Ceftriaxone Degradation in the Presence of Sodium Halides Investigated by Electrochemical Methods Assisted by UV-Vis Spectrophotometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrochemical Measurements
2.3. UV-Vis Spectrophotometry
3. Results and Discussion
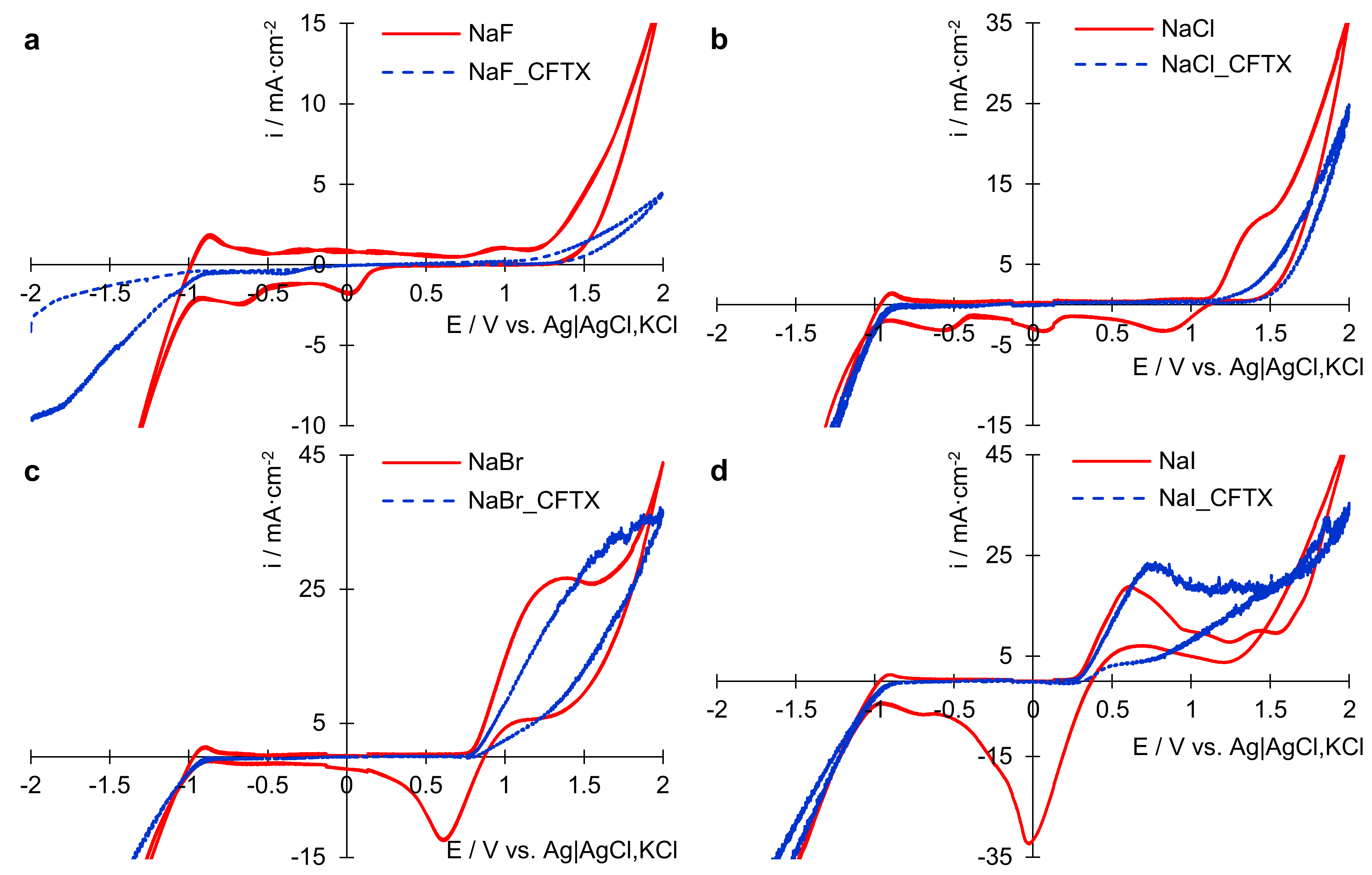
3.1. Electrochemical Behavior of CFTX in the Presence of Different Ions
3.1.1. Study by Cyclic Voltammetry (CV)
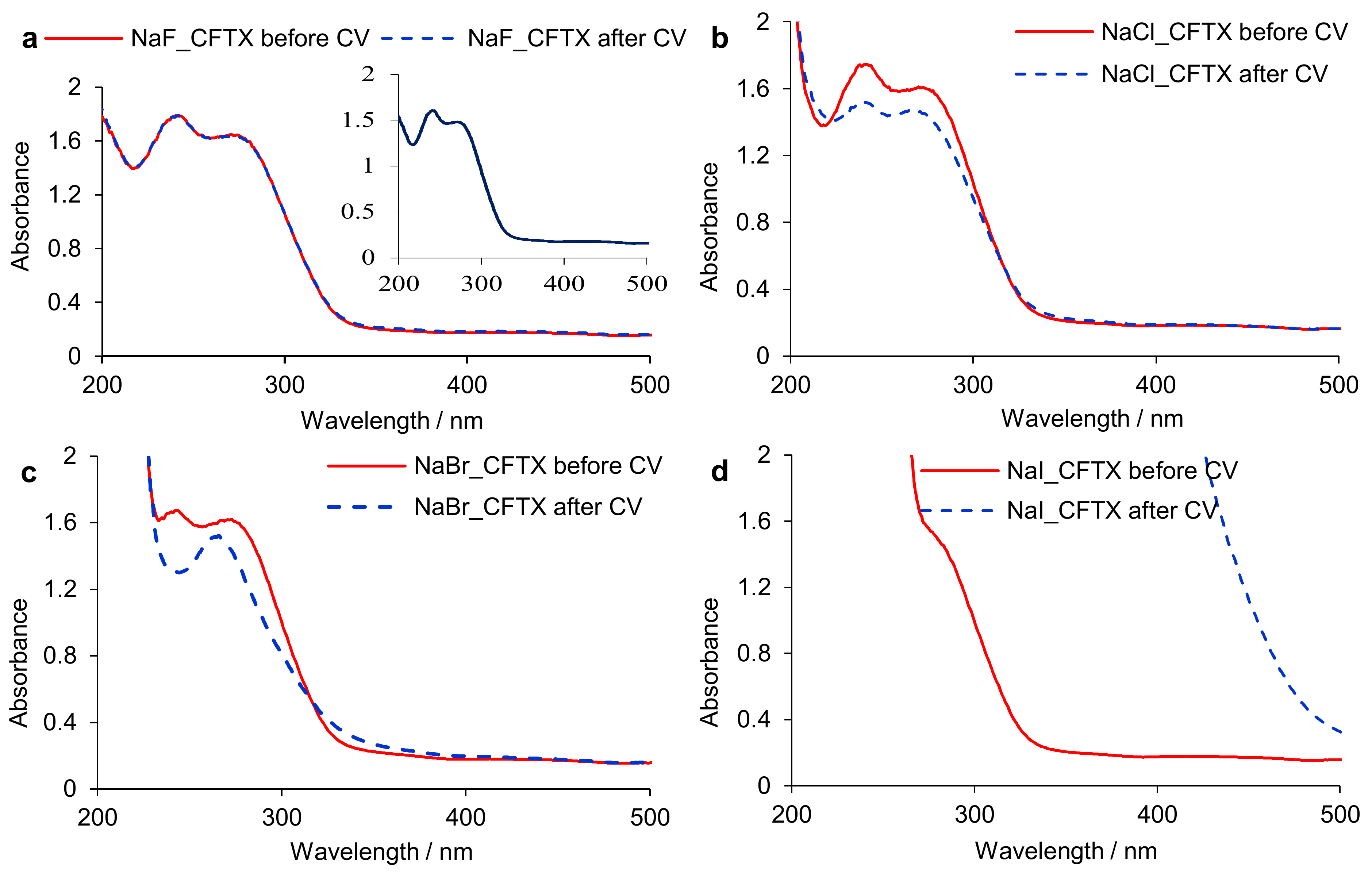
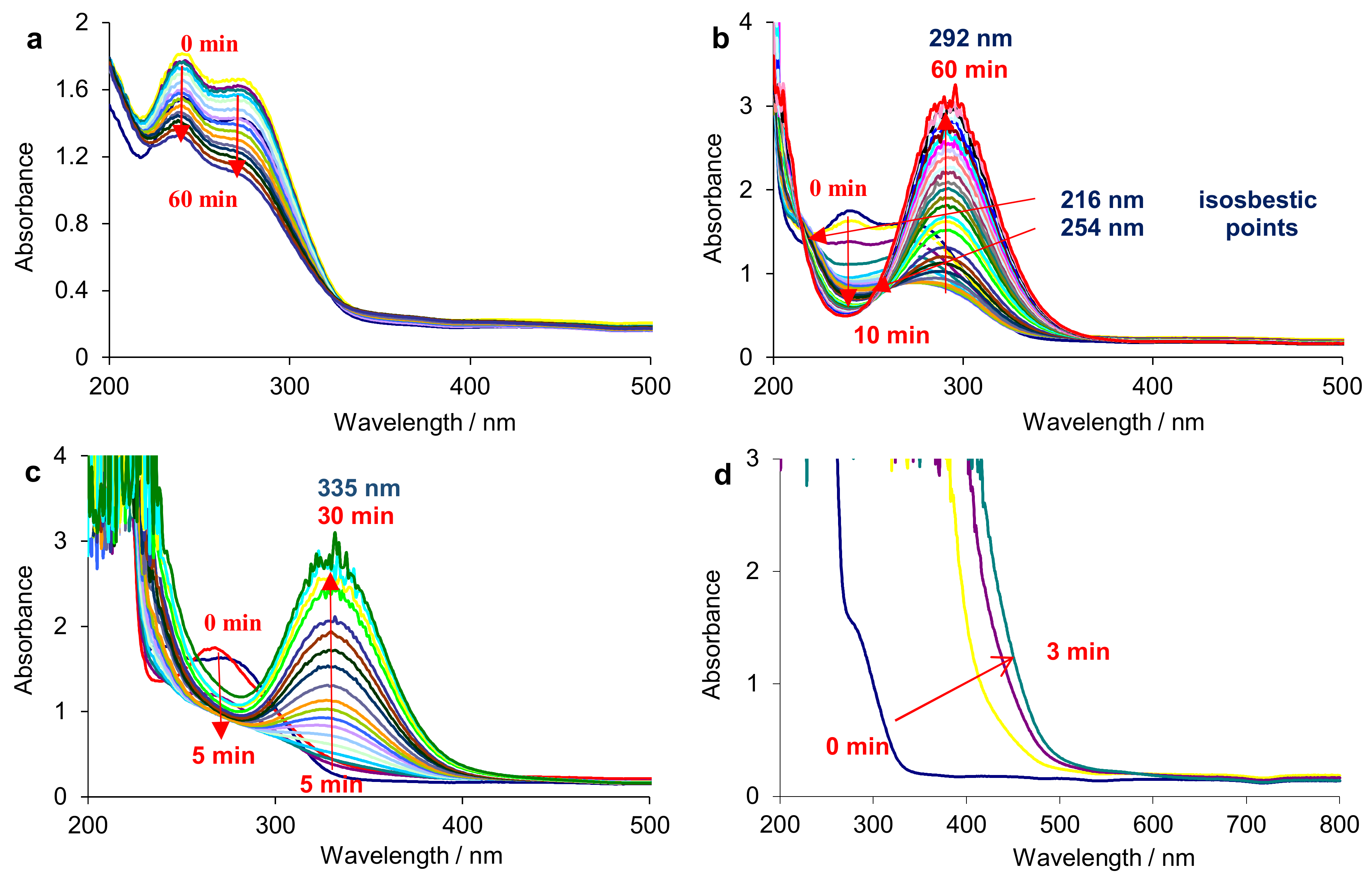
3.1.2. Spectrophotometric Study
3.1.3. Electrolysis Assisted by UV-Vis Spectrophotometry
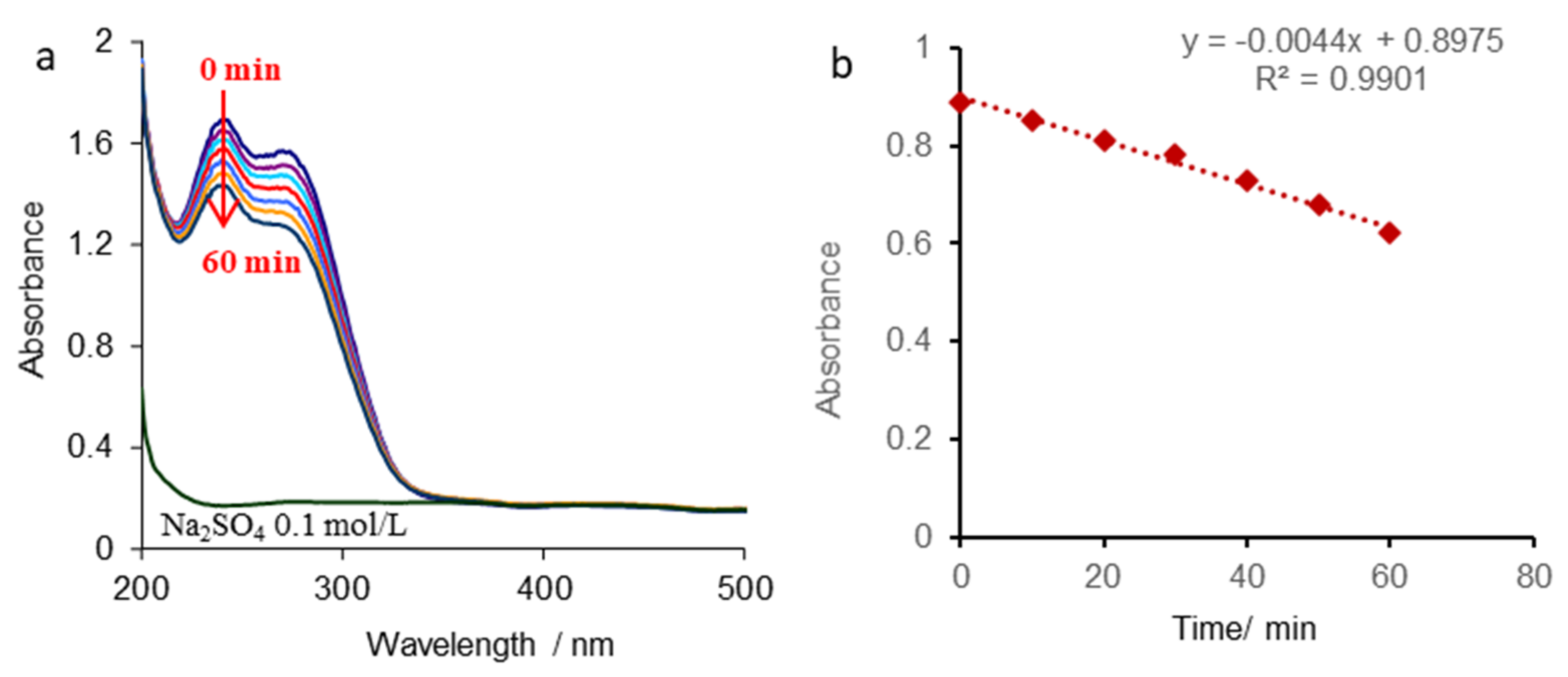
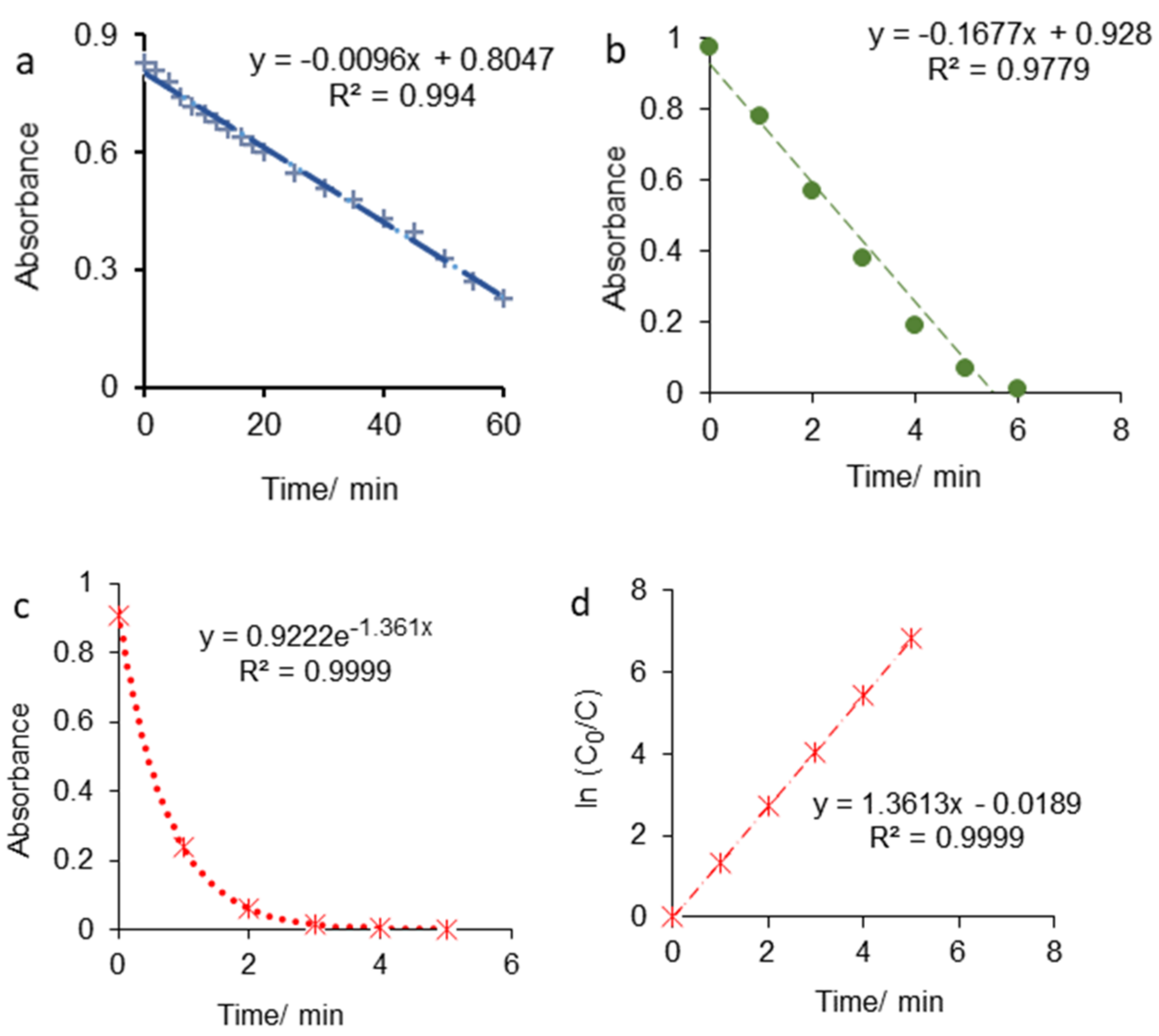
3.2. Kinetic Study of CFTX Degradation Reaction
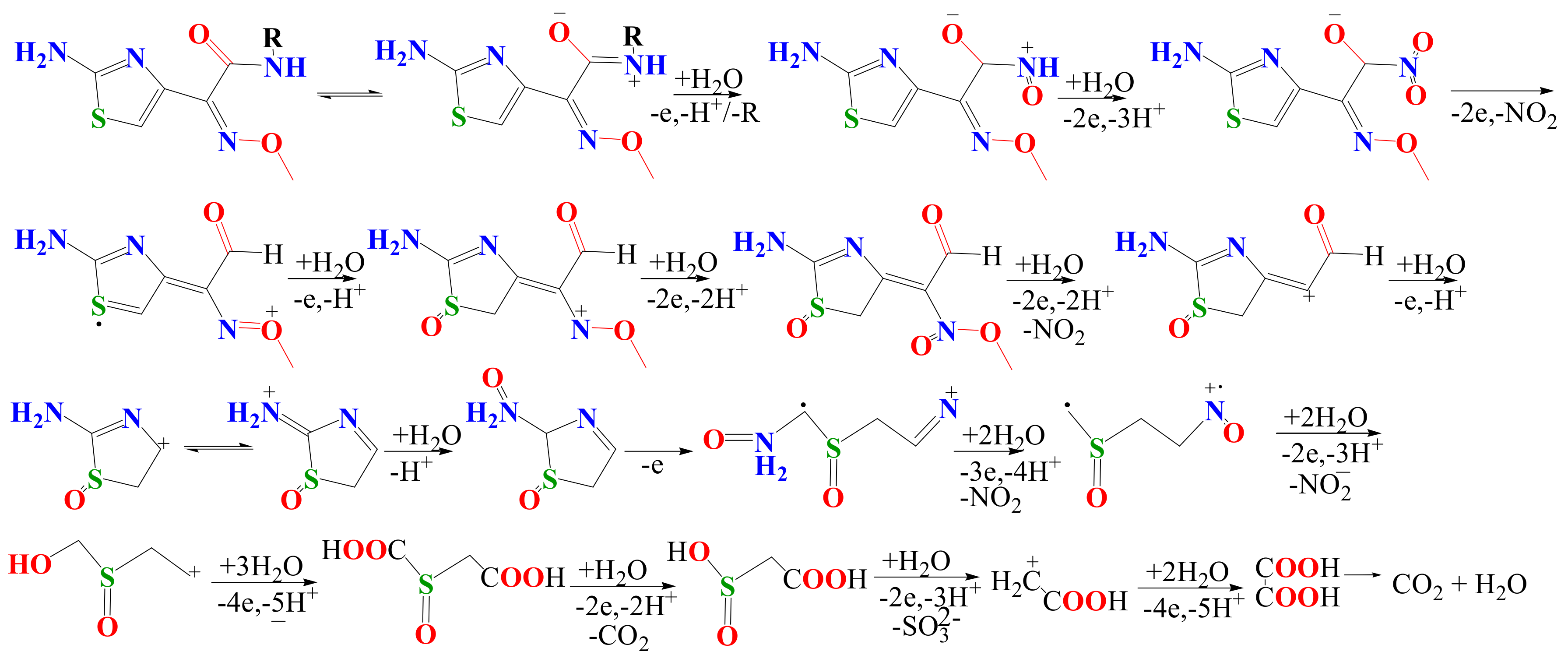
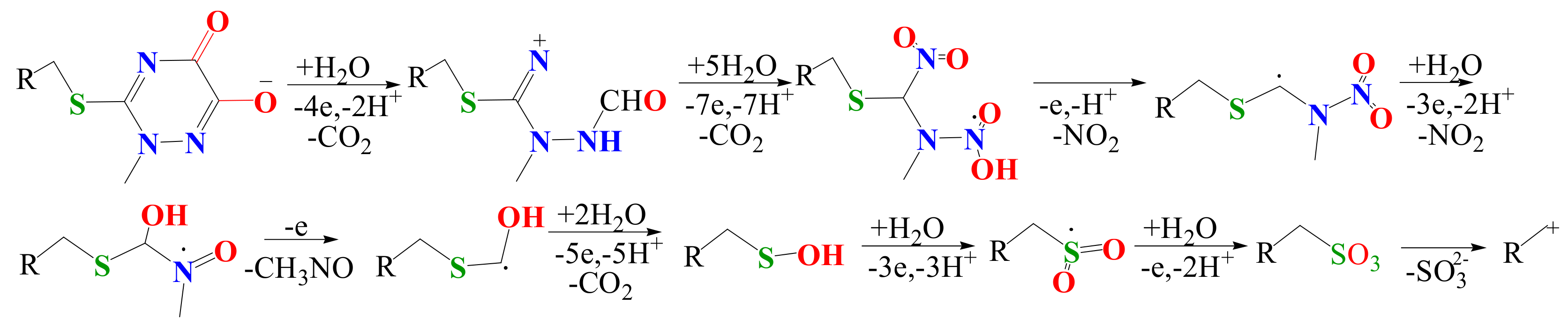
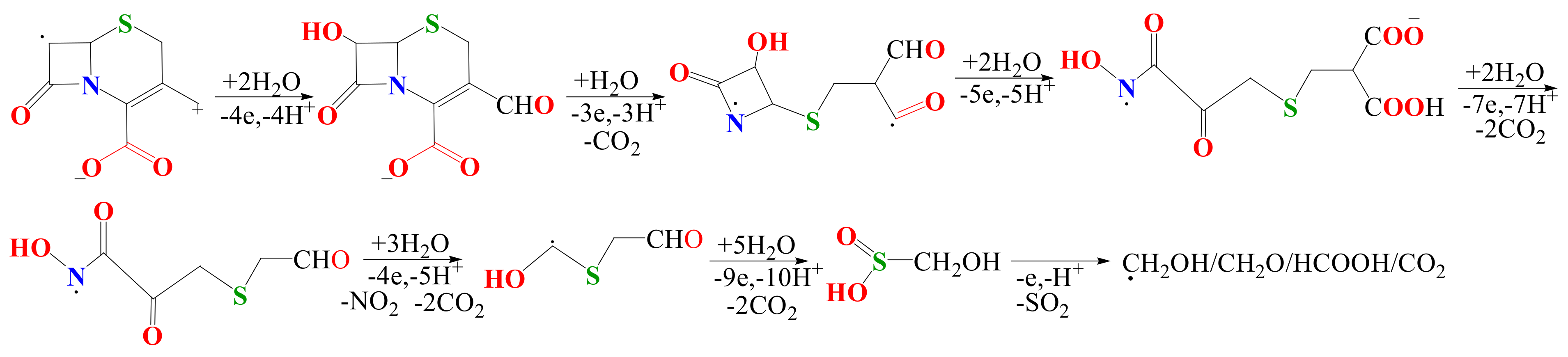
3.3. CFTX Electrochemical Degradation Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ribeiro, A.R.; Sures, B.; Schmidt, T.C. Cephalosporin antibiotics in the aquatic environment: A critical review of occurrence, fate, ecotoxicity and removal technologies. Environ. Pollut. 2018, 241, 1153–1166. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Tang, X.; Zuo, J.; Zhang, M.; Chen, L.; Li, Z. Distribution and persistence of cephalosporins in cephalosporin producing wastewater using SPE and UPLC–MS/MS method. Sci. Total Environ. 2016, 569–570, 23–30. [Google Scholar] [CrossRef] [PubMed]
- El-Shaboury, S.R.; Saleh, G.A.; Mohamed, F.A.; Rageh, A.H. Analysis of cephalosporin antibiotics. J. Pharm. Biomed. Anal. 2007, 45, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sengun, F.I.; Ulas, K. Analytical investigations on cephalosporins—I Spectrophotometric determination of ceftriaxone. Talanta 1986, 33, 363–365. [Google Scholar] [CrossRef]
- Ivaska, A.; Nordstrom, F. Determination of some cephalosporins by differential pulse polarography and linear scan voltammetry. Anal. Chim. Acta 1983, 146, 87–95. [Google Scholar] [CrossRef]
- Foog, A.G.; Fayad, N.M. Differential pulse polarography determination of cephalosporins and their degradation products. Anal. Chim. Acta 1979, 108, 205–211. [Google Scholar] [CrossRef]
- Feier, B.; Gui, A.; Cristea, C.; Săndulescu, R. Electrochemical determination of cephalosporins using a bare boron-doped diamond electrode. Anal. Chim. Acta 2017, 976, 25–34. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, L.; Ji, R. Biotic and abiotic degradation of four cephalosporin antibiotics in a lake surface water and sediment. Chemosphere 2010, 80, 1399–1405. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, Y.; Li, Q. Indirect spectrophotometric determination of sodium ceftriaxone with n-propyl alcohol-ammonium sulfate–water system by extraction flotation of copper(II). Clin. Chim. Acta 2008, 391, 80–84. [Google Scholar] [CrossRef]
- Reynoso, E.; Spesia, M.B.; García, N.A.; Biasutti, M.A.; Criado, S. Riboflavin-sensitized photooxidation of Ceftriaxone and Cefotaxime. Kinetic study and effect on Staphylococcus aureus. J. Photoch. Photobio. B 2015, 142, 35–42. [Google Scholar] [CrossRef]
- Yang, B.; Zuo, J.; Li, P.; Wang, K.; Yu, X.; Zhang, M. Effective ultrasound electrochemical degradation of biological toxicity and refractory cephalosporin pharmaceutical wastewater. Chem. Eng. J. 2016, 287, 30–37. [Google Scholar] [CrossRef]
- Sacco, O.; Vaiano, V.; Rizzo, L.; Sannino, D. Intensification of ceftriaxone degradation under UV and solar light irradiation in presence of phosphors based structured catalyst. Chem. Eng. Process. 2019, 137, 12–21. [Google Scholar] [CrossRef]
- Kordestani, B.; Yengejeh, R.J.; Takdastan, A.; Neisi, A.K. A new study on photocatalytic degradation of meropenem and ceftriaxone antibiotics based on sulfate radicals: Influential factors, biodegradability, mineralization approach. Microchem. J. 2019, 146, 286–292. [Google Scholar] [CrossRef]
- Kaur, B.; Kuntus, L.; Tikker, P.; Kattel, E.; Trapido, M.; Dulova, N. Photo-induced oxidation of ceftriaxone by persulfate in the presence of iron oxides. Sci. Total Environ. 2019, 676, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Badi, M.Y.; Azari, A.; Pasalari, H.; Esrafili, A.; Farzadkia, M. Modification of activated carbon with magnetic Fe3O4 nanoparticle composite for removal of ceftriaxone from aquatic solutions. J. Mol. Liq. 2018, 261, 146–154. [Google Scholar] [CrossRef]
- Qiao, J.; Lv, M.; Qu, Z.; Zhang, M.; Cui, X.; Wang, D.; Piao, C.; Liu, Z.; Wang, J.; Song, Y. Preparation of a novel Z-scheme KTaO3/FeVO4/Bi2O3 nanocomposite for efficient sonocatalytic degradation of ceftriaxone sodium. Sci. Total Environ. 2019, 689, 178–192. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Liang, X.; Shi, H.; Wang, C.; Fan, J.; Hu, X.; Liu, E. Enhanced photocatalytic activity of Ag-CsPbBr3/CN composite for broad spectrum photocatalytic degradation of cephalosporin antibiotics 7-ACA. Appl. Catal. B-Environ. 2019, 247, 57–69. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Shi, H.; Liu, E.; Fan, J.; Hu, X. Enhanced photocatalytic activity of ZnSe QDs/g-C3N4 composite for Ceftriaxone sodium degradation under visible light. Mat. Lett. 2018, 231, 150–153. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, X.; Shi, H.; Wang, Y.; Ren, Y.; Liu, E.; Zhang, X.; Fan, J.; Hu, X. Photocatalytic activity enhanced by synergistic effects of nano-silver and ZnSe quantum dots co-loaded with bulk g-C3N4 for Ceftriaxone sodium degradation in aquatic environment. Chem. Eng. J. 2018, 353, 56–68. [Google Scholar] [CrossRef]
- Shi, X.; Karachi, A.; Hosseini, M.; Yazd, M.S.; Kamyab, H.; Ebrahimi, M.; Parsaee, Z. Ultrasound wave assisted removal of Ceftriaxone sodium in aqueous media with novel nano composite g-C3N4/MWCNT/Bi2WO6 based on CCD-RSM model. Ultrason. Sonochem. 2019, 104460. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, X.; Wang, Y.; Shi, H.; Liu, E.; Fan, J.; Hu, X. Degradation and removal of Ceftriaxone sodium in aquatic environment with Bi2WO6/g-C3N4 photocatalyst. J. Colloid Interf. Sci. 2018, 523, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Tutunaru, B.; Samide, A.; Neamțu, C.; Prunaru, I. Electrochemical and thermal stability of Brown HT food additive. Chem. Ind. Chem. Eng. Q. 2019, 25, 89–96. [Google Scholar] [CrossRef]
- Tutunaru, B.; Samide, A.; Neamtu, C.; Tigae, C. Spectroelectrochemical studies of interactions between vitamin A and nanocolloidal silver. Int. J. Electrochem. Sci. 2018, 13, 5850–5859. [Google Scholar] [CrossRef]
- Samide, A.; Tutunaru, B.; Bratulescu, G.; Ionescu, C. Electrochemical synthesis and characterization of new electrodes based on poly-hematoxylin film. J. Appl. Polym. Sci. 2013, 30, 687–697. [Google Scholar] [CrossRef]
- Samide, A.; Tutunaru, B.; Cioateră, N.; Vladu, A.C.; Spînu, C.; Tigae, C. Catalytic activity of thallium on electrochemical degradation of Metronidazole from aqueous solutions. Chem. Eng. Commun. 2016, 203, 1572–1581. [Google Scholar] [CrossRef]
- Samide, A.; Tutunaru, B. Interactions between vitamin C and nanocolloidal silver particles studied by cyclic voltammetry and UV-Vis spectrophotometry. Electroanalysis 2017, 29, 2498–2506. [Google Scholar] [CrossRef]
- Tutunaru, B.; Samide, A.; Moanţă, A.; Ionescu, C.; Tigae, C. Electrochemical study of metribuzin pesticide degradation on Bi electrode in aqueous solution. Int. J. Electroch. Sci. 2015, 10, 223–234. [Google Scholar]
- Samide, A.; Tutunaru, B.; Tigae, C.; Efrem, R.; Moanţă, A.; Dumitru, M. The removal of dyes from wastewater by electrochemical degradation. Environ. Prot. Eng. 2014, 40, 93–104. [Google Scholar]
- Zhao, Y.; Wang, Y.; Liu, E.; Fan, J.; Hu, X. Bi2WO6 nanoflowers: An efficient visible light photocatalytic activity for ceftriaxone sodium degradation. Appl. Surf. Sci. 2018, 436, 854–864. [Google Scholar] [CrossRef]
- Guo, X.; Wan, J.; Yu, X.; Lin, Y. Study on preparation of SnO2-TiO2/Nano-graphite composite anode and electro-catalytic degradation of ceftriaxone sodium. Chemosphere 2016, 164, 421–429. [Google Scholar] [CrossRef]
- Guo, X.; Li, D.; Wan, J.; Yu, X. Preparation and electrochemical property of TiO2/Nano-graphite composite anode for electro-catalytic degradation of ceftriaxone sodium. Electrochim. Acta 2015, 180, 957–964. [Google Scholar] [CrossRef]
- Li, D.; Guo, X.; Song, H.; Sun, T.; Wan, J. Preparation of RuO2-TiO2/Nano-graphite composite anode for electrochemical degradation of ceftriaxone sodium. J. Hazard. Mater. 2018, 351, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Jayasankar, B.; Karan, K. O2 electrochemistry on Pt: A unified multi-step model for oxygen reduction and oxide growth. Electrochim. Acta 2018, 273, 367–378. [Google Scholar] [CrossRef]
- Rinaldo, S.G.; Lee, W.; Stumper, J.; Eikerling, M. Mechanistic principles of platinum oxide formation and reduction. Electrocatalysis 2014, 5, 262–272. [Google Scholar] [CrossRef]
- Birss, V.I.; Chang, M.; Segal, J. Platinum oxide film formation-reduction: An in-situ mass measurement study. J. Electroanal. Chem. 1993, 355, 181–191. [Google Scholar] [CrossRef]
- Jaksic, M.M.; Johansen, B.; Tunold, R. Electrochemical behaviour of platinum in alkaline and acidic solutions of heavy and regular water. Int. J. Hydrogen Energ. 1993, 10, 817–837. [Google Scholar] [CrossRef]
- Coustan, L.; Shul, G.; Bélanger, D. Electrochemical behavior of platinum, gold and glassy carbon electrodes in water-in-salt electrolyte. Electrochem. Commun. 2017, 77, 89–92. [Google Scholar] [CrossRef]
- Pöpke, H.; Mutoro, E.; Luerßen, B.; Janek, J. Oxygen reduction and oxidation at epitaxial model-type Pt(O2)/YSZ electrodes—On the role of PtOx formation on activation, passivation, and charge transfer. Catal. Today 2013, 202, 12–19. [Google Scholar] [CrossRef]
- Alsabet, M.; Grden, M.; Jerkiewicz, G. Comprehensive study of the growth of thin oxide layers on Pt electrodes under well-defined temperature, potential, and time conditions. J. Electroanal. Chem. 2006, 589, 120–127. [Google Scholar] [CrossRef]
- Huang, X.; Qu, Y.; Cid, C.A.; Finke, C.; Hoffmann, M.R.; Lim, K.; Jiang, S.C. Electrochemical disinfection of toilet wastewater using wastewater electrolysis cell. Water Res. 2016, 92, 164–172. [Google Scholar] [CrossRef]
- Mastragostino, M.; Gramellini, C. Kinetic study of the electrochemical processes of the bromine/bromine aqueous system on vitreous carbon electrodes. Electrochim. Acta 1985, 30, 373–380. [Google Scholar] [CrossRef]
- Denaro, A.R.; Mitchell, A.; Richardson, M.R. Glow-discharge electrolysis of iodide solutions. Electrochim. Acta 1971, 16, 755–762. [Google Scholar] [CrossRef]
- Abo El Maali, N.; Ali, A.M.M.; Khodari, M.; Ghandour, M.A. Cathodic stripping voltammetric determination of the cephalosporin antibiotic Ceftriaxone at the mercury electrode in aqueous and biological media. Bioelectrochem. Bioenerg. 1991, 26, 485–492. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Schmidt, T.C. Determination of acid dissociation constant (pKa) of cephalosporin antibiotics: Computational and experimental approaches. Chemosphere 2017, 169, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Lu, L.; Chang, Y.; Zhang, D.S.; Li, J.; Feng, Y.C.; Hu, C.Q. Identification of a new isomer from a reversible isomerization of ceftriaxone in aqueous solution. J. Pharm. Biomed. Anal. 2015, 102, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Canton, E.; Esteban, M.J.; Rius, F. Factors affecting the stability of ceftriaxone sodium in solution on storage. Int. J. Pharm. 1993, 92, 47–53. [Google Scholar] [CrossRef]

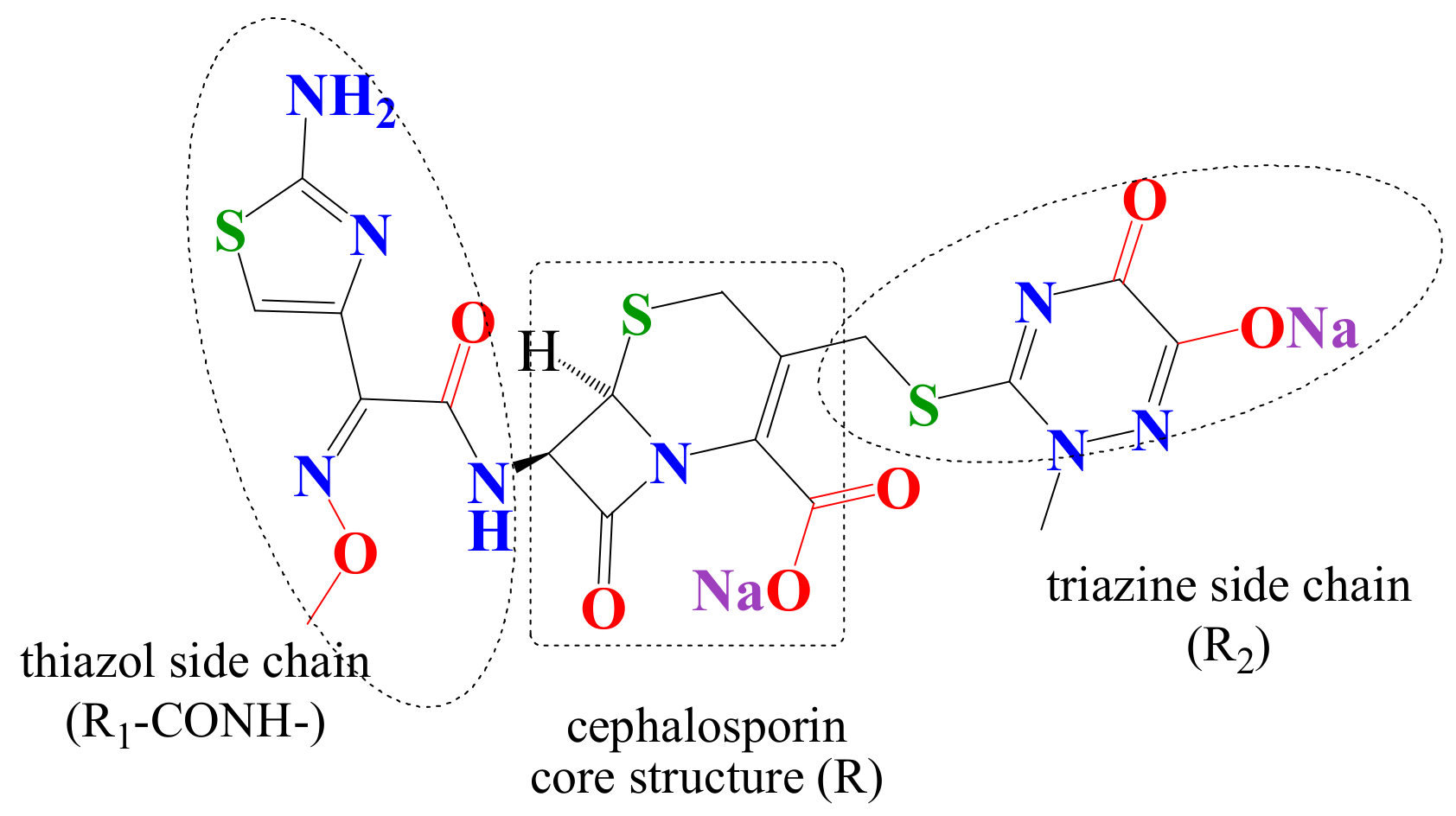
| disodium(6R,7R)-7-[[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-methoxyiminoacetyl] amino]-3-[(2-methyl-6-oxido-5-oxo-1,2,4-triazin-3-yl)sulfanylmethyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene−2-carboxylate | |
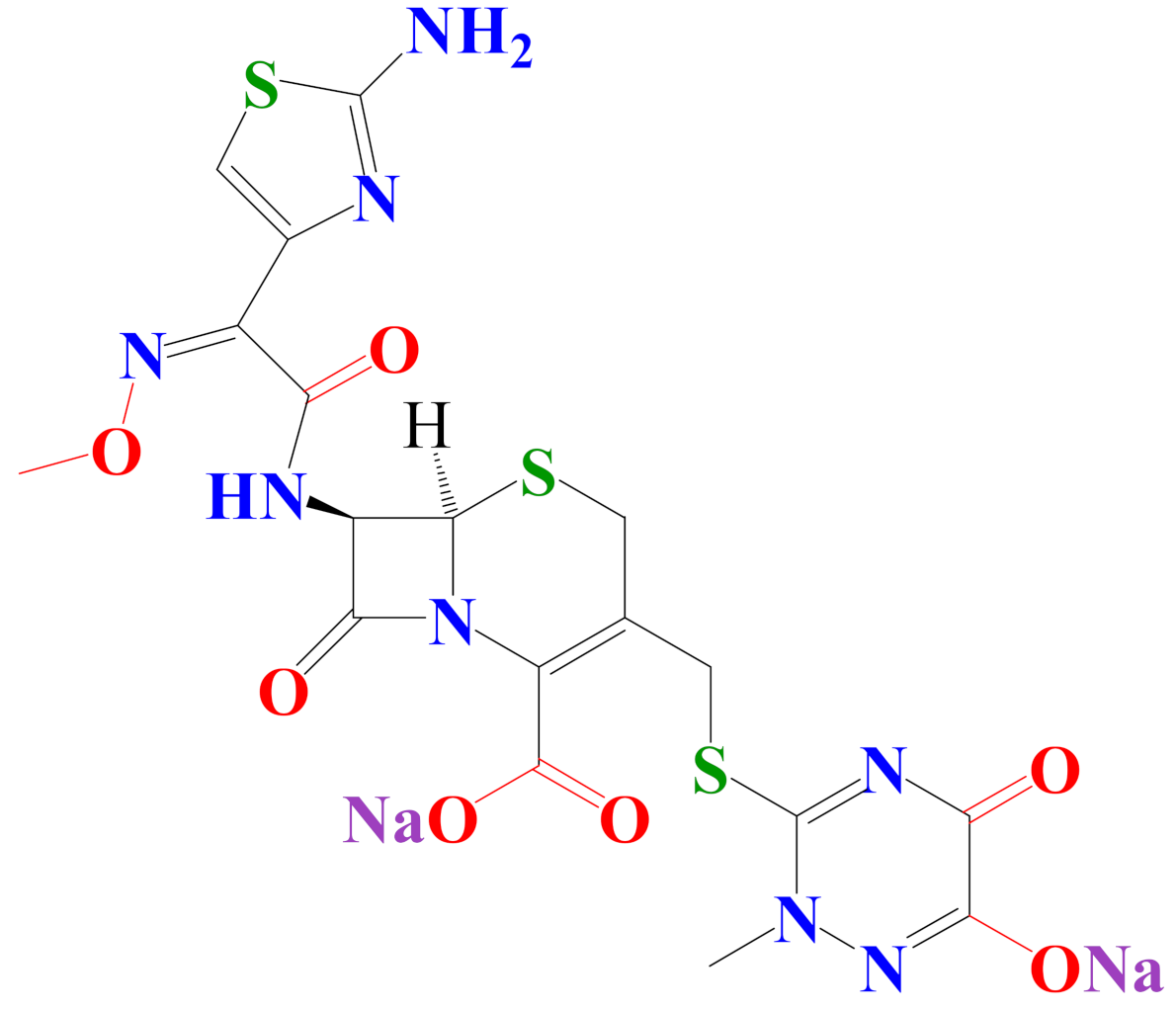 | C18H16N8 Na2O7S3 Molecular weight: 598.6 g·mol−1 Melting point: 155 °C Solubility: 0.105 g·L−1 Biological half-life: 5.8–8.7 h |
| CFTX/Supporting Electrolyte | Applied Kinetic Model | k/min−1 | Final Degradation Time/min |
|---|---|---|---|
| CFTX/SO42− | Zero-order reaction kinetics | 0.0044 | 204 |
| CFTX/F− | Zero-order reaction kinetics | 0.0096 | 84 |
| CFTX/Cl− | Zero-order reaction kinetics | 0.1677 | 5.5 |
| CFTX/Br− | First-order reaction kinetics | 1.361 | 3 min (degradation percentage close to 100%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tutunaru, B.; Samide, A.; Iordache, S.; Tigae, C.; Simionescu, A.; Popescu, A. Ceftriaxone Degradation in the Presence of Sodium Halides Investigated by Electrochemical Methods Assisted by UV-Vis Spectrophotometry. Appl. Sci. 2021, 11, 1376. https://doi.org/10.3390/app11041376
Tutunaru B, Samide A, Iordache S, Tigae C, Simionescu A, Popescu A. Ceftriaxone Degradation in the Presence of Sodium Halides Investigated by Electrochemical Methods Assisted by UV-Vis Spectrophotometry. Applied Sciences. 2021; 11(4):1376. https://doi.org/10.3390/app11041376
Chicago/Turabian StyleTutunaru, Bogdan, Adriana Samide, Simona Iordache, Cristian Tigae, Andreea Simionescu, and Alexandru Popescu. 2021. "Ceftriaxone Degradation in the Presence of Sodium Halides Investigated by Electrochemical Methods Assisted by UV-Vis Spectrophotometry" Applied Sciences 11, no. 4: 1376. https://doi.org/10.3390/app11041376
APA StyleTutunaru, B., Samide, A., Iordache, S., Tigae, C., Simionescu, A., & Popescu, A. (2021). Ceftriaxone Degradation in the Presence of Sodium Halides Investigated by Electrochemical Methods Assisted by UV-Vis Spectrophotometry. Applied Sciences, 11(4), 1376. https://doi.org/10.3390/app11041376





