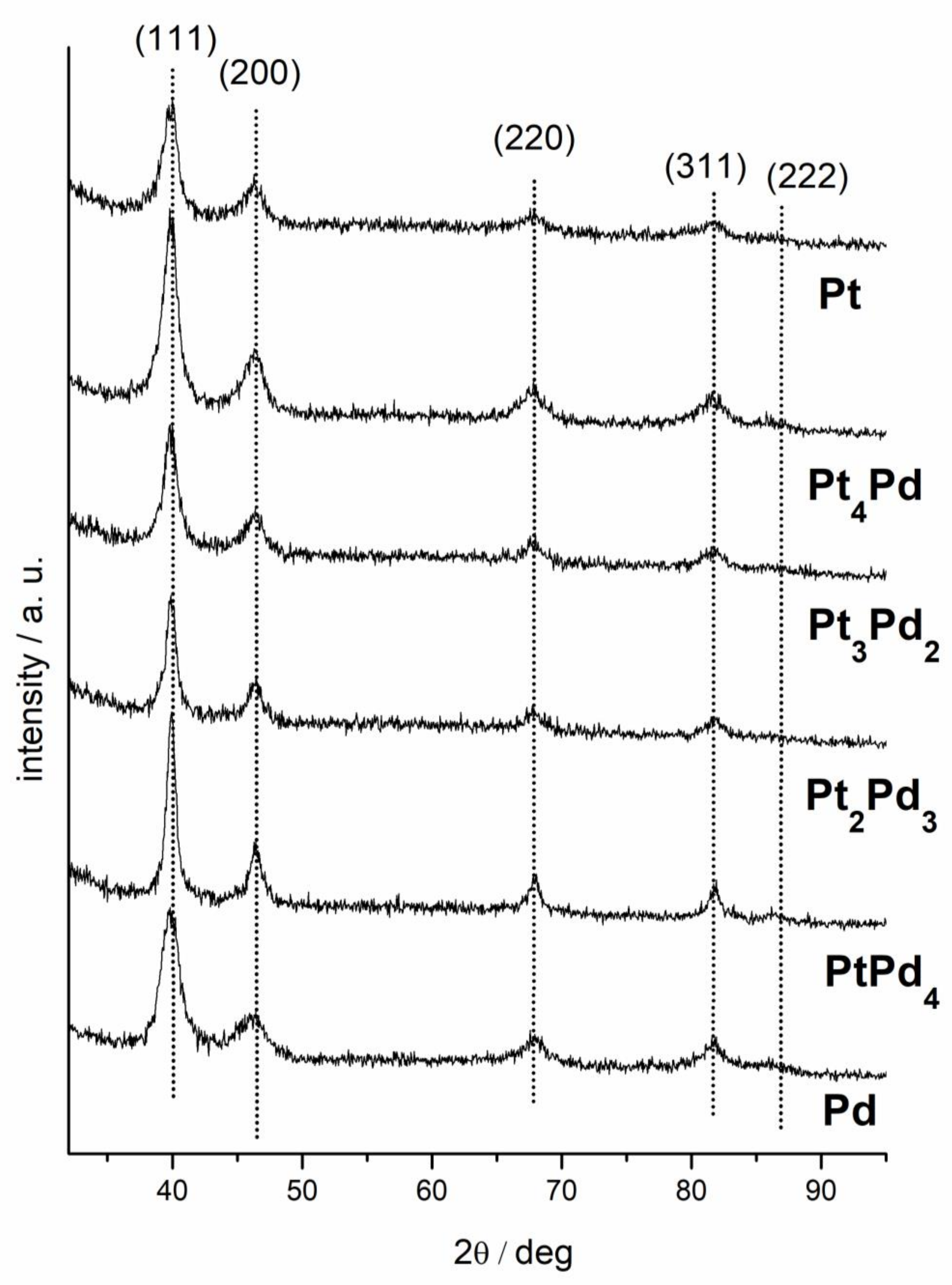
3.1. Characterization of the Nanoparticles
3.2. Electrochemical Experiments
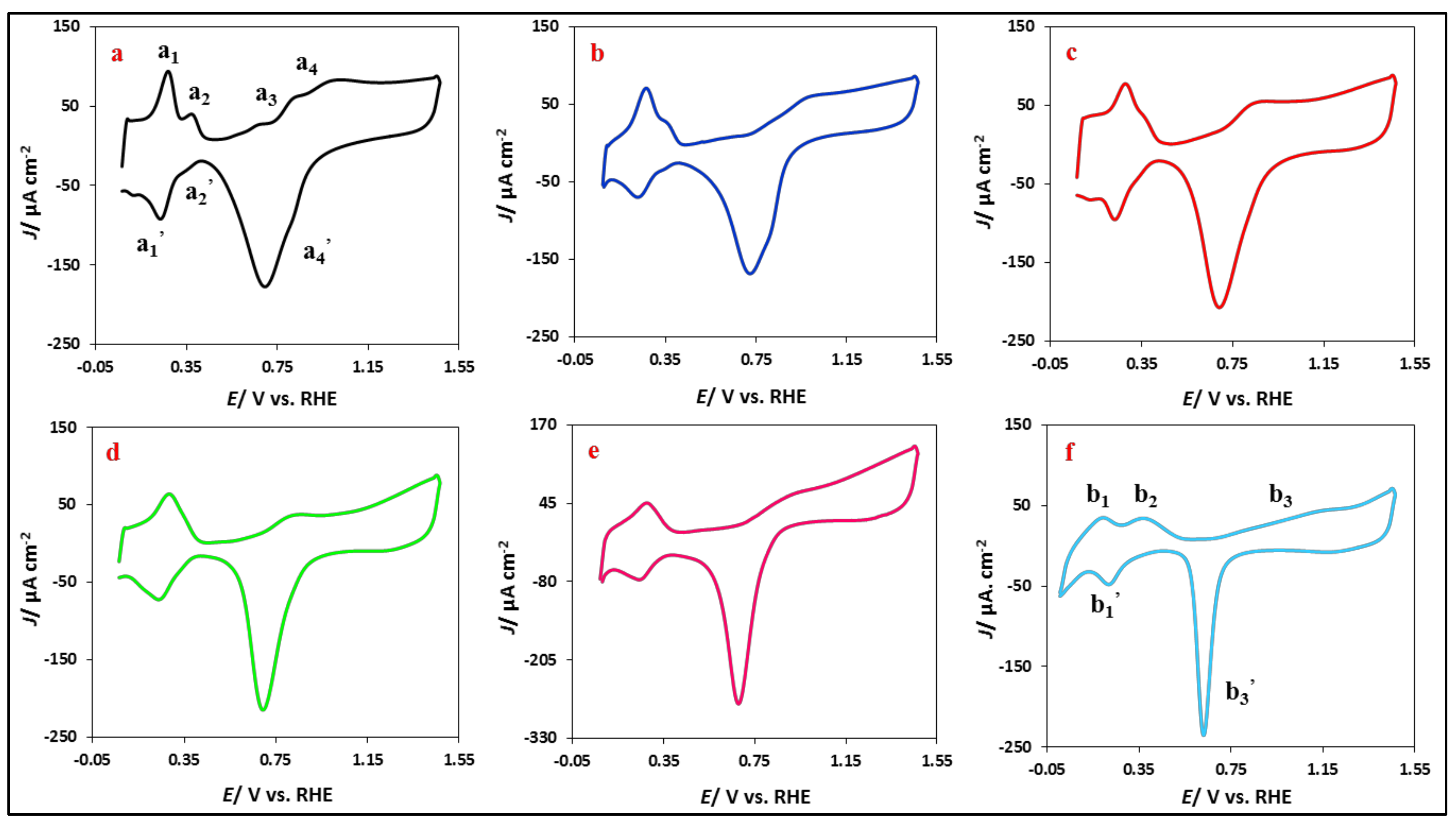
3.2.1. Electrochemical Characterization: Background Examination in the Alkaline Media
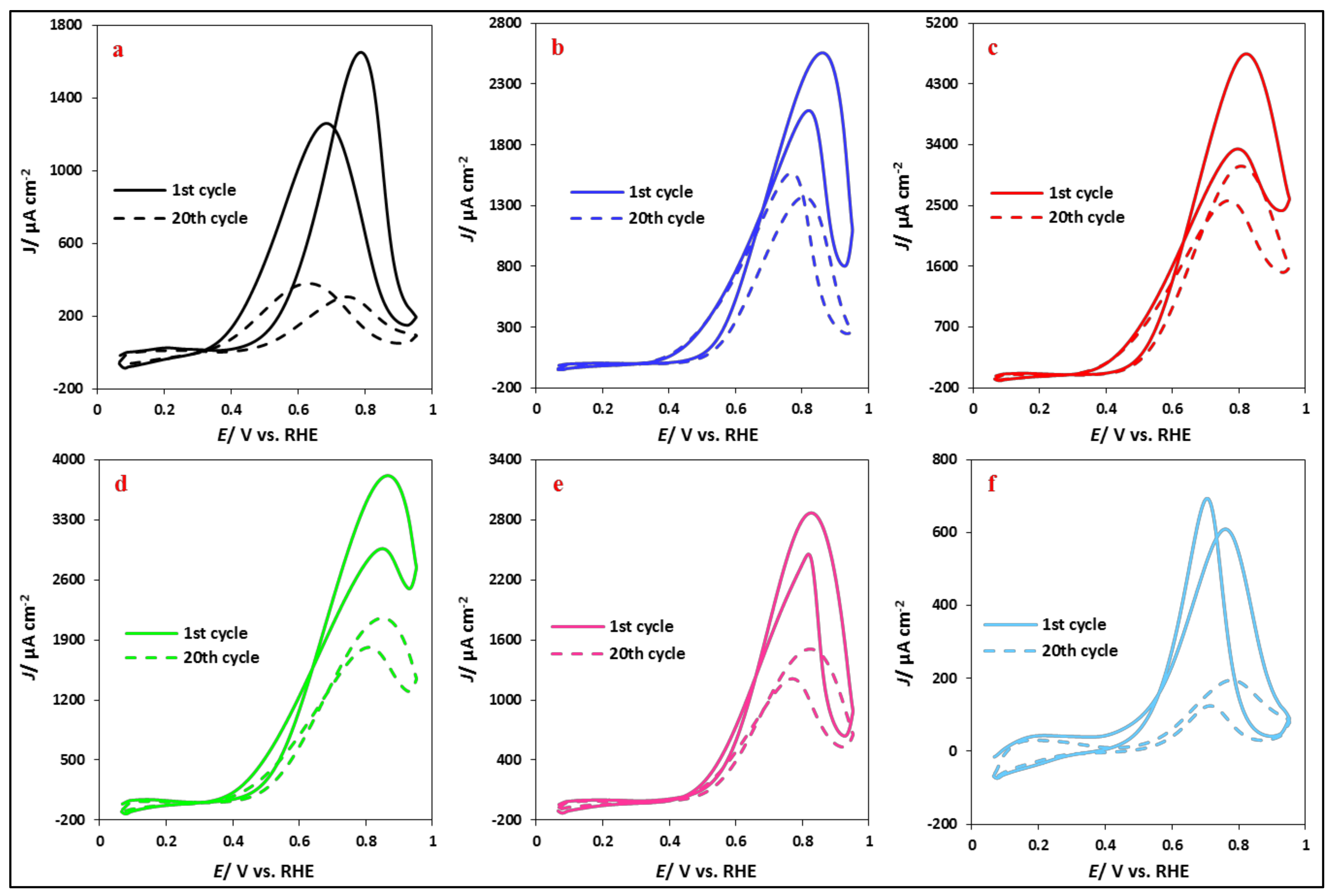
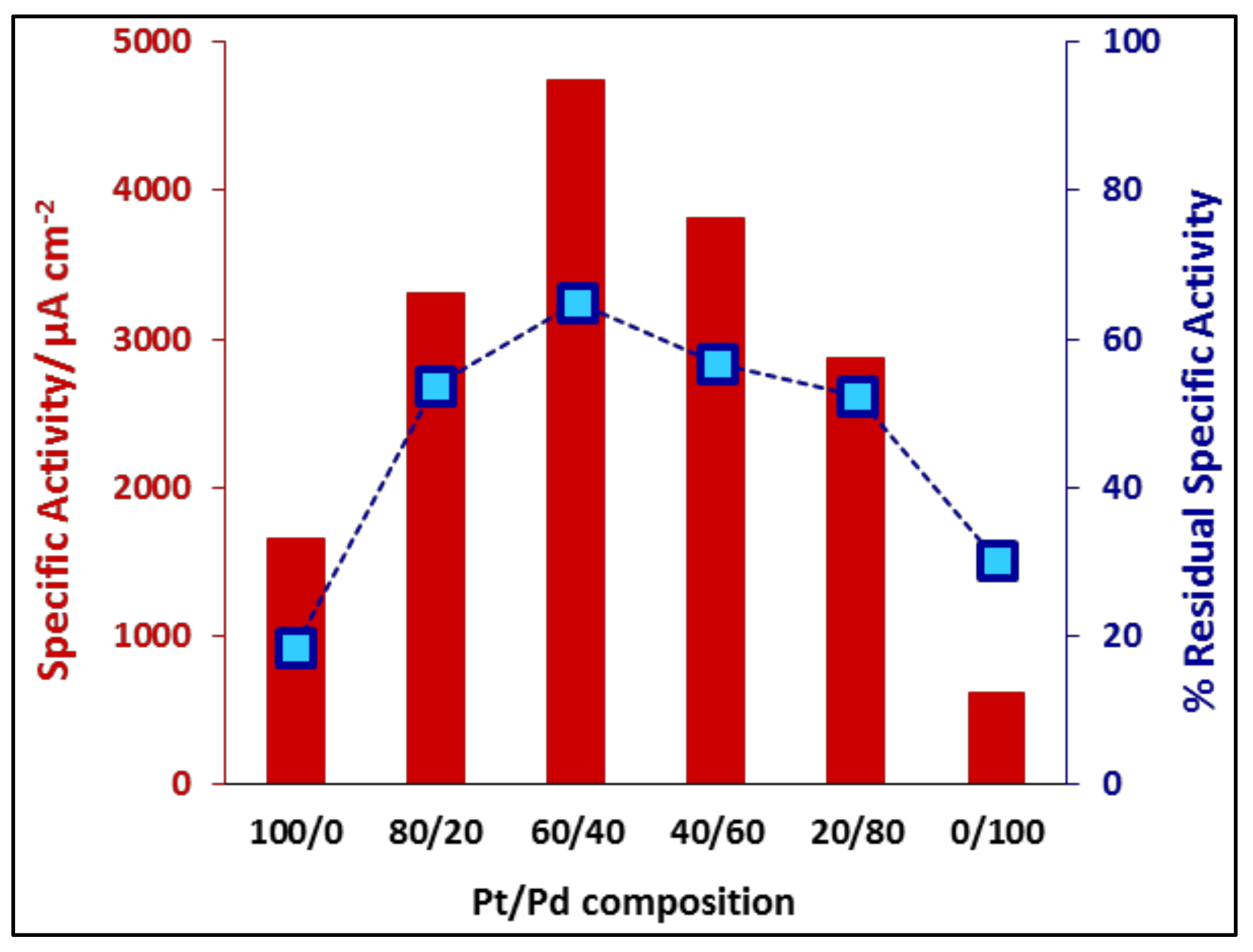
3.2.2. Ethanol Electrooxidation: Potentiodynamic Measurements
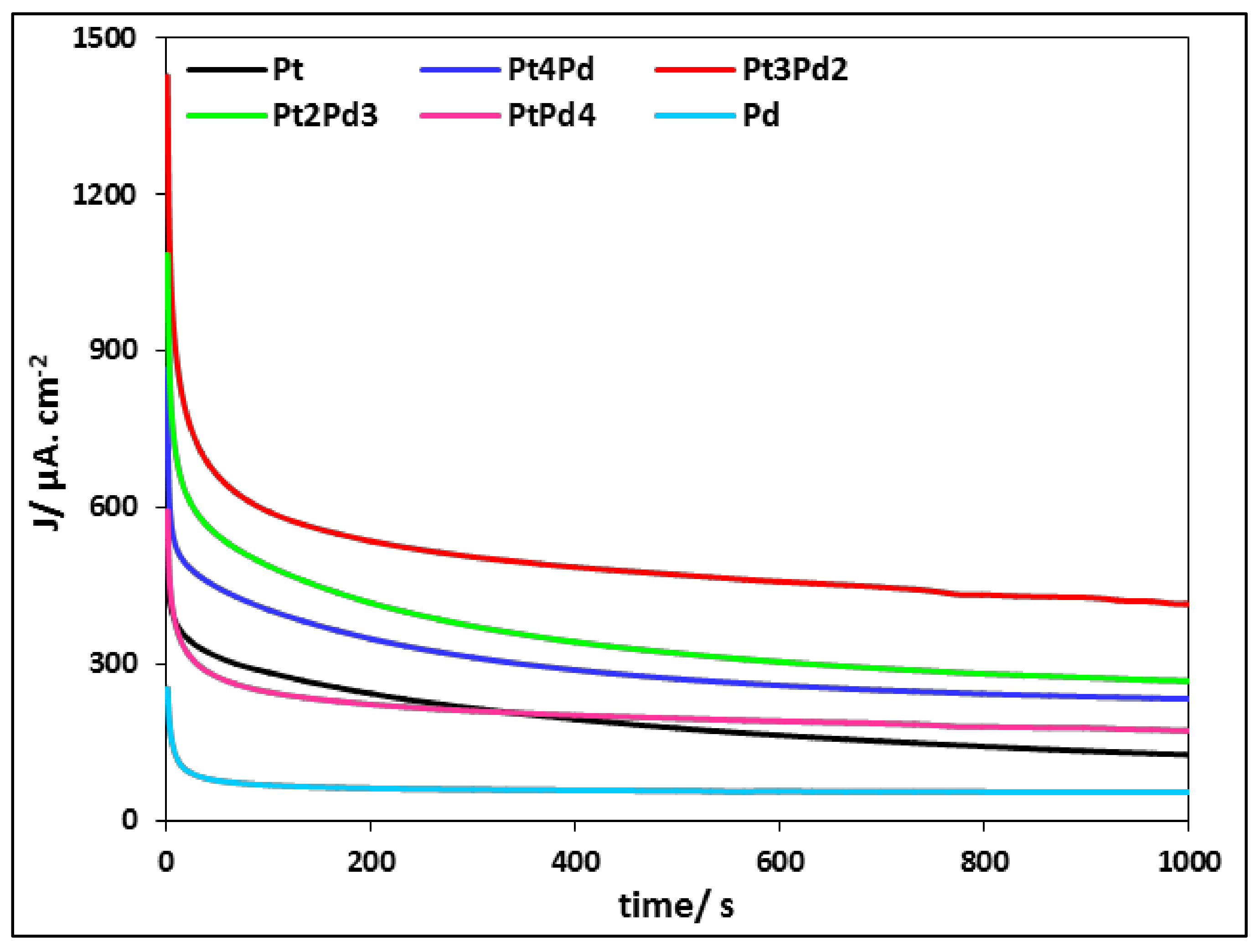
3.2.3. Ethanol Electrooxidation: Potentiostatic Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Inci, M.; Türksoy, O. Review of fuel cells to grid interface: Configurations, technical challenges and trends. J. Clean. Prod. 2019, 213, 1353–1370. [Google Scholar] [CrossRef]
- Rana, M.; Patil, P.K.; Chhetri, M.; Dileep, K.; Datta, R.; Gautam, U.K. Pd-Pt alloys nanowires as support-less electrocatalyst with high synergistic enhancement in efficiency for methanol oxidation in acidic medium. J. Colloid Interface Sci. 2016, 463, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhang, X.; Wang, Q.; Han, Y.; Fang, Y.; Dong, S. Shape-Control of Pt-Ru nanocrystals: Tuning surface structure for enhanced electrocatalytic methanol oxidation. J. Am. Chem. Soc. 2018, 140, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Ong, B.C.; Kamarudin, S.K.; Basri, S. Direct liquid fuel cells: A review. Int. J. Hydrog. Energy 2017, 42, 10142–10157. [Google Scholar] [CrossRef]
- Fadzillah, D.M.; Kamarudin, S.K.; Zainoodin, M.A.; Masdar, M.S. Critical challenges in the system development of direct alcohol fuel cells as portable power supplies: An overview. Int. J. Hydrog. Energy 2019, 44, 3031–3054. [Google Scholar] [CrossRef]
- Christensen, P.A.; Jones, S.W.M. An in situ FTIR study of ethanol oxidation at polycrystalline platinum in 0.1 M KOH at 25 and 50 °C. J. Phys. Chem. C 2014, 118, 29760–29769. [Google Scholar] [CrossRef]
- Jua, K.J.; Liu, L.; Feng, J.J.; Zhang, Q.L.; Wei, J.; Wang, A.J. Bio-Directed one-pot synthesis of Pt-Pd alloyed nanoflowers supported on reduced graphene oxide with enhanced catalytic activity for ethylene glycol oxidation. Electrochim. Acta 2016, 188, 696–703. [Google Scholar] [CrossRef]
- Qian, W.; Wilkinson, D.P.; Shen, J.; Wang, H.; Zhang, J. Architecture for portable direct liquid fuel cells. J. Power Sources 2006, 154, 202–213. [Google Scholar] [CrossRef]
- Li, N.H.; Sun, S.G. In situ FTIR spectroscopic studies of the electrooxidation of C4 alcohol on a platinum electrode in acid solutions Part, I. Reaction mechanism of 1-butanol oxidation. J. Electroanal. Chem. 1997, 436, 65–72. [Google Scholar]
- Jin, Z.; Wang, Q.; Zheng, W.; Cui, X. Highly ordered periodic Au/TiO2 hetero-nanostructures for plasmon-induced enhancement of the activity and stability for ethanol electro-oxidation. ACS Appl. Mater. Interfaces 2016, 8, 5273–5279. [Google Scholar] [CrossRef]
- Kamyabi, M.A.; Mohammadian, H.; Jadali, S.; Moharramnezhad, M. Hydrothermal syntheses of NiO-GO nanocomposite on 3D nickel foam as a support for Pt nanoparticles and its superior electrocatalytic activity towards methanol oxidation. Electroanalysis 2019, 31, 1484–1493. [Google Scholar] [CrossRef]
- Kamyabi, M.A.; Nosratabad, E.T.; Jadali, S. A Pt-Polymer nanocomposite as the excellent electro-catalyst: Synthesis, characterization, and electrochemical behavior towards methanol oxidation in the alkaline media. Synth. Met. 2019, 255, 116110–116117. [Google Scholar] [CrossRef]
- Kamyabi, M.A.; Ebrahimi Qaratapeh, K.; Jadali, S.; Moharramnezhad, M. Decorating the carbon felt electrode with polymeric platinize nanocomposite: Characterization and electrocatalytic activity towards methanol oxidation reaction. J. Chem. Sci. 2019, 131, 1–9. [Google Scholar] [CrossRef]
- Kamyabi, M.A.; Jadali, S. A sponge like Pd arrays on Ni foam substrate: Highly active non-platinum electrocatalyst for methanol oxidation in alkaline media. Mater. Chem. Phys. 2021, 257, 123626. [Google Scholar] [CrossRef]
- Chumillas, S.; Busó-Rogero, C.; Solla-Gullón, J.; Vidal-Iglesias, F.J.; Herrero, E.; Feliu, J.M. Size and diffusion effects on the oxidation of formic acid and ethanol on platinum nanoparticles. Electrochem. Commun. 2011, 13, 1194–1197. [Google Scholar] [CrossRef]
- Kowal, A.; Li, M.; Shao, M.; Sasaki, K.; Vukmirovic, M.B.; Zhang, J.; Marinkovic, N.S.; Liu, P.; Frenkel, A.I.; Adzic, R.R. Ternary Pt/Rh/SnO2 electrocatalysts for oxidizing ethanol to CO2. Nat. Mater. 2009, 8, 325–330. [Google Scholar] [CrossRef]
- Li, M.; Zhou, W.P.; Marinkovic, N.S.; Sasaki, K.; Adzic, R.R. The role of rhodium and tin oxide in the platinum-based electrocatalysts for ethanol oxidation to CO2. Electrochim. Acta 2013, 104, 454–461. [Google Scholar] [CrossRef]
- Figueiredo, M.C.; Santasalo-Aarnio, A.; Vidal-Iglesias, F.J.; Solla-Gullón, J.; Feliu, J.M.; Kontturi, K.; Kallio, T. Tailoring properties of platinum supported catalysts by irreversible adsorbed adatoms toward ethanol oxidation for direct ethanol fuel cells. Appl. Catal. B Environ. 2013, 140–141, 378–385. [Google Scholar] [CrossRef]
- Busó-Rogero, C.; Herrero, E.; Feliu, J.M. Ethanol oxidation on Pt single-crystal electrodes: Surface-structure effects in alkaline Medium. ChemPhysChem 2014, 15, 2019–2028. [Google Scholar] [CrossRef]
- Busó-Rogero, C.; Solla-Gullón, J.; Vidal-Iglesias, F.J.; Herrero, E.; Feliu, J.M. Oxidation of ethanol on platinum nanoparticles: Surface structure and aggregation effects in alkaline medium. J. Solid State Electrochem. 2016, 20, 1095–1106. [Google Scholar] [CrossRef]
- Liang, Z.X.; Zhao, T.S.; Xu, J.B.; Zhu, L.D. Mechanism study of the ethanol oxidation reaction on palladium in alkaline media. Electrochim. Acta 2009, 54, 2203–2208. [Google Scholar] [CrossRef]
- Cheng, Y.; Guo, M.; Yu, Y.; Zhai, M.; Guo, R.; Hu, J. Fabrication of coral-like Pd based porous MnO2 nanosheet arrays on nickel foam for methanol electrooxidation. Ind. Eng. Chem. Res. 2018, 57, 10893–10904. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, N.; Banis, M.N.; Xiao, B.; Riese, A.; Sun, X. Comparative study to understand the intrinsic properties of Pt and Pd catalysts for methanol and ethanol oxidation in alkaline media. Electrochim. Acta 2015, 185, 267–275. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Q.; Xu, J.; Lee, C.S. Bimetallic PtPd nanoparticles on nafion–graphene film as catalyst for ethanol electro-oxidation. J. Mater. Chem. 2012, 22, 8057–8062. [Google Scholar] [CrossRef]
- Ren, F.; Wang, H.; Zhai, C.; Zhu, M.; Yue, R.; Du, Y.; Yang, P.; Xu, J.; Lu, W. Clean method for the synthesis of reduced graphene oxide-supported PtPd alloys with high electrocatalytic activity for ethanol oxidation in alkaline medium. ACS Appl. Mater. Interfaces 2014, 6, 3607–3614. [Google Scholar] [CrossRef]
- Chen, X.; Cai, Z.; Chen, X.; Oyama, M. Green synthesis of graphene-PtPd alloy nanoparticles with high electrocatalytic performance for ethanol oxidation. J. Mater. Chem. A 2014, 2, 315–320. [Google Scholar] [CrossRef]
- Li, S.S.; Zheng, J.N.; Ma, X.; Hu, Y.Y.; Wang, A.J.; Chen, J.R.; Feng, J.J. Facile synthesis of hierarchical dendritic PtPd nanogarlands supported on reduced graphene oxide with enhanced electrocatalytic properties. Nanoscale 2014, 6, 5708–5713. [Google Scholar] [CrossRef]
- Yang, G.; Zhou, Y.; Pan, H.B.; Zhu, C.; Fu, S.; Wai, C.M.; Du, D.; Zhu, J.J.; Lin, Y. Ultrasonic-assisted synthesis of Pd-Pt/carbon nanotubes nanocomposites for green enhanced electro-oxidation of ethanol and methanol in alkaline medium. Ultrason. Sonochem. 2016, 28, 192–198. [Google Scholar] [CrossRef]
- Fan, J.; Qi, K.; Zhang, L.; Zhang, H.; Yu, S.; Cui, X. Engineering Pt/Pd interfacial electronic structures for highly efficient hydrogen evolution and alcohol oxidation. ACS Appl. Mater. Interfaces 2017, 9, 18008–18014. [Google Scholar] [CrossRef]
- Huang, Z.; Zhou, H.; Chang, Y.; Fu, C.; Zeng, F.; Kuang, Y. Improved catalytic performance of Pd nanowires for ethanol oxidation by monolayer of Pt. Chem. Phys. Lett. 2013, 585, 128–132. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, T.; Jiang, R.; Jiang, F. Comparison of electrocatalytic activity of Pt1-xPdx/C catalysts for ethanol electro-oxidation in acidic and alkaline media. RSC Adv. 2020, 10, 10134–10143. [Google Scholar] [CrossRef]
- Luo, W.; Zhou, H.; Fu, C.; Huang, Z.; Gao, N.; Kuang, Y. Preparation and characterization of porous sponge-like Pd@Pt nanotubes with high catalytic activity for ethanol oxidation. Mater. Lett. 2016, 173, 43–46. [Google Scholar] [CrossRef]
- Solla-Gullón, J.; Rodes, A.; Montiel, V.; Aldaz, A.; Clavilier, J. Electrochemical characterisation of platinum/palladium nanoparticles prepared in a water-in-oil microemulsion. J. Electroanal. Chem. 2003, 554–555, 273–284. [Google Scholar] [CrossRef]
- Solla-Gullón, J.; Montiel, V.; Aldaz, A.; Clavilier, J. Synthesis and electrochemical decontamination of platinum-palladium nanoparticles prepared by water-in-oil microemulsion. J. Electrochem. Soc. 2003, 150, E104–E109. [Google Scholar] [CrossRef]
- Vidal-Iglesias, F.J.; Solla-Gullon, J.; Montiel, V.; Feliu, J.M.; Aldaz, A. Screening of electrocatalysts for direct ammonia fuel cell: Ammonia oxidation on PtMe (Me: Ir, Rh, Pd, Ru) and preferentially oriented Pt(1 0 0) nanoparticles. J. Power Sources 2007, 171, 448–456. [Google Scholar] [CrossRef]
- Vidal-Iglesias, F.J.; Aran-Ais, R.M.; Solla-Gullo, J.; Herrero, E.; Feliu, J.M. Electrochemical characterization of shape-controlled Pt nanoparticles in different supporting electrolytes. ACS Catal. 2012, 2, 901–910. [Google Scholar] [CrossRef]
- Solla-Gullón, J.; Vidal-Iglesias, F.J.; Montiel, V.; Aldaz, A. Electrochemical characterization of platinum-ruthenium nanoparticles prepared by water-in-oil microemulsion. Electrochim. Acta 2004, 49, 5079–5088. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, L.; Walton, J.; Liu, Z.; Tang, Z. Facile fabrication of PtPd alloyed worm-like nanoparticles for electrocatalytic reduction of oxygen. Int. J. Hydrog. Energy 2017, 42, 17112–17121. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, L.M.; Guo, Y.F.; Dai, Z.X.; Zhang, R.H.; Sun, C.; Zhou, X.W. In situ synthesis of PtPd bimetallic nanocatalysts supported on graphene nanosheets for methanol oxidation using triblock copolymer as reducer and stabilizer. J. Electroanal. Chem. 2016, 783, 132–139. [Google Scholar] [CrossRef]
- Xue, Q.; Bai, J.; Han, C.; Chen, P.; Jiang, J.X.; Chen, Y. Au nanowires@Pd-polyethylenimine nanohybrids as highly active and methanol-tolerant electrocatalysts toward oxygen reduction reaction in alkaline media. ACS Catal. 2018, 8, 11287–11295. [Google Scholar] [CrossRef]
- Farias, M.J.S.; Vidal-Iglesias, F.J.; Solla-Gullón, J.; Herrero, E.; Feliu, J.M. On the behavior of CO oxidation on shape-controlled Pt nanoparticles in alkaline medium. J. Electroanal. Chem. 2014, 716, 16–22. [Google Scholar] [CrossRef]
- Montiel, M.A.; Vidal-Iglesias, F.J.; Montiel, V.; Solla-Gullón, J. Electrocatalysis on shape-controlled metal nanoparticles: Progress in surface cleaning methodologies. Curr. Opin. Electrochem. 2017, 1, 34–39. [Google Scholar] [CrossRef]
- Solla-Gullón, J.; Montiel, V.; Aldaz, A.; Clavilier, J. Electrochemical and electrocatalytic behaviour of platinum-palladium nanoparticle alloys. Electrochem. Commun. 2002, 4, 716–721. [Google Scholar] [CrossRef]
- Solla-Gullón, J.; Montiel, V.; Aldaz, A.; Clavilier, J. Electrochemical characterisation of platinum nanoparticles prepared by microemulsion: How to clean them without loss of crystalline surface structure. J. Electroanal. Chem. 2000, 491, 69–77. [Google Scholar] [CrossRef]
- Guerin, S.; Attard, G.S. Electrochemical behavior of electrodeposited nanostructured palladium + platinum films in 2 M H2SO4. Electrochem. Commun. 2001, 3, 544–548. [Google Scholar] [CrossRef]
- Alexeyeva, N.; Sarapuu, A.; Tammeveski, K.; Vidal-Iglesias, F.J.; Solla-Gullón, J.; Feliu, J.M. Electroreduction of oxygen on Vulcan carbon supported Pd nanoparticles and Pd–M nanoalloys in acid and alkaline solutions. Electrochim. Acta 2011, 56, 6702–6708. [Google Scholar] [CrossRef]
- Erikson, H.; Sarapuu, A.; Alexeyeva, N.; Tammeveski, K.; Solla-Gullón, J.; Feliu, J.M. Electrochemical reduction of oxygen on palladium nanocubes in acid and alkaline solutions. Electrochim. Acta 2012, 59, 329–335. [Google Scholar] [CrossRef]
- Pandey, R.K.; Lakshminarayanan, V. Electro-Oxidation of formic acid, methanol, and ethanol on electrodeposited Pd-polyaniline nanofiber films in acidic and alkaline medium. J. Phys. Chem. C 2009, 113, 21596–21603. [Google Scholar] [CrossRef]
- Jiang, R.; Tran, D.T.; McClure, J.P.; Chu, D. A class of (Pd-Ni-P) electrocatalysts for the ethanol oxidation reaction in alkaline media. ACS Catal. 2014, 4, 2577–2586. [Google Scholar] [CrossRef]
- Chen, Z.; He, Y.C.; Chen, J.H.; Fu, X.Z.; Sun, R.; Chen, Y.X.; Wong, C.P. PdCu alloy flower-like nanocages with high electrocatalytic performance for methanol oxidation. J. Phys. Chem. C 2018, 122, 8976–8983. [Google Scholar] [CrossRef]
- Lin, Y.; Tian, N.; Xiao, C.; Sheng, T.; Li, G.; Zhang, F.; Ye, J.; Xu, B.; Zhou, Z.; Sun, S. Effects of atom arrangement and thickness of Pt atomic layers on Pd nanocrystals for electrocatalysis. Electrochim. Acta 2018, 271, 519–525. [Google Scholar] [CrossRef]
- Ma, L.; Chu, D.; Chen, R. Comparison of ethanol electro-oxidation on Pt/C and Pd/C catalysts in alkaline media. Int. J. Hydrog. Energy 2012, 37, 11185–11194. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, L.; Tian, Y.; Jiao, A.; Li, S.; Liu, X.; Chen, M.; Chen, F. Convenient synthesis of 3D fluffy PtPd nanocorals loaded on 2D h-BN supports as highly efficient and stable electrocatalysts for alcohol oxidation reaction. ACS Omega 2019, 4, 11163–11172. [Google Scholar] [CrossRef] [PubMed]
- Buso-Rogero, C.; Brimaud, S.; Solla-Gullon, J.; Vidal-Iglesias, F.J.; Herrero, E.; Behm, J.R.; Feliu, J.M. Ethanol oxidation on shape-controlled platinum nanoparticles at different pHs: A combined in situ IR spectroscopy and online mass spectrometry study. J. Electroanal. Chem. 2015, 763, 116–124. [Google Scholar] [CrossRef]
- Busó-Rogero, C.; Solla-Gullón, J.; Vidal-Iglesias, F.J.; Herrero, E.; Feliu, J.M. Adatom modified shape-controlled platinum nanoparticles towards ethanol oxidation. Electrochim. Acta 2016, 196, 270–279. [Google Scholar] [CrossRef]
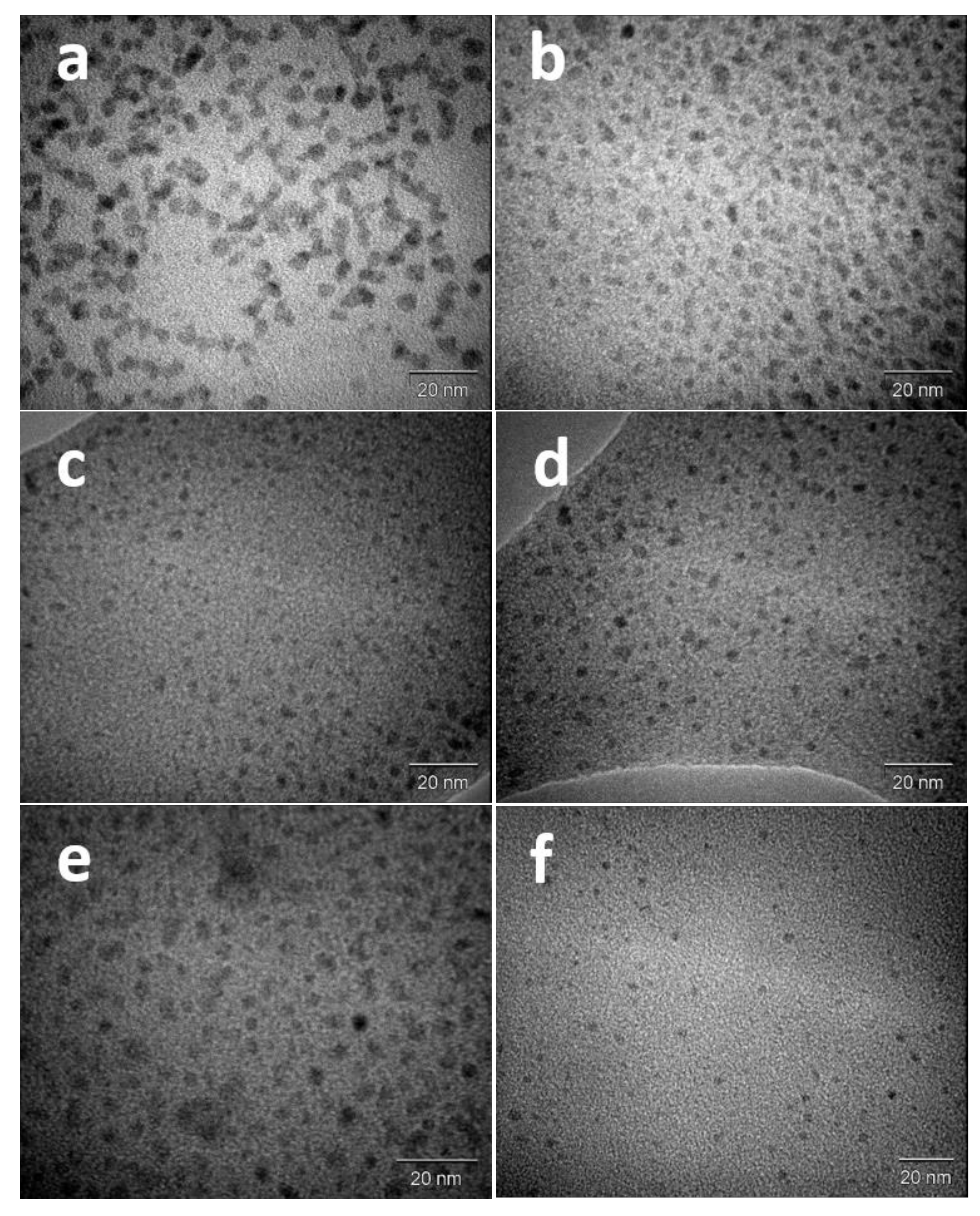


| Nominal Atomic Composition Pt/Pd | Particle Size (TEM)/nm | Particle Size (XRD)/nm |
|---|---|---|
| Pt Pt4Pd | 4.5 ± 0.7 3.5 ± 0.7 | 3.2 ± 0.4 3.8 ± 0.4 |
| Pt3Pd2 | 3.4 ± 0.5 | 4.2 ± 0.4 |
| Pt2Pt3 | 4.1 ± 0.6 | 4.9 ± 0.5 |
| PtPd4 Pd | 3.4 ± 0.5 1.6 ± 0.4 | 4 ± 0.4 2.7 ± 0.4 |
| Nominal Atomic Composition Pt/Pd | Calculated with AAS (Pd) UV-Vis (Pt) | Calculated with EDX | Calculated with XPS Pt (4f7/2) Pd (3d5/2) |
|---|---|---|---|
| Pt4Pd | 79.6 ± 0.3/20.4 ± 0.3 | 82 ± 0.5/18 ± 0.5 | 75.3 ± 0.5/24.7 ± 0.5 |
| Pt3Pd2 | 59.4 ± 0.3/40.6 ± 0.3 | 62 ± 0.5/38 ± 0.5 | 56.6 ± 0.5/43.4 ± 0.5 |
| Pt2Pd3 | 40.6 ± 0.3/59.4 ± 0.3 | 40 ± 0.5/60 ± 0.5 | 38 ± 0.5/62 ± 0.5 |
| PtPd4 | 17.8 ± 0.3/82.2 ± 0.3 | 20.6 ± 0.5/79.4 ± 0.5 | 21.4 ± 0.5/78.6 ± 0.5 |
| Nanoparticles | Q/μC | Width at Half Heigh/V | Epeak/V |
|---|---|---|---|
| Pt | 120.4 | 0.24 | 0.72 |
| Pt4Pd | 83.5 | 0.24 | 0.72 |
| Pt3Pd2 | 305.5 | 0.22 | 0.70 |
| Pt2Pd3 | 377.8 | 0.15 | 0.70 |
| PtPd4 | 64.1 | 0.15 | 0.70 |
| Pd | 25.0 | 0.10 | 0.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jadali, S.; Kamyabi, M.A.; Solla-Gullón, J.; Herrero, E. Effect of Pd on the Electrocatalytic Activity of Pt towards Oxidation of Ethanol in Alkaline Solutions. Appl. Sci. 2021, 11, 1315. https://doi.org/10.3390/app11031315
Jadali S, Kamyabi MA, Solla-Gullón J, Herrero E. Effect of Pd on the Electrocatalytic Activity of Pt towards Oxidation of Ethanol in Alkaline Solutions. Applied Sciences. 2021; 11(3):1315. https://doi.org/10.3390/app11031315
Chicago/Turabian StyleJadali, Salma, Mohammad Ali Kamyabi, José Solla-Gullón, and Enrique Herrero. 2021. "Effect of Pd on the Electrocatalytic Activity of Pt towards Oxidation of Ethanol in Alkaline Solutions" Applied Sciences 11, no. 3: 1315. https://doi.org/10.3390/app11031315
APA StyleJadali, S., Kamyabi, M. A., Solla-Gullón, J., & Herrero, E. (2021). Effect of Pd on the Electrocatalytic Activity of Pt towards Oxidation of Ethanol in Alkaline Solutions. Applied Sciences, 11(3), 1315. https://doi.org/10.3390/app11031315







