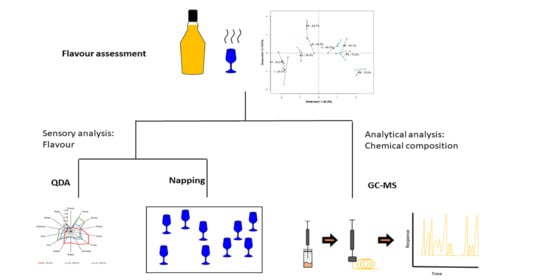
Comparison of Three Approaches to Assess the Flavour Characteristics of Scotch Whisky Spirit
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Spirit Samples
2.2. Sensory Evaluation
2.2.1. Panellists
2.2.2. Spirit Preparation and Presentation
2.2.3. Quantitative Descriptive Analysis
2.2.4. Napping
2.3. Gas-Chromatography-Mass Spectrometry
2.4. Statistical Comparison of the Three Methods Using Hierarchical Multiple Factor Analysis
3. Results
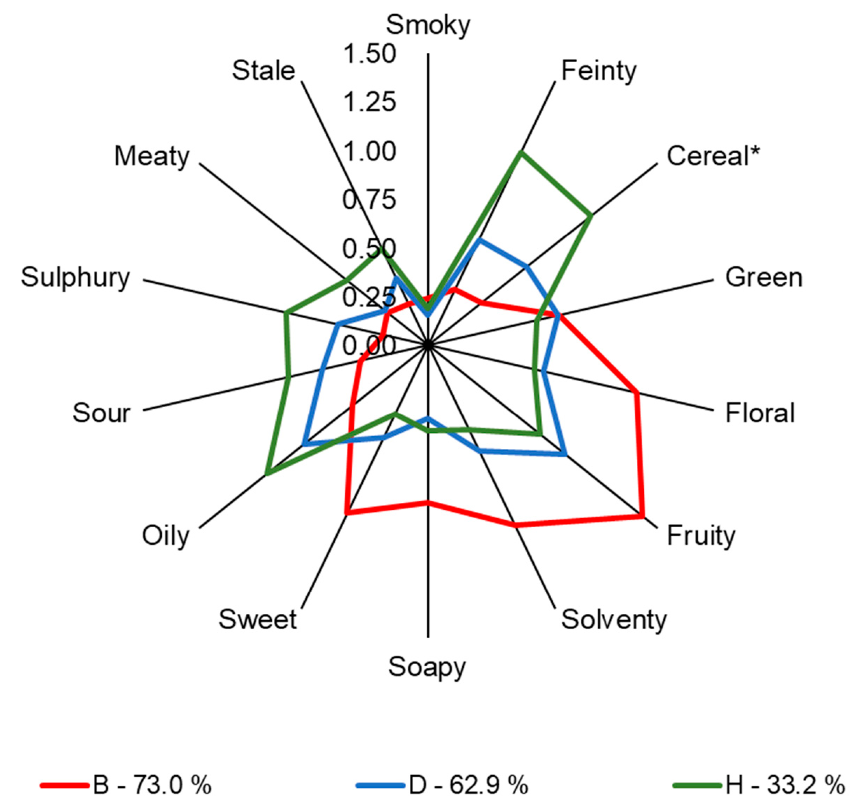
3.1. Quantitative Descriptive Analysis
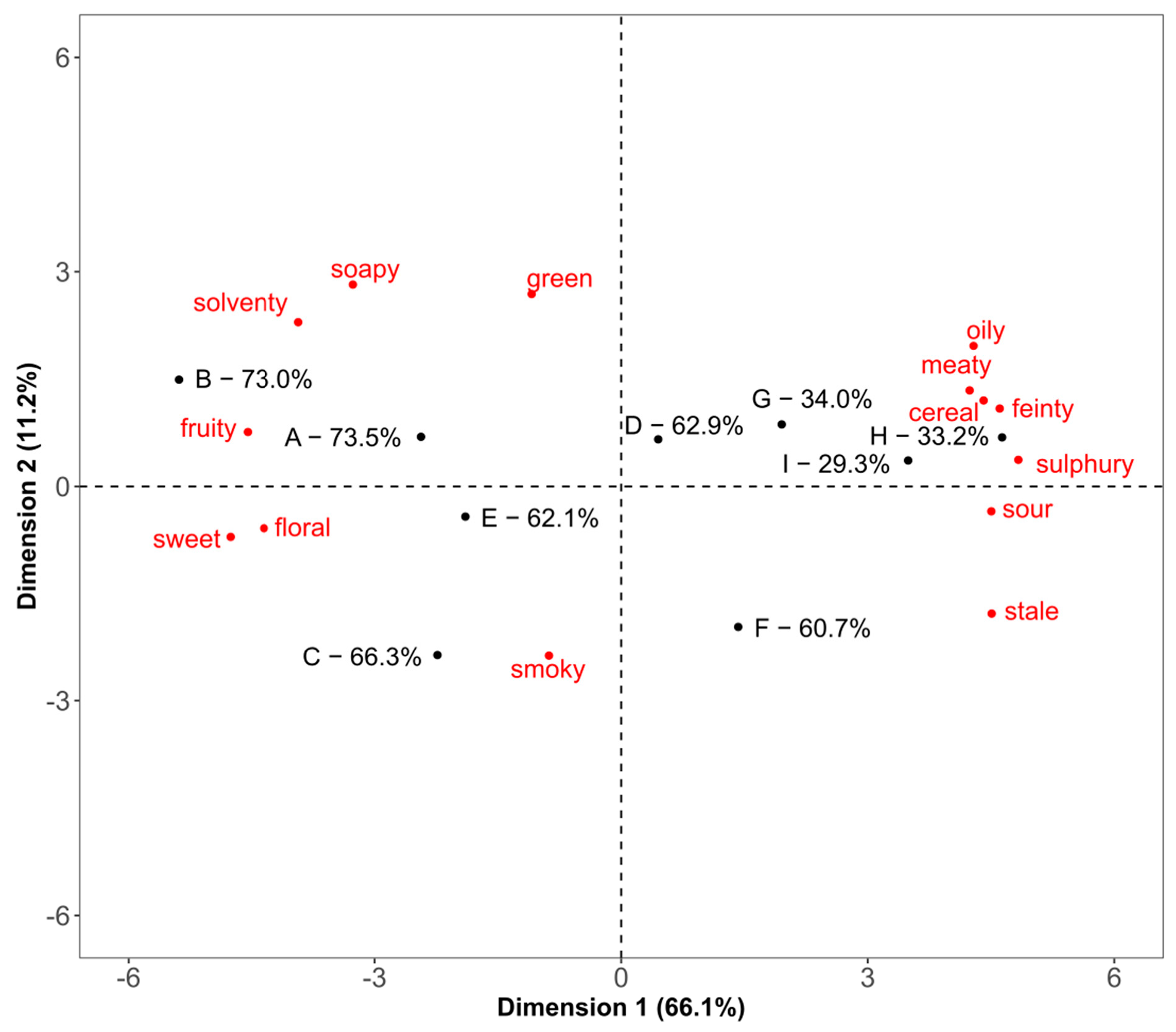
3.2. Napping
3.3. Time Expenditure for QDA Versus Napping
3.4. Gas-Chromatography-Mass Spectrometry
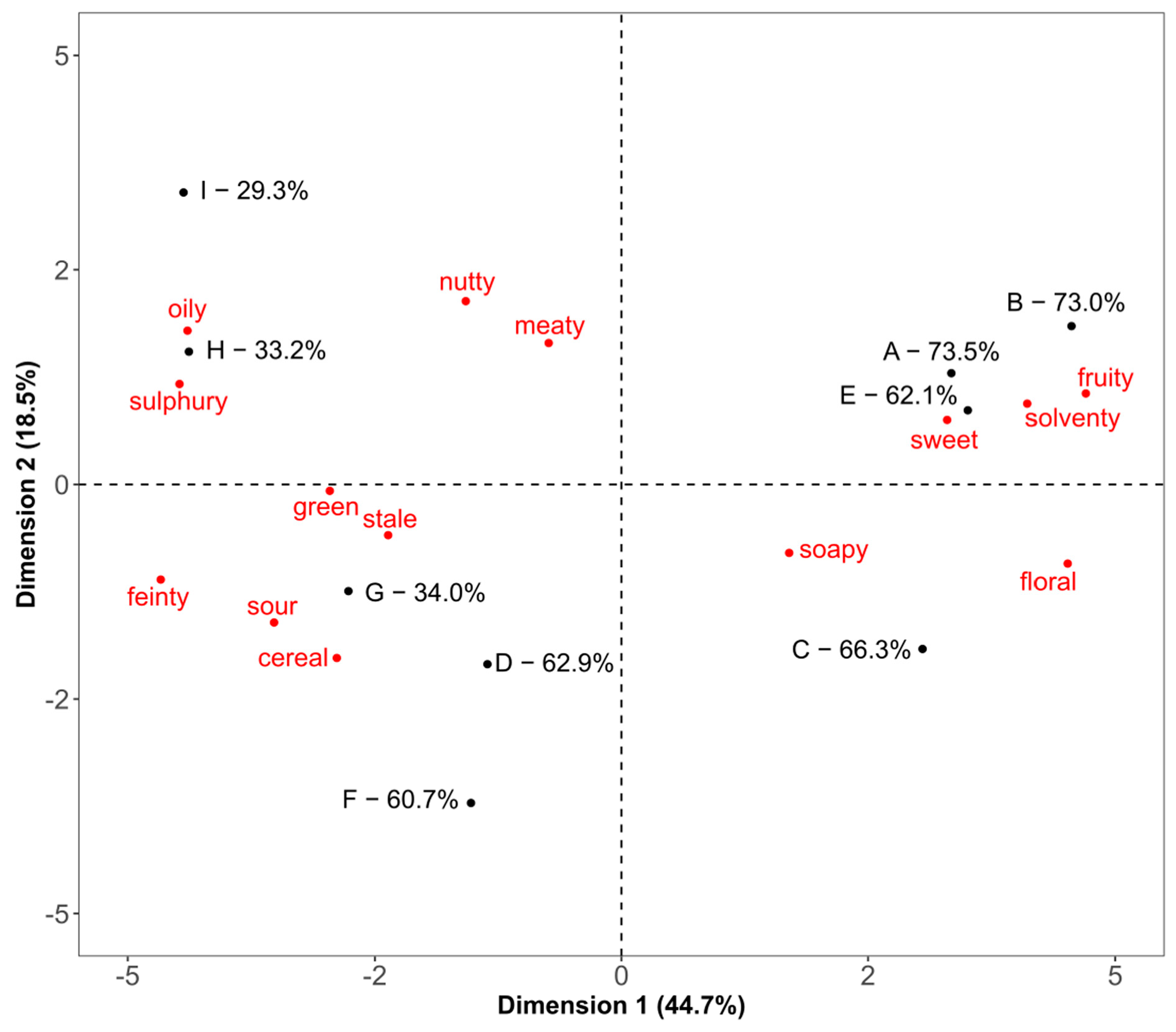
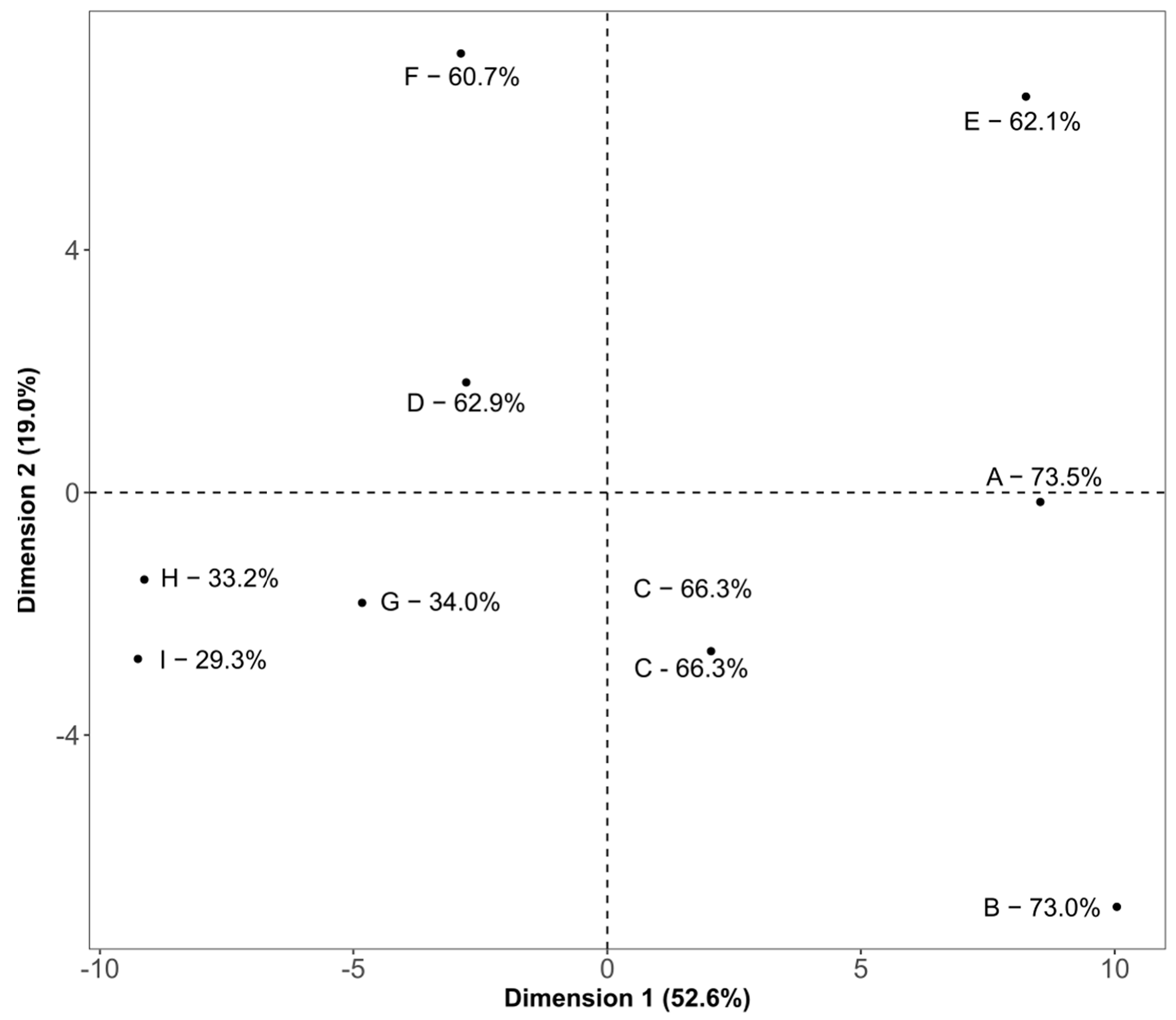
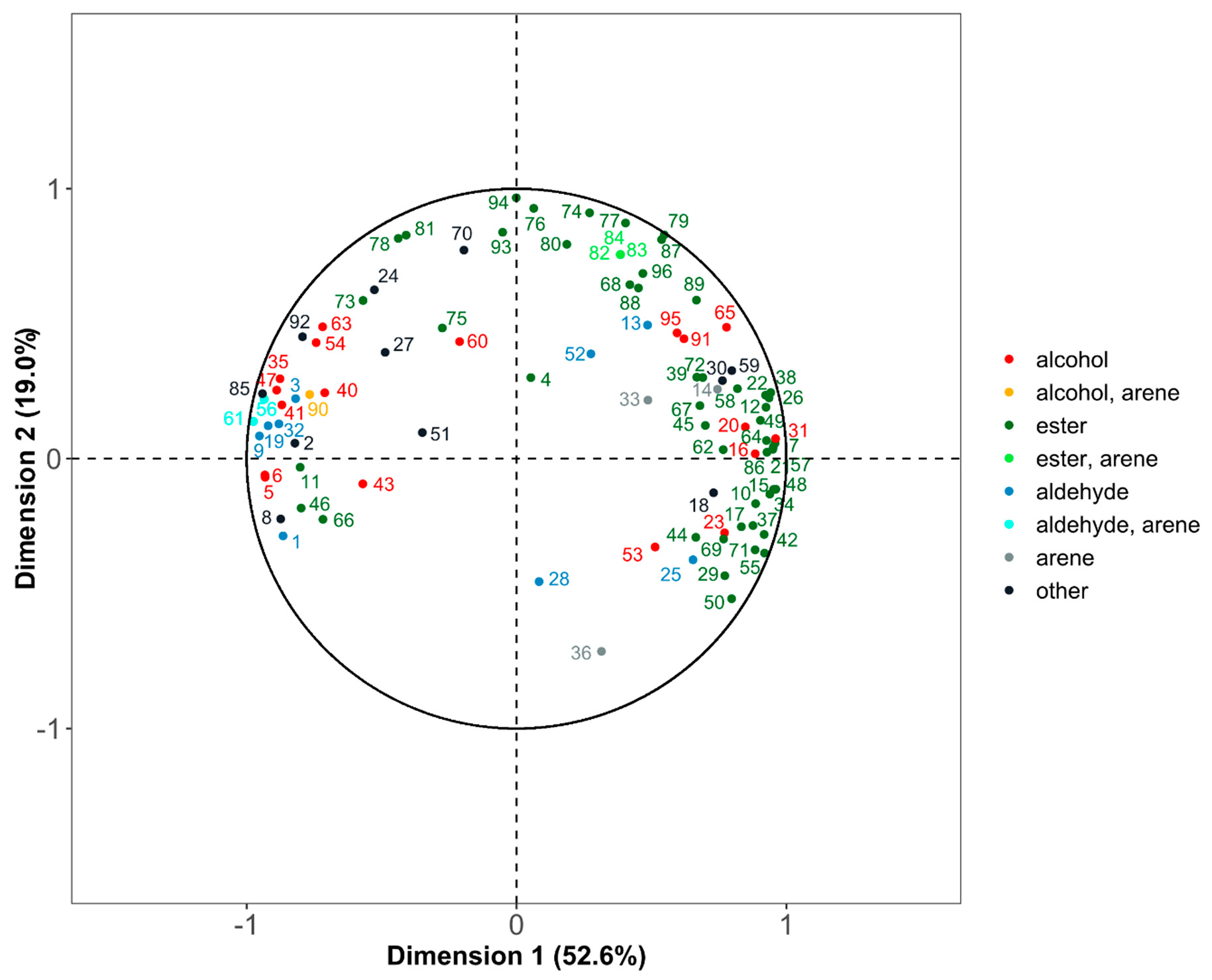
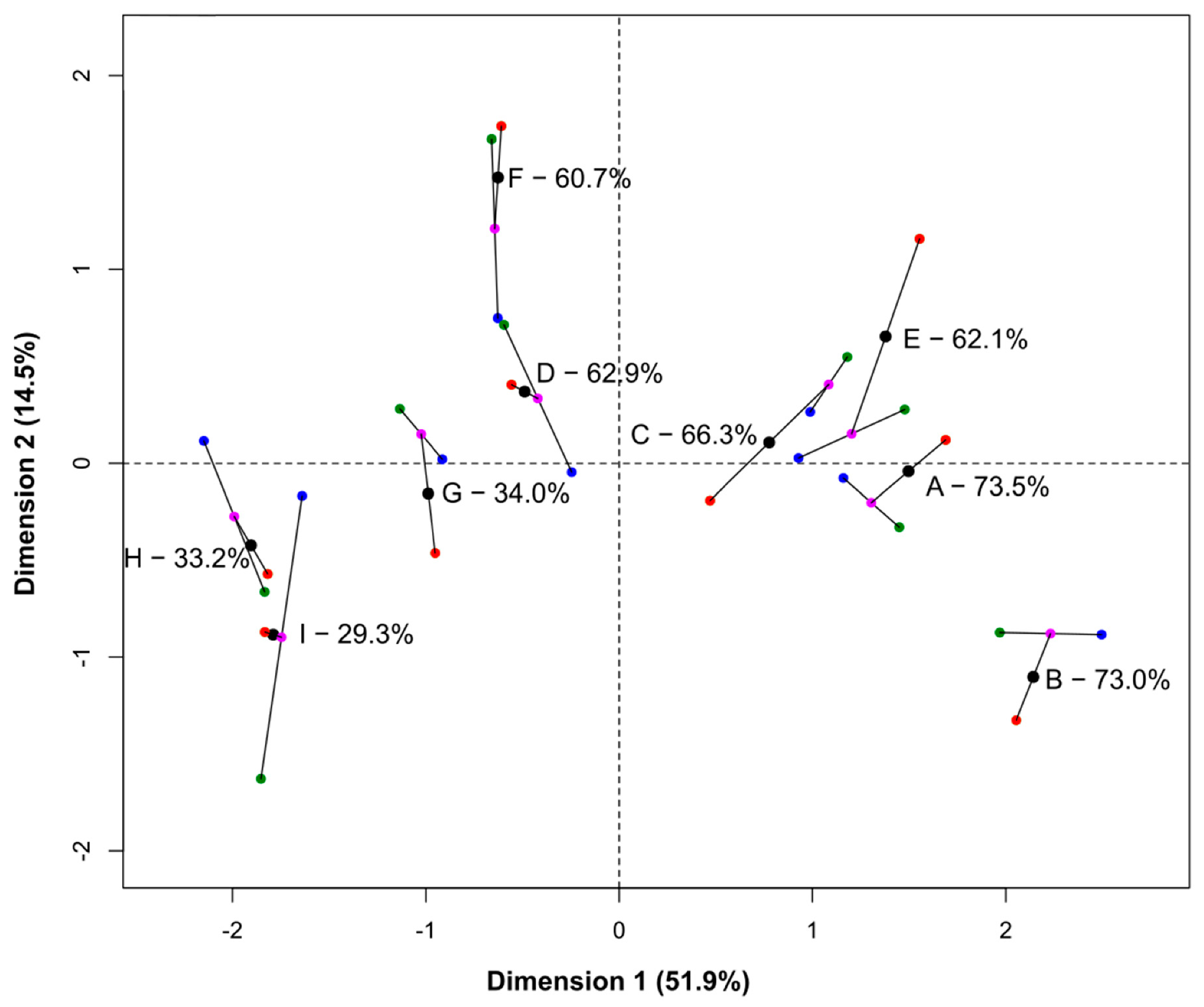
3.5. Statistical Comparison of the Three Methods Using Hierarchical Multiple Factor Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Flavour Attribute | A—73.5% | B—73.0% | C—66.3% | D—62.9% | E—62.1% | F—60.7% | G—34.0% | H—33.2% | I—29.3% |
|---|---|---|---|---|---|---|---|---|---|
| Smoky | 0.15 ± 0.3 a | 0.24 ± 0.42 a | 0.3 ± 0.5 a | 0.15 ± 0.28 a | 0.37 ± 0.44 a | 0.24 ± 0.36 a | 0.15 ± 0.23 a | 0.19 ± 0.29 a | 0.31 ± 0.36 a |
| Feinty * | 0.31 ± 0.31 c,d | 0.32 ± 0.29 c,d | 0.22 ± 0.28 d | 0.6 ± 0.47 a,b,c,d | 0.6 ± 0.47 b,c,d | 0.71 ± 0.53 a,b,c,d | 0.78 ± 0.39 a,b,c | 1.1 ± 0.62 a | 0.97 ± 0.67 a,b |
| Cereal * | 0.55 ± 0.59 a,b,c | 0.35 ± 0.45 c | 0.42 ± 0.46 b,c | 0.65 ± 0.51 a,b,c | 0.65 ± 0.51 a,b,c | 0.65 ± 0.45 a,b,c | 0.98 ± 0.8 a,b | 1.06 ± 0.55 a | 1.15 ± 0.57 a |
| Green | 0.56 ± 0.53 a | 0.69 ± 0.63 a | 0.61 ± 0.44 a | 0.68 ± 0.42 a | 0.58 ± 0.55 a | 0.49 ± 0.47 a | 0.61 ± 0.56 a | 0.57 ± 0.49 a | 0.67 ± 0.54 a |
| Floral | 0.73 ± 0.45 a | 1.09 ± 0.73 a | 1.02 ± 0.62 a | 0.61 ± 0.52 a | 0.72 ± 0.52 a | 0.6 ± 0.53 a | 0.63 ± 0.6 a | 0.56 ± 0.55 a | 0.49 ± 0.57 a |
| Fruity * | 1.32 ± 0.8 a,b | 1.4 ± 0.84 a | 1.03 ± 0.63 a,b,c | 0.89 ± 0.54 a,b,c | 0.89 ± 0.54 a,b,c | 0.7 ± 0.49 b,c | 0.62 ± 0.34 c | 0.73 ± 0.64 a,b,c | 0.61 ± 0.5 c |
| Solventy * | 0.73 ± 0.57 a,b | 1.02 ± 0.79 a | 0.53 ± 0.4 a,b | 0.6 ± 0.37 a,b | 0.6 ± 0.37 a,b | 0.49 ± 0.38 a,b | 0.67 ± 0.49 a,b | 0.48 ± 0.33 b | 0.51 ± 0.47 a,b |
| Soapy | 0.74 ± 0.68 a | 0.81 ± 0.73 a | 0.35 ± 0.38 a | 0.38 ± 0.36 a | 0.57 ± 0.5 a | 0.35 ± 0.4 a | 0.39 ± 0.41 a | 0.44 ± 0.42 a | 0.48 ± 0.54 a |
| Sweet | 0.65 ± 0.47 a | 0.95 ± 0.79 a | 0.78 ± 0.58 a | 0.52 ± 0.47 a | 0.72 ± 0.55 a | 0.56 ± 0.63 a | 0.39 ± 0.47 a | 0.39 ± 0.53 a | 0.38 ± 0.54 a |
| Oily | 0.66 ± 0.61 a | 0.49 ± 0.59 a | 0.49 ± 0.47 a | 0.81 ± 0.51 a | 0.63 ± 0.54 a | 0.66 ± 0.51 a | 0.89 ± 0.61 a | 1.05 ± 0.52 a | 0.77 ± 0.59 a |
| Sour | 0.46 ± 0.46 a | 0.36 ± 0.43 a | 0.51 ± 0.44 a | 0.56 ± 0.48 a | 0.56 ± 0.43 a | 0.54 ± 0.36 a | 0.54 ± 0.32 a | 0.74 ± 0.51 a | 0.63 ± 0.48 a |
| Sulphury * | 0.34 ± 0.36 a,b | 0.24 ± 0.3 b | 0.29 ± 0.35 a,b | 0.48 ± 0.47 a,b | 0.48 ± 0.47 a,b | 0.58 ± 0.52 a,b | 0.51 ± 0.51 a,b | 0.75 ± 0.62 a | 0.64 ± 0.54 a,b |
| Meaty | 0.23 ± 0.47 a | 0.27 ± 0.51 a | 0.24 ± 0.37 a | 0.28 ± 0.37 a | 0.2 ± 0.41 a | 0.32 ± 0.47 a | 0.38 ± 0.5 a | 0.53 ± 0.58 a | 0.5 ± 0.56 a |
| Stale | 0.32 ± 0.29 a | 0.24 ± 0.37 a | 0.44 ± 0.42 a | 0.38 ± 0.32 a | 0.35 ± 0.39 a | 0.51 ± 0.51 a | 0.43 ± 0.41 a | 0.55 ± 0.42 a | 0.56 ± 0.41 a |
| Compound | A 73.5% | B 73.0% | C 66.3% | D 62.9% | E 62.1% | F 60.7% | G 34.0% | H 33.2% | I 29.3% |
|---|---|---|---|---|---|---|---|---|---|
| 1. Acetaldehydealdehyde alcoholic, floral fruity | 481,908 ± 54,424 c | 521,324 ± 52,570 c | 659,602 ± 64,200 c | 575,229 ± 159,495 c | 462,804 ± 25,557 c | 730,904 ± 67,474 c | 1,149,399 ± 263,756 b | 1,441,220 ± 115,373 a,b | 1,580,086 ± 255,833 a |
| 2. Dimethyl sulfidedisulfide sweet, sulphury, green | 488,557 ± 147,809 b,c | 463,450 ± 59,197 c | 831,790 ± 47,118 a | 646,831 ± 334,386 a,b,c | 550,063 ± 29,253 a,b,c | 771,636 ± 56,293 a,b,c | 673,223 ± 66,403 a,b,c | 808,655 ± 142,510 a,b | 822,607 ± 111,136 a,b |
| 3. Isobutyraldehydealdehyde floral, green | 56,538 ± 17,203 d | 50,548 ± 3244 d | 99,743 ± 3424 a,b,c | 76,706 ± 38,572 c,d | 68,910 ± 5008 c,d | 123,065 ± 14,296 a | 79,920 ± 11,498 b,c,d | 119,969 ± 10,856 a | 117,905 ± 11,806 a,b |
| 4. Methyl acetateester alcoholic, sweet, fruity | 101,359 ± 20,659 a,b | 117,666 ± 11,182 a,b | 112,418 ± 26,146 a,b | 81,077 ± 29,053 b | 119,322 ± 21,799 a,b | 141,544 ± 21,077 a | 105,035 ± 31,487 a,b | 110,870 ± 11,829 a,b | 114,212 ± 27,776 a,b |
| 5. trans-2-Methyl-4-hexen-3-olalcohol not described | 5979 ± 1587 d | 3202 ± 545 d | 12,643 ± 982 c,d | 27,021 ± 13,501 b,c | 9751 ± 2943 c,d | 39,575 ± 6681 b | 44,934 ± 13,128 b | 73,897 ± 8422 a | 83,902 ± 11,460 a |
| 6. 2-Methyl-4-hexen-3-olalcohol not described | 31,077 ± 8052 c,d | 15,360 ± 2621 d | 54,540 ± 4627 c,d | 117,107 ± 60,489 b,c | 43,430 ± 12,407 c,d | 178,104 ± 37,429 b | 200,046 ± 61,292 b | 323,561 ± 44,658 a | 360,150 ± 54,919 a |
| 7. Ethyl acetateester alcoholic, sweet, green, fruity | 18,382,122 ± 3,730,553 a | 22,321,690 ± 383,721 a | 11,053,271 ± 376,456 b | 9,781,320 ± 3,917,725 b | 19,020,913 ± 1,954,200 a | 10,743,941 ± 1,859,787 b | 2,508,092 ± 758,621 c | 3,973,210 ± 348,385 c | 3,723,184 ± 439,572 c |
| 8. 1,1-Diethoxyethaneacetal alcoholic, sweet, sulphury, green, nutty | 395,771 ± 111,620 c | 303,824 ± 37,278 c | 625,277 ± 82,896 c | 676,984 ± 202,277 c | 435,590 ± 83,210 c | 925,422 ± 174,163 c | 1,965,337 ± 713,235 b | 2,308,725 ± 96,073 a,b | 2,876,673 ± 686,735 a |
| 9. Isovaleraldehydealdehyde alcoholic, floral, sweet, nutty, oily, fruity | 267,053 ± 19,695 d | 224,790 ± 21,939 d | 453,132 ± 29,579 c,d | 680,272 ± 360,328 b,c | 312,757 ± 29,686 d | 945,977 ± 109,025 a,b | 694,170 ± 126,614 b,c | 1,204,443 ± 131,856 a | 1,174,573 ± 110,932 a |
| 10. Propyl acetateester alcoholic, sulphury, feinty, fruity | 244,492 ± 90,151 b | 443,962 ± 33,857 a | 49,898 ± 2894 c | 48,993 ± 21,442 c | 270,535 ± 22,953 b | 70,599 ± 17,901 c | 14,271 ± 4554 c | 16,485 ± 1035 c | 16,312 ± 1821 c |
| 11. Methyl 2-methylbutyrateester alcoholic, green, oily, fruity | 3223 ± 467 c,d,e | 2894 ± 391 d,e | 2247 ± 901 e | 4939 ± 1549 c | 3681 ± 208 c,d,e | 4473 ± 240 c,d | 4786 ± 259 c,d | 9666 ± 705 a | 7071 ± 1491 b |
| 12. Isobutyl acetateester alcoholic, sweet, fruity | 1,975,886 ± 841,217 b | 2,418,168 ± 202,003 a,b | 513,693 ± 24,473 c | 546,970 ± 273,726 c | 3,159,200 ± 334,715 a | 434,861 ± 128,777 c | 164,831 ± 61,002 c | 85,453 ± 13,519 c | 85,611 ± 9792 c |
| 13. 1-Pentene-3-onealdehyde sulphury, meaty | 13,664 ± 3088 a,b | 11,493 ± 796 b,c | 9690 ± 1076 c | 9415 ± 2079 c | 17,437 ± 1564 a | 12,077 ± 1578 b,c | 8968 ± 795 c | 11,236 ± 1219 b,c | 12,189 ± 1469 b,c |
| 14. 2-Ethyl-5-methylfuranarene green, meaty | 78,998 ± 42,083 a | 63,287 ± 12,084 a | 75,955 ± 11,011 a | 49,306 ± 32,684 a | 63,610 ± 6642 a | 71,385 ± 16,008 a | 31,385 ± 3850 a | 38,328 ± 10,687 a | 35,159 ± 8913 a |
| 15. Ethyl butyrateester alcoholic, fruity | 1,557,492 ± 548,733 a | 1,705,076 ± 133,103 a | 490,967 ± 31,338 b | 334,084 ± 173,138 b | 1,304,489 ± 112,516 a | 302,383 ± 87,024 b | 201,152 ± 62,397 b | 115,315 ± 15,095 b | 113,868 ± 13,781 b |
| 16. 1-Propanolalcohol alcoholic, sulphury, feinty, sour | 666,958 ± 82,445 b | 866,202 ± 48,897 a | 229,968 ± 5890 e | 385,333 ± 46,031 d | 555,349 ± 26,746 c | 439,314 ± 56,070 d | 121,130 ± 29,508 f | 121,258 ± 12,576 f | 116,833 ± 15,323 f |
| 17. Ethyl isovalerateester sweet, fruity | 69,540 ± 28,504 a,b | 81,615 ± 5173 a | 52,892 ± 1805 b,c | 18,450 ± 8870 d | 66,520 ± 8929 a,b | 27,107 ± 4546 c,d | 14,343 ± 3368 d | 37,112 ± 2914 c,d | 35,741 ± 7217 c,d |
| 18. Dimethyl disulfidesulfide sulphury | 245,936 ± 47,623 a | 244,873 ± 23,530 a | 138,845 ± 37,223 b | 105,460 ± 70,434 b | 204,624 ± 13,460 a,b | 174,837 ± 58,260 a,b | 178,734 ± 42,061 a,b | 110,281 ± 17,298 b | 173,003 ± 50,611 a,b |
| 19. Hexanalaldehyde floral, green, oily, fruity | 88,813 ± 34,412 c,d | 68,190 ± 6782 d | 109,882 ± 2099 a,b,c,d | 103,170 ± 53,972 b,c,d | 79,626 ± 10,708 c,d | 155,460 ± 13,097 a,b | 132,003 ± 18,382 a,b,c | 154,091 ± 15,653 a,b | 163,701 ± 16,008 a |
| 20. 2-Methyl-1-propanolalcohol alcoholic | 10,587,847 ± 838,584 a,b | 9,884,785 ± 581,965 a,b,c | 7,826,962 ± 1,235,571 b,c,d | 7,834,439 ± 917,929 b,c,d | 11,496,815 ± 431,102 a | 5,988,978 ± 1,378,002 d,e | 4,205,263 ± 1,078,625 e | 7,248,326 ± 875,791 c,d | 5,314,747 ± 2,219,781 d,e |
| 21. Isoamyl acetateester alcoholic, sweet, fruity | 35,620,257 ± 11,700,931 b | 53,609,891 ± 1,105,546 a | 18,204,837 ± 508,238 c | 14,049,416 ± 6,462,066 c,d | 50,960,037 ± 6,037,004 a | 15,717,204 ± 3,151,031 c,d | 7,460,715 ± 2,390,776 c,d | 3,628,765 ± 824,823 d | 3,663,686 ± 470,296 d |
| 22. Ethyl valerateester sweet, green, fruity | 120,237 ± 23,133 a | 98,923 ± 4823 a,b,c | 63,994 ± 11,711 c,d,e | 47,432 ± 26,293 d,e | 111,105 ± 12,852 a,b | 77,297 ± 25,304 b,c,d | 40,686 ± 11,490 d,e | 38,253 ± 4028 e | 37,144 ± 4759 e |
| 23. 1-Butanolalcohol alcoholic, sweet, oily, feinty | 221,621 ± 109,583 a | 159,773 ± 8053 a | 35,027 ± 1008 b | 26,833 ± 887 b | 47,278 ± 4203 b | 24,135 ± 4819 b | 11,233 ± 2224 b | 12,675 ± 974 b | 12,439 ± 1159 b |
| 24. 5-methyl-5-hepten-3-oneketone not described | 18,090 ± 5096 c,d | 12,391 ± 1085 d | 19,517 ± 240 b,c | 24,516 ± 1901 a,b | 17,077 ± 1062 c,d | 26,453 ± 3632 a | 16,855 ± 2150 c,d | 19,557 ± 2739 b,c | 20,309 ± 1716 a,b,c |
| 25. 5-Methyl-2,4-Hexenalaldehyde not described | 51,021 ± 12,868 b | 303,174 ± 127,475 a | 26,022 ± 8956 b | 24,214 ± 16,534 b | 65,551 ± 20,616 b | 60,221 ± 35,190 b | 8579 ± 3169 b | 4586 ± 1595 b | 5822 ± 5390 b |
| 26. Pentyl acetateester alcoholic, fruity | 90,060 ± 17,714 a | 93,897 ± 7777 a | 40,028 ± 2711 b,c | 47,514 ± 25,183 b,c | 111,553 ± 8927 a | 50,758 ± 14,555 b | 36,333 ± 8528 b,c | 19,668 ± 4415 c | 19,475 ± 2242 c |
| 27. 2-Heptanoneketone green, nutty, meaty, sour, fruity | 40,689 ± 3588 b,c | 18,150 ± 1673 d | 30,143 ± 809 c,d | 24,009 ± 7829 d | 49,723 ± 4798 a,b | 44,036 ± 4874 a,b | 43,271 ± 3644 a,b | 55,271 ± 10,628 a | 50,527 ± 2656 a,b |
| 28. Heptanalaldehyde alcoholic, floral, green, oily | 19,474 ± 5909 a | 33,914 ± 39,102 a | 20,984 ± 2415 a | 15,534 ± 8230 a | 18,011 ± 4885 a | 26,753 ± 8272 a | 20,667 ± 3846 a | 23,720 ± 1445 a | 25,293 ± 2673 a |
| 29. 1-Butanol, 3-methyl-, propanoateester not described | 93,842 ± 76,204 b | 179,940 ± 14,247 a | 10,327 ± 1670 c | 3297 ± 1265 c | 38,891 ± 4406 b,c | 10,242 ± 2928 c | 3490 ± 502 c | 3278 ± 243 c | 3886 ± 726 c |
| 30. D-Limoneneterpene sweet, green, fruity | 76,550 ± 29,999 a | 54,222 ± 24,589 a,b | 55,420 ± 7020 a,b | 41,451 ± 31,326 a,b | 48,187 ± 4425 a,b | 64,299 ± 24,031 a | 14,922 ± 3397 b | 17,295 ± 7110 b | 12,526 ± 6172 b |
| 31. 3-Methyl-1-butanol / 2-Methyl-1-butanolalcohol alcoholic, feinty, sour, fruity | 35,207,874 ± 4,630,155 b | 43,035,303 ± 882,122 a | 29,870,260 ± 846,324 c | 25,569,536 ± 1,433,457 c | 38,863,229 ± 3,081,251 a,b | 25,827,190 ± 1,670,510 c | 13,077,795 ± 1,721,053 d | 13,790,941 ± 868,228 d | 13,022,207 ± 801,278 d |
| 32. 2-Hexenalaldehyde sweet, sulphury, green, nutty | 4399 ± 1364 d | 0 ± 0 e | 5583 ± 385 c,d | 7108 ± 750 a,b,c | 0 ± 0 e | 9039 ± 596 a | 6745 ± 1454 b,c | 8215 ± 1136 a,b | 8386 ± 875 a,b |
| 33. 2-Pentylfuranarene sulphury, green, nutty, fruity | 601,438 ± 223,825 a,b | 536,551 ± 90,592 a,b | 784,789 ± 181,074 a | 511,350 ± 388,544 a,b | 430,067 ± 54,886 a,b | 723,665 ± 79,541 a | 281,067 ± 34,991 b | 303,600 ± 94,489 b | 265,816 ± 77,408 b |
| 34. Ethyl hexanoateester sweet, green, oily, fruity | 21,361,104 ± 4,467,181 a | 21,754,800 ± 320,144 a | 20,696,226 ± 3,610,088 a | 9,072,237 ± 5,481,090 c | 19,791,349 ± 1,593,226 a,b | 10,575,207 ± 3,221,552 c | 12,879,040 ± 2,526,623 b,c | 6,048,507 ± 1,124,185 c | 5,907,996 ± 445,776 c |
| 35. 1-Pentanolalcohol sweet, oily, feinty | 59,470 ± 21,837 b,c | 38,153 ± 2780 c | 65,040 ± 3961 b,c | 106,426 ± 15,577 a | 56,454 ± 6016 b,c | 110,575 ± 17,646 a | 70,278 ± 6796 b | 119,105 ± 19,147 a | 110,749 ± 9634 a |
| 36. Styrenearene floral, sweet | 1,218,132 ± 888,865 a,b | 1,584,839 ± 282,254 a,b | 1,725,646 ± 382,468 a | 99,985 ± 72,226 c | 79,701 ± 20,940 c | 133,142 ± 56,968 c | 75,142 ± 15,304 c | 1,061,644 ± 354,016 a,b | 748,193 ± 176,825 b,c |
| 37. Isoamyl butyrateester green, fruity | 84,318 ± 47,794 b | 131,369 ± 7041 a | 31,650 ± 3875 c | 6814 ± 4290 c | 85,595 ± 10,581 b | 17,430 ± 6634 c | 10,072 ± 3036 c | 19,193 ± 3709 c | 20,362 ± 1209 c |
| 38. Hexyl acetateester sweet, green, fruity | 489,986 ± 125,749 a | 453,761 ± 43,030 a,b | 226,494 ± 20,254 c,d | 240,480 ± 137,346 c,d | 513,665 ± 37,021 a | 304,411 ± 95,689 b,c | 215,861 ± 58,764 c,d | 108,461 ± 28,101 d | 107,566 ± 12,342 d |
| 39. Ethyl 3-hexenoateester sweet, green, fruity | 7667 ± 3510 a,b | 6417 ± 704 a,b,c | 8345 ± 1506 a | 6014 ± 2116 a,b,c | 6160 ± 336 a,b,c | 7958 ± 2260 a | 3693 ± 1328 b,c | 3357 ± 478 c | 2786 ± 411 c |
| 40. Prenolalcohol green, sour, fruity | 2079 ± 1581 c,d | 478 ± 90 d | 4666 ± 158 a | 3635 ± 947 a,b,c | 2106 ± 473 c,d | 3984 ± 1004 a,b | 2300 ± 317 b,c,d | 4243 ± 642 a | 3997 ± 337 a,b |
| 41. 2-Heptanolalcohol sweet, green, fruity | 33,288 ± 18,193 c,d | 25,143 ± 2093 d | 45,455 ± 1046 b,c,d | 48,723 ± 5772 a,b,c | 24,663 ± 2909 d | 67,250 ± 13,562 a | 43,599 ± 4313 b,c,d | 63,191 ± 9954 a,b | 57,734 ± 5064 a,b |
| 42. Propyl hexanoateester sweet, green, fruity | 39,592 ± 15,104 b | 57,188 ± 1569 a | 17,122 ± 4462 c,d | 6222 ± 4376 d | 30,246 ± 4788 b,c | 9959 ± 3427 d | 8365 ± 2652 d | 3887 ± 705 d | 3834 ± 205 d |
| 43. 7-Octen-2-onealcohol not described | 8349 ± 6150 b,c | 1360 ± 158 d | 1500 ± 489 d | 1428 ± 602 d | 11,224 ± 2137 b | 2713 ± 575 c,d | 11,783 ± 1149 b | 22,898 ± 3783 a | 19,904 ± 929 a |
| 44. 2,4-Hexadienedioic acid, dimethyl esterester not described | 14,918 ± 5514 a | 12,934 ± 862 a,b | 11,247 ± 1268 a,b | 10,704 ± 2265 a,b | 8864 ± 1090 b | 10,124 ± 2775 a,b | 8339 ± 1222 b | 8798 ± 819 b | 9405 ± 770 a,b |
| 45. Ethyl heptanoateester alcoholic, sweet, green, nutty, fruity | 1,130,004 ± 376,482 a | 923,846 ± 16,928 a | 1,179,993 ± 231,889 a | 832,334 ± 571,592 a | 898,959 ± 143,947 a | 980,882 ± 347,887 a | 752,067 ± 250,901 a | 665,463 ± 52,407 a | 639,422 ± 37,235 a |
| 46. 2-Hydroxyethyl propionateester sweet, oily, meaty, fruity | 127,963 ± 64,010 b | 77,485 ± 2973 b | 208,029 ± 54,664 b | 209,560 ± 137,062 b | 100,126 ± 8933 b | 340,314 ± 48,466 b | 245,003 ± 74,830 b | 1,082,241 ± 247,838 a | 1,176,303 ± 229,384 a |
| 47. 1-Hexanolalcohol alcoholic, sweet, green, oily, feinty | 127,988 ± 40,711 c,d | 88,862 ± 6286 d | 139,573 ± 3181 c,d | 200,877 ± 13,888 a,b | 113,253 ± 9525 c,d | 234,965 ± 38,079 a | 150,543 ± 13,001 b,c | 250,166 ± 39,329 a | 231,403 ± 15,459 a |
| 48. Isobutyl hexanoateester sweet, green, fruity | 312,155 ± 157,080 a | 306,401 ± 27,847 a | 234,753 ± 63,284 a | 45,044 ± 33,334 b | 304,888 ± 71,109 a | 49,764 ± 18,255 b | 71,353 ± 26,067 b | 23,765 ± 4610 b | 22,920 ± 895 b |
| 49. Heptyl acetateester alcoholic, green, nutty, fruity | 34,713 ± 10,593 a | 30,435 ± 2844 a,b | 14,052 ± 2177 c,d | 7986 ± 4892 d | 33,429 ± 2836 a | 20,138 ± 6954 b,c | 13,275 ± 3980 c,d | 5627 ± 1789 d | 5242 ± 254 d |
| 50. Methyl octanoateester sweet, sulphury, green, oily, fruity | 120,997 ± 38,764 b,c | 171,994 ± 12,957 a | 137,223 ± 20,989 a,b | 39,251 ± 26,270 e | 91,125 ± 18,279 b,c,d | 49,606 ± 12,407 d,e | 77,193 ± 22,890 c,d,e | 51,048 ± 6883 d,e | 50,174 ± 5706 d,e |
| 51. 2-Nonanoneketone sweet, green, nutty, fruity | 28,549 ± 13,708 a,b,c | 14,431 ± 1176 c,d | 25,227 ± 1525 a,b,c,d | 13,269 ± 5314 d | 18,449 ± 2299 b,c,d | 30,168 ± 8241 a,b | 36,125 ± 3001 a | 24,635 ± 4434 a,b,c,d | 24,440 ± 365 a,b,c,d |
| 52. Nonanalaldehyde floral, green, oily | 2590 ± 1134 a | 2482 ± 307 a | 2539 ± 291 a | 1788 ± 526 a | 2417 ± 619 a | 3330 ± 1502 a | 2392 ± 541 a | 2157 ± 514 a | 2081 ± 113 a |
| 53. 3,5-Octadien-2-olalcohol not described | 2968 ± 668 a,b | 4738 ± 745 a | 3710 ± 910 a,b | 2432 ± 695 b | 2780 ± 380 b | 3745 ± 1305 a,b | 2617 ± 878 b | 2673 ± 352 b | 2757 ± 134 b |
| 54. (R)-(-)-2-Octanolalcohol green, oily, sour | 38,783 ± 13,899 b,c | 26,017 ± 1741 c | 44,156 ± 1294 a,b,c | 50,719 ± 12,475 a,b | 31,972 ± 3025 b,c | 60,976 ± 15,354 a | 40,962 ± 4199 a,b,c | 50,615 ± 8095 a,b | 47,893 ± 3902 a,b |
| 55. Ethyl octanoateester alcoholic, sweet, oily, fruity | 92,505,435 ± 19,005,128 b | 121,187,111 ± 8,707,498 a | 88,065,236 ± 7,990,917 b | 30,061,202 ± 18,622,279 c | 73,315,080 ± 10,859,990 b | 29,630,811 ± 7,412,592 c | 38,817,582 ± 9,917,716 c | 20,086,095 ± 3,843,883 c | 19,176,119 ± 932,389 c |
| 56. Furfuralaldehyde, arene sweet, nutty | 324,984 ± 233,569 d | 126,495 ± 18,722 d | 462,183 ± 87,303 d | 1,132,087 ± 146,477 b,c | 466,830 ± 121,216 d | 1,449,314 ± 237,701 a,b | 951,675 ± 149,006 c | 1,792,410 ± 304,014 a | 1,580,711 ± 160,346 a |
| 57. Isopentyl hexanoateester green, fruity | 746,539 ± 351,540 a | 727,214 ± 46,122 a | 627,587 ± 171,197 a,b | 157,157 ± 117,441 c | 880,603 ± 83,331 a | 284,669 ± 96,362 b,c | 224,690 ± 81,035 c | 122,222 ± 24,065 c | 109,750 ± 9450 c |
| 58. Octyl acetateester floral, green, nutty, oily, fruity | 47,978 ± 28,343 a | 22,687 ± 5789 b,c | 6414 ± 1534 c | 7420 ± 4217 c | 36,685 ± 6362 a,b | 14,623 ± 5039 b,c | 8923 ± 2890 c | 3468 ± 970 c | 3275 ± 392 c |
| 59. 2-Methyldecan-3-oneketone not described | 647,645 ± 258,166 c | 1,059,357 ± 102,156 b | 155,332 ± 19,523 d,e | 418,000 ± 240,079 c,d,e | 1,619,000 ± 234,068 a | 445,728 ± 58,106 c,d | 166,035 ± 64,743 d,e | 81,845 ± 13,125 e | 87,047 ± 19,372 e |
| 60. 2-Nonanolalcohol green, oily, sour, fruity | 28,739 ± 22,768 a,b,c | 9704 ± 2414 c | 32,115 ± 3349 a,b | 12,580 ± 4034 b,c | 17,353 ± 3351 b,c | 43,136 ± 10,736 a | 19,392 ± 1110 b,c | 24,715 ± 4144 a,b,c | 22,083 ± 1673 b,c |
| 61. Benzaldehydearene, aldehyde sweet, nutty, meaty, fruity | 119,214 ± 62,943 b | 65,242 ± 9967 b | 282,004 ± 91,678 b | 718,428 ± 94,484 a | 205,568 ± 75,371 b | 799,130 ± 217,023 a | 723,093 ± 161,943 a | 919,547 ± 234,254 a | 1,007,765 ± 169,758 a |
| 62. Ethyl nonanoateester alcoholic, oily, fruity | 1,926,162 ± 568,332 a | 1,051,770 ± 87,136 b,c | 1,221,447 ± 154,442 a,b | 788,277 ± 586,952 b,c | 926,694 ± 27,400 b,c | 893,458 ± 327,051 b,c | 479,690 ± 169,360 c | 498,155 ± 86,631 b,c | 410,091 ± 70,947 c |
| 63. Linaloolalcohol floral, sweet, green, nutty, oily | 56,095 ± 42,410 b,c,d | 21,981 ± 4351 d | 75,726 ± 9786 b,c | 87,435 ± 18,664 b,c | 45,791 ± 9559 c,d | 135,643 ± 17,382 a | 64,661 ± 5776 b,c,d | 96,904 ± 15,112 a,b | 89,060 ± 5574 b |
| 64. Octyl octanoateester nutty, oily, fruity | 1,600,274 ± 654,651 b | 1,705,887 ± 107,776 b | 1,379,790 ± 231,816 b | 341,077 ± 255,506 c | 2,413,979 ± 329,573 a | 321,637 ± 129,965 c | 304,044 ± 126,200 c | 150,270 ± 40,266 c | 135,021 ± 33,558 c |
| 65. 3-Methylbutyl heptanoatealcohol green, fruity | 8481 ± 5136 a,b | 8144 ± 1023 a,b | 9547 ± 2469 a,b | 5795 ± 4775 b | 14,067 ± 2170 a | 8648 ± 3249 a,b | 3581 ± 1342 b | 3949 ± 834 b | 3494 ± 837 b |
| 66. Isoamyl lactateester nutty, fruity | 30,090 ± 22,925 b | 14,295 ± 2080 b | 42,389 ± 12,843 b | 59,798 ± 18,494 b | 38,945 ± 11,941 b | 71,170 ± 7398 b | 36,082 ± 9195 b | 735,431 ± 132,659 a | 766,027 ± 110,054 a |
| 67. Ethyl 3-nonanoateester not described | 46,814 ± 24,299 a | 30,506 ± 2140 a,b | 44,532 ± 7714 a | 14,860 ± 10,601 b | 38,884 ± 6130 a,b | 36,266 ± 11,194 a,b | 26,386 ± 7833 a,b | 23,247 ± 2992 a,b | 17,972 ± 672 b |
| 68. Ethyl 8-nonenoateester not described | 9707 ± 3888 a | 6591 ± 358 a | 10,179 ± 914 a | 8619 ± 5824 a | 9157 ± 1852 a | 11,386 ± 3315 a | 6551 ± 1929 a | 5989 ± 1187 a | 5204 ± 171 a |
| 69. Methyl decanoateester alcoholic, floral, oily, fruity | 136,805 ± 71,439 a,b | 151,708 ± 5522 a | 153,104 ± 15,713 a | 48,190 ± 35,636 c | 74,446 ± 3784 b,c | 90,924 ± 27,602 a,b,c | 59,963 ± 16,278 c | 43,801 ± 6568 c | 39,743 ± 8555 c |
| 70. 2-Undecanoneketone floral, oily, fruity | 63,260 ± 72,561 b,c | 20,931 ± 20,419 b,c | 86,076 ± 73,537 b,c | 126,252 ± 67,766 b,c | 169,327 ± 46,660 a,b | 292,273 ± 117,557 a | 147,695 ± 67,327 a,b,c | 14,057 ± 1560 c | 139,823 ± 14,448 b,c |
| 71. Ethyl decanoateester alcoholic, sweet, oily, fruity | 116,133,681 ± 19,769,284 b | 157,344,789 ± 15,847,183 a | 109,626,744 ± 6,589,724 b | 32,082,437 ± 22,333,847 d,e | 68,126,749 ± 8,045,711 c | 48,195,673 ± 13,593,134 c,d | 26,093,987 ± 6,329,905 d,e | 15,121,898 ± 4,628,880 e | 13,059,109 ± 3,307,716 e |
| 72. Isopentyl octanoateester sweet, green, nutty, oily, soapy, fruity | 750,276 ± 539,105 c | 2,390,522 ± 311,955 b | 883,884 ± 243,086 c | 566,012 ± 434,133 c | 4,841,560 ± 1301,331 a | 585,569 ± 171,591 c | 409,120 ± 158,046 c | 238,795 ± 121,416 c | 193,168 ± 96,340 c |
| 73. Ethyl trans-4-decanoateester not described | 5530 ± 6772 b | 3430 ± 1098 b | 3444 ± 1292 b | 5244 ± 3047 b | 7570 ± 2040 a,b | 14,705 ± 3104 a | 8804 ± 2638 a,b | 10,167 ± 2055 a,b | 8966 ± 1612 a,b |
| 74. Decyl acetateester green, oily, soapy | 68,334 ± 48,626 b | 19,151 ± 1166 b | 10,976 ± 2962 b | 64,762 ± 45,846 b | 278,529 ± 102,001 a | 253,256 ± 65,939 a | 36,177 ± 13,494 b | 18,566 ± 10,300 b | 13,864 ± 4173 b |
| 75. Ethyl-9-decanoateester alcoholic, sweet, oily | 388,165 ± 54,518 b,c | 505,882 ± 66,888 a,b,c | 213,146 ± 63,611 c | 960,037 ± 778,852 a,b | 311,145 ± 40,259 b,c | 1166,768 ± 304,610 b,c | 343,276 ± 332,721 b,c | 549,674 ± 153,802 a,b,c | 426,589 ± 64,466 b,c |
| 76. Isopropyl decanoateester sulphury, green, oily, fruity | 45,054 ± 41,191 c,d | 13,577 ± 2929 d | 11,651 ± 4819 d | 112,823 ± 67,092 b,c | 148,479 ± 62,231 a,b | 217,807 ± 19,412 a | 34,228 ± 9661 c,d | 21,423 ± 7897 d | 20,051 ± 4339 d |
| 77. Propyl decanoateester sulphury, green, oily, fruity | 24,992 ± 21,435 a,b,c | 8500 ± 2096 b,c | 7546 ± 2948 c | 16,487 ± 12,259 a,b,c | 30,868 ± 9152 a,b | 33,965 ± 10,246 a | 9534 ± 3354 b,c | 6276 ± 4365 c | 5280 ± 3413 c |
| 78. Ethyl undecanoateester nutty, oily, soapy | 4349 ± 2662 a,b | 466 ± 158 b | 1482 ± 660 b | 5884 ± 4024 a,b | 5209 ± 1826 a,b | 7685 ± 2264 a | 4554 ± 1291 a,b | 5677 ± 3577 a,b | 3714 ± 2203 a,b |
| 79. Isobutyl decanoateester alcoholic, sweet, oily, fruity | 300,289 ± 219,409 a,b,c | 129,725 ± 22,169 b,c | 172,082 ± 49,544 b,c | 247,318 ± 161,342 a,b,c | 497,603 ± 178,774 a | 346,599 ± 89,573 a,b | 113,646 ± 40,382 b,c | 81,469 ± 49,235 b,c | 65,083 ± 33,798 c |
| 80. 3-Methylbutyl nonanoateester floral, oily, fruity | 7757 ± 5802 b,c | 5005 ± 4982 b,c | 5732 ± 2208 b,c | 22,249 ± 4020 a | 20,673 ± 8306 a | 15,663 ± 5277 a,b | 4363 ± 1372 b,c | 5941 ± 4496 b,c | 3654 ± 2475 c |
| 81. Methyl dodecanoateester nutty, oily, meaty, soapy | 34,021 ± 24,736 a,b | 10,354 ± 472 c | 35,553 ± 9312 a,b | 102,217 ± 83,053 a,b | 100,946 ± 24,772 a,b | 163,632 ± 22,873 a | 100,405 ± 37,590 a,b | 78,447 ± 32,177 a,b,c | 54,590 ± 24,549 a,b |
| 82. Phenethyl acetateester, arene floral, sweet, fruity | 683,823 ± 456,857 b,c | 161,435 ± 28,242 c | 239,346 ± 58,795 b,c | 734,898 ± 25,371 b,c | 2,329,105 ± 534,582 a | 767,940 ± 97,739 b | 402,793 ± 139,518 b,c | 305,361 ± 66,151 b,c | 292,177 ± 27,306 b,c |
| 83. 2-Phenylethyl hexanoateester, arene floral, sweet, sulphury, green, oily | 683,805 ± 456,822 b,c | 161,435 ± 28,242 c | 239,346 ± 58,795 b,c | 734,870 ± 25,423 b,c | 2,329,100 ± 534,574 a | 767,931 ± 97,737 b | 402,789 ± 139,522 b,c | 305,353 ± 66,162 b,c | 292,166 ± 27,328 b,c |
| 84. Phenethyl isobutyrateester, arene floral, fruity | 683,805 ± 456,822 b,c | 161,435 ± 28,242 c | 239,339 ± 58,795 b,c | 734,870 ± 25,423 b,c | 2,328,598 ± 534,482 a | 767,905 ± 97,689 b | 402,789 ± 139,522 b,c | 305,334 ± 66,173 b,c | 292,142 ± 27,330 b,c |
| 85. Hexanoic acidcarboxylic acid sulphury, oily, sour | 1764 ± 1260 c,d | 0 ± 0 d | 22,336 ± 5937 b,c,d | 34,378 ± 8615 a,b,c | 21,026 ± 11,822 b,c,d | 56,916 ± 28,989 a | 43,045 ± 9235 a,b | 66,369 ± 7485 a | 60,122 ± 24,856 a |
| 86. Ethyl dodecanoateester floral, sweet, oily, soapy | 39,313,957 ± 4,699,832 a,b | 37,292,487 ± 2,796,072 a,b | 40,693,658 ± 2,667,062 a | 23,549,146 ± 14,984,160 b,c | 37,069,182 ± 2,103,565 a,b | 23,900,201 ± 5,477,888 b,c | 19,616,178 ± 5,261,140 c | 13,204,315 ± 7,417,775 c | 9,534,318 ± 4,343,818 c |
| 87. Isoamyl decanoateester alcoholic, sweet, green, oily, fruity | 43,540 ± 29,955 a,b,c | 36,563 ± 12,682 a,b,c | 35,040 ± 9967 a,b,c | 46,993 ± 30,979 a,b,c | 82,611 ± 36,874 a | 72,219 ± 17,665 a,b | 23,851 ± 10,901 b,c | 16,840 ± 14,118 c | 12,590 ± 9912 c |
| 88. Ethyl isopentyl succinateester not described | 19,936 ± 4579 b | 7213 ± 1188 b | 11,089 ± 3307 b | 12,041 ± 7962 b | 101,223 ± 23,769 a | 19,158 ± 5798 b | 9027 ± 8100 b | 9783 ± 6009 b | 6090 ± 2075 b |
| 89. Ethyl palmitateester oily, sour, fruity | 52,964 ± 6905 b | 36,174 ± 3559 b,c | 34,767 ± 4511 b,c | 27,736 ± 22,109 b,c | 106,991 ± 19,913 a | 41,298 ± 11,891 b,c | 22,407 ± 22,527 b,c | 24,279 ± 14,063 b,c | 15,228 ± 5218 c |
| 90. Phenylethyl alcoholalcohol, arene floral | 313,498 ± 229,653 c,d,e | 75,354 ± 1845 e | 160,682 ± 37,998 d,e | 594,364 ± 232,741 c,d,e | 835,052 ± 481,591 b,c | 777,606 ± 403,726 c | 669,356 ± 99,565 c,d | 1,422,707 ± 104,397 a | 1,377,261 ± 113,270 a,b |
| 91. 1-Dodecanolalcohol sweet, nutty, oily, soapy | 103,458 ± 48,322 a | 22,892 ± 3936 b | 16,404 ± 5358 b | 36,847 ± 17,654 b | 87,958 ± 24,496 a | 29,529 ± 5992 b | 16,758 ± 5634 b | 24,703 ± 10,875 b | 16,111 ± 6321 b |
| 92. Octanoic acidcarboxylic acid sulphury, oily, sour | 35,646 ± 28,778 b,c | 4169 ± 1434 c | 202,987 ± 65,586 a,b,c | 169,648 ± 69,450 a,b,c | 180,250 ± 96,869 a,b,c | 395,738 ± 277,591 a | 198,817 ± 12,281 a,b,c | 332,090 ± 23,916 a | 303,876 ± 163,549 a,b |
| 93. Ethyl tetradecanoateester sweet, oily | 330,543 ± 149,195 a,b | 102,320 ± 30,277 b | 300,722 ± 61,556 a,b | 571,487 ± 366,616 a | 582,094 ± 129,115 a | 493,222 ± 120,580 a,b | 453,966 ± 190,638 a,b | 318,223 ± 229,844 a,b | 181,455 ± 113,184 a,b |
| 94. Ethyl 9-tetradecanoateester sweet, oily | 6089 ± 1960 a,b | 1450 ± 483 b | 3052 ± 915 b | 8286 ± 5556 a,b | 14,487 ± 4289 a | 13,359 ± 3371 a | 4789 ± 3125 b | 7726 ± 5965 a,b | 4042 ± 2405 b |
| 95. 2-Dodecanolalcohol not described | 355,693 ± 105,182 a | 65,427 ± 11,207 c,d | 42,179 ± 10,385 d | 174,186 ± 68,697 b,c | 263,712 ± 32,928 a,b | 108,203 ± 28,715 c,d | 72,619 ± 34,477 c,d | 45,895 ± 17,641 d | 36,128 ± 12,572 d |
| 96. Ethyl-9-hexanoateester sweet, green, oily, fruity | 173,174 ± 42,301 a,b | 96,397 ± 6544 c,d | 107,734 ± 27,922 b,c,d | 110,130 ± 54,113 b,c,d | 141,237 ± 14,123 a,b,c | 186,540 ± 16,840 a | 71,620 ± 26,218 c,d | 95,337 ± 38,507 c,d | 59,687 ± 28,428 d |
References
- The Scotch Whisky Association. Facts & Figures. Available online: https://www.scotch-whisky.org.uk/insights/facts-figures/ (accessed on 26 August 2020).
- The Scotch Whisky Regulations. 2009. Available online: http://www.legislation.gov.uk/uksi/2009/2890/pdfs/uksi_20092890_en.pdf (accessed on 2 December 2020).
- Lee, K.-Y.M.; Paterson, A.; Piggott, J.R.; Richardson, G.D. Origins of Flavour in Whiskies and a Revised Flavour Wheel: A Review. J. Inst. Brew. 2001, 107, 287–313. [Google Scholar] [CrossRef] [Green Version]
- Olaniran, A.O.; Hiralal, L.; Mokoena, M.P.; Pillay, B. Flavour-Active Volatile Compounds in Beer: Production, Regulation and Control. J. Inst. Brew. 2017, 123, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Depraetere, S.A.; Delvaux, F.; Schutter, D.D.; Williams, I.S.; Winderickx, J.; Delvaux, F.R. The Influence of Wort Aeration and Yeast Preoxygenation on Beer Staling Processes. Food Chem. 2008, 107, 242–249. [Google Scholar] [CrossRef]
- Harrison, B.; Fagnen, O.; Jack, F.; Brosnan, J. The Impact of Copper in Different Parts of Malt Whisky Pot Stills on New Make Spirit Composition and Aroma. J. Inst. Brew. 2012, 117, 106–112. [Google Scholar] [CrossRef]
- Wanikawa, A. Flavors in Malt Whisky: A Review. J. Am. Soc. Brew. Chem. 2020, 78, 260–278. [Google Scholar] [CrossRef]
- Aylott, R.I.; MacKenzie, W.M. Analytical Strategies to Confirm the Generic Authenticity of Scotch Whisky. J. Inst. Brew. 2010, 116, 215–229. [Google Scholar] [CrossRef]
- Neto, H.B.d.A.; Yohannan, B.K.; Bringhurst, T.A.; Brosnan, J.M.; Pearson, S.Y.; Walker, J.W.; Walker, G.M. Evaluation of a Brazilian Fuel Alcohol Yeast Strain for Scotch Whisky Fermentations. J. Inst. Brew. 2012. [Google Scholar] [CrossRef] [Green Version]
- Bathgate, G.N. A Review of Malting and Malt Processing for Whisky Distillation. J. Inst. Brew. 2016, 122, 197–211. [Google Scholar] [CrossRef]
- Walker, G.; Brosnan, J.; Bringhurst, T.; Jack, F. Selecting New Distilling Yeasts for Improved Fermentation and for Sustainability. Worldw. Distill. Spirits Conf. 2011, 1–11. [Google Scholar] [CrossRef]
- Martin, P.; Chang, X. Bere Whisky–Rediscovering the Spirit of an Old Barley. Brew. Distill. Int. 2008, 4, 41–43. [Google Scholar]
- Pires, E.J.; Teixeira, J.A.; Brányik, T.; Vicente, A.A. Yeast: The Soul of Beer’s Aroma—a Review of Flavour-Active Esters and Higher Alcohols Produced by the Brewing Yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef] [Green Version]
- Stone, H.; Sidel, J.; Oliver, S.; Woolsey, A.; Singleton, R.C. Sensory Evaluation by Quantitative Descriptive Analysis. Food Technol. 1974, 8, 24–32. [Google Scholar] [CrossRef]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food. In Food Science Text Series, 2nd ed.; Heldman, D.R., Ed.; Springer: New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- Shortreed, G.W.; Rickards, P.; Swan, J.; Burtles, S. The Flavour Terminology of Scotch Whisky. Brew. Guard. 1979, 108, 2–6. [Google Scholar]
- Piggott, J. Whiskies: Composition, Sensory Properties and Sensory Analysis. In Alcoholic Beverages; Piggott, J. Woodhead Publishing Limited: Cambridge, UK, 2012; pp. 379–392. [Google Scholar] [CrossRef]
- Holt, H.; Pearson, W. Napping–a Rapid Method for Sensory Analysis of Wines. Aust. Wine Res. Inst. Tech. Rev. 2014, 208, 10–14. [Google Scholar]
- Liu, J.; Grønbeck, M.S.; Di Monaco, R.; Giacalone, D.; Bredie, W.L.P. Performance of Flash Profile and Napping with and without Training for Describing Small Sensory Differences in a Model Wine. Food Qual. Prefer. 2016, 48, 41–49. [Google Scholar] [CrossRef]
- Liu, J.; Bredie, W.L.P.; Sherman, E.; Harbertson, J.F.; Heymann, H. Comparison of Rapid Descriptive Sensory Methodologies: Free-Choice Profiling, Flash Profile and Modified Flash Profile. Food Res. Int. 2018, 106, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Giacalone, D.; Ribeiro, L.M.; Frøst, M.B. Consumer-Based Product Profiling: Application of Partial Napping ®® for Sensory Characterization of Specialty Beers by Novices and Experts. J. Food Prod. Mark. 2013, 19, 201–218. [Google Scholar] [CrossRef]
- Lelièvre, M.; Chollet, S.; Abdi, H.; Valentin, D. What Is the Validity of the Sorting Task for Describing Beers? A Study Using Trained and Untrained Assessors. Food Qual. Prefer. 2008, 19, 697–703. [Google Scholar] [CrossRef]
- Giacalone, D.; Bredie, W.L.P.; Frøst, M.B. “All-In-One Test” (AI1): A Rapid and Easily Applicable Approach to Consumer Product Testing. Food Qual. Prefer. 2013, 27, 108–119. [Google Scholar] [CrossRef]
- Risvik, E. Sensory Properties and Preferences. Meat Sci. 1994, 36, 67–77. [Google Scholar] [CrossRef]
- Pagès, J. Collection and Analysis of Perceived Product Inter-Distances Using Multiple Factor Analysis: Application to the Study of 10 White Wines from the Loire Valley. Food Qual. Prefer. 2005, 16, 642–649. [Google Scholar] [CrossRef]
- Pagès, J. Direct Collection of Sensory Distances: Application to the Evaluation of Ten White Wines of the Loire Valley. Sci. Aliment. 2003, 23, 679–688. [Google Scholar] [CrossRef]
- Valentin, D.; Chollet, S.; Lelièvre, M.; Abdi, H. Quick and Dirty but Still Pretty Good: A Review of New Descriptive Methods in Food Science. Int. J. Food Sci. Technol. 2012, 47, 1563–1578. [Google Scholar] [CrossRef]
- Albert, A.; Varela, P.; Salvador, A.; Hough, G.; Fiszman, S. Overcoming the Issues in the Sensory Description of Hot Served Food with a Complex Texture. Application of QDA ®®, Flash Profiling and Projective Mapping Using Panels with Different Degrees of Training. Food Qual. Prefer. 2011, 22, 463–473. [Google Scholar] [CrossRef]
- Aguiar, L.A.d.; Melo, L.; de Lacerda de Oliveira, L. Validation of Rapid Descriptive Sensory Methods against Conventional Descriptive Analyses: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2535–2552. [Google Scholar] [CrossRef] [PubMed]
- Cartier, R.; Rytz, A.; Lecomte, A.; Poblete, F.; Krystlik, J.; Belin, E.; Martin, N. Sorting Procedure as an Alternative to Quantitative Descriptive Analysis to Obtain a Product Sensory Map. Food Qual. Prefer. 2006, 17, 562–571. [Google Scholar] [CrossRef]
- Oliver, P.; Cicerale, S.; Pang, E.; Keast, R. Comparison of Quantitative Descriptive Analysis to the Napping Methodology with and without Product Training. J. Sens. Stud. 2018, 33, e12331. [Google Scholar] [CrossRef]
- Aylott, R. Whisky Analysis. In Whisky; Russell, I., Stewart, G., Eds.; Elsevier: Oxford, UK, 2014; pp. 243–270. [Google Scholar] [CrossRef]
- Stupak, M.; Goodall, I.; Tomaniova, M.; Pulkrabova, J.; Hajslova, J. A Novel Approach to Assess the Quality and Authenticity of Scotch Whisky Based on Gas Chromatography Coupled to High Resolution Mass Spectrometry. Anal. Chim. Acta 2018, 1042, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Pryde, J.; Conner, J.; Jack, F.; Lancaster, M.; Meek, L.; Owen, C.; Paterson, R.; Steele, G.; Strang, F.; Woods, J. Sensory and Chemical Analysis of “Shackleton’s” Mackinlay Scotch Whisky. J. Inst. Brew. 2011, 117, 156–165. [Google Scholar] [CrossRef]
- Reid, S.J.; Speers, R.A.; Willoughby, N.; Lumsden, W.B.; Maskell, D.L. Pre-Fermentation of Malt Whisky Wort Using Lactobacillus Plantarum and Its Influence on New-Make Spirit Character. Food Chem. 2020, 320, 126605. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Zheng, X.P.; Song, P.; Sun, Z.L.; Tian, T.T. Characterization of Volatiles in the Six Most Well-Known Distilled Spirits. J. Am. Soc. Brew. Chem. 2013, 71, 161–169. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Perrin, L.; Symoneaux, R.; Maître, I.; Asselin, C.; Jourjon, F.; Pagès, J. Comparison of Three Sensory Methods for Use with the Napping ®® Procedure: Case of Ten Wines from Loire Valley. Food Qual. Prefer. 2008, 19, 1–11. [Google Scholar] [CrossRef]
- Louw, L.; Malherbe, S.; Naes, T.; Lambrechts, M.; van Rensburg, P.; Nieuwoudt, H. Validation of Two Napping®® Techniques as Rapid Sensory Screening Tools for High Alcohol Products. Food Qual. Prefer. 2013, 30, 192–201. [Google Scholar] [CrossRef]
- Le Dien, S.; Pagès, J. Hierarchical Multiple Factor Analysis: Application to the Comparison of Sensory Profiles. Food Qual. Prefer. 2003, 14, 397–403. [Google Scholar] [CrossRef]
- Pagès, J.; Cadoret, M.; Lê, S. The Sorted Napping: A New Holistic Approach in Sensory Evaluation. J. Sens. Stud. 2010, 25, 637–658. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J.; Valentin, D. Multiple Factor Analysis: Principal Component Analysis for Multitable and Multiblock Data Sets. Wiley Interdiscip. Rev. Comput. Stat. 2013, 5, 149–179. [Google Scholar] [CrossRef]
- Dehlholm, C.; Brockhoff, P.B.; Meinert, L.; Aaslyng, M.D.; Bredie, W.L.P. Rapid Descriptive Sensory Methods–Comparison of Free Multiple Sorting, Partial Napping, Napping, Flash Profiling and Conventional Profiling. Food Qual. Prefer. 2012, 26, 267–277. [Google Scholar] [CrossRef]
- Josse, J.; Pagès, J.; Husson, F. Testing the Significance of the RV Coefficient. Comput. Stat. Data Anal. 2008, 53, 82–91. [Google Scholar] [CrossRef]
- Torri, L.; Dinnella, C.; Recchia, A.; Naes, T.; Tuorila, H.; Monteleone, E. Projective Mapping for Interpreting Wine Aroma Differences as Perceived by Naïve and Experienced Assessors. Food Qual. Prefer. 2013, 29, 6–15. [Google Scholar] [CrossRef] [Green Version]
- Louw, L.; Oelofse, S.; Naes, T.; Lambrechts, M.; van Rensburg, P.; Nieuwoudt, H. Trained Sensory Panellists’ Response to Product Alcohol Content in the Projective Mapping Task: Observations on Alcohol Content, Product Complexity and Prior Knowledge. Food Qual. Prefer. 2014, 34, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Barcenas, P.; Elortondo, F.J.P.; Albisu, M. Projective Mapping in Sensory Analysis of Ewes Milk Cheeses: A Study on Consumers and Trained Panel Performance. Food Res. Int. 2004, 37, 723–729. [Google Scholar] [CrossRef]
- Delarue, J. The Use of Rapid Sensory Methods in R&D and Research: An Introduction. In In Rapid Sensory Profiling Techniques and Related, Methods; Delarue, J., Lawlor, L.B., Rogeaux, M., Eds.; Woodhead Publishing: Cambridge, UK, 2015; pp. 3–25. [Google Scholar] [CrossRef]
- Gawel, R. The Use of Language by Trained and Untrained Experienced Wine Tasters. J. Sens. Stud. 1997, 12, 267–284. [Google Scholar] [CrossRef]
- Kennedy, J.; Heymann, H. Projective Mapping and Descriptive Analysis of Milk and Dark Chocolates. J. Sens. Stud. 2009, 24, 220–233. [Google Scholar] [CrossRef]
- Moelich, E.I.; Muller, M.; Joubert, E.; Næs, T.; Kidd, M. Validation of Projective Mapping as Potential Sensory Screening Tool for Application by the Honeybush Herbal Tea Industry. Food Res. Int. 2017, 99, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Tian, X.; Ma, Y.; Sun, Y.; Qi, X.; Miu, C.; Xu, Y. Aroma Characteristics of Cabernet Sauvignon Wines from Loess Plateau in China by QDA ®®, Napping ®® and GC–O Analysis. Eur. Food Res. Technol. 2020, 246, 821–832. [Google Scholar] [CrossRef]
- Mielby, L.H.; Hopfer, H.; Jensen, S.; Thybo, A.K.; Heymann, H. Comparison of Descriptive Analysis, Projective Mapping and Sorting Performed on Pictures of Fruit and Vegetable Mixes. Food Qual. Prefer. 2014, 35, 86–94. [Google Scholar] [CrossRef]
- Pickup, W.; Bremer, P.; Peng, M. Comparing Conventional Descriptive Analysis and Napping ®®-UFP against Physiochemical Measurements: A Case Study Using Apples. J. Sci. Food Agric. 2018, 98, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Conner, J.M.; Birkmyre, L.; Paterson, A.; Piggott, J.R. Headspace Concentrations of Ethyl Esters at Different Alcoholic Strengths. J. Sci. Food Agric. 1998, 77, 121–126. [Google Scholar] [CrossRef]
- Wanikawa, A.; Hosoi, K.; Kato, T.; Nakagawa, K. Identification of Green Note Compounds in Malt Whisky Using Multidimensional Gas Chromatography. Flavour Fragr. J. 2002, 17, 207–211. [Google Scholar] [CrossRef]
- Poisson, L.; Schieberle, P. Characterization of the Key Aroma Compounds in an American Bourbon Whisky by Quantitative Measurements, Aroma Recombination, and Omission Studies. J. Agric. Food Chem. 2008, 56, 5820–5826. [Google Scholar] [CrossRef] [PubMed]
- Boothroyd, E.; Linforth, R.S.T.; Jack, F.; Cook, D.J. Origins of the Perceived Nutty Character of New-Make Malt Whisky Spirit. J. Inst. Brew. 2014, 120, 16–22. [Google Scholar] [CrossRef]
- Piggot, R. Chapter 17—From Pot Stills to Continuous Stills: Flavor Modification by Distillation. In The Alcohol Textbook 4 th Edition A Reference for the Beverage, Fuel and Industrial Alcohol Industries; Nottingham University Press: Nottingham, UK, 2003; pp. 255–266. [Google Scholar]

| Spirits | Alcohol by Volume (ABV) [%] |
|---|---|
| A | 73.5 |
| B | 73.0 |
| C | 66.3 |
| D | 62.9 |
| E | 62.1 |
| F | 60.7 |
| G | 34.0 |
| H | 33.2 |
| I | 29.3 |
| Methodology Comparison | RV Coefficient |
|---|---|
| QDA versus Napping | 0.906 |
| QDA versus GC-MS | 0.895 |
| Napping versus GC-MS | 0.927 |
| GC-MS | QDA | Napping | |
|---|---|---|---|
| Nature of Analysis | Analytical | Sensory | Sensory |
| Costs | medium (after initial capital cost of the GC-MS): regular replacement of column, fibre, septa, no reuse of vials possible, internal standard | low: sensory glasses | low: sensory glasses, nappé |
| Preparation time | medium: diluting samples, preparing GC-MS (column, injector), runtime of the method | medium: diluting samples for each session, pre-select vocabulary | low: diluting samples |
| Sample volume required | 1 to 5 mL at 20% ABV | 20 mL 20% ABV multiplied by number of sessions | 20 mL at 20% ABV |
| Suitable sample set size | 1 to >100 | 1 to 10 | 6 to 20 |
| Comparison between sample sets | yes | yes | no |
| Panellist requirements | none | >8 trained | >15 more panellists needed if not trained |
| Time for data analysis | high: peaks can be automatically integrated but have to be manually checked before statistical analysis | low: statistically analysis directly possible | medium: measuring coordinates if recorded on paper, followed by statistical analysis |
| Panellist time | not applicable | ~7 min per session, analysis often split into several sessions | ~20 min |
| Nature of results | quantitative or qualitative | quantitative | semi-quantitative, qualitative |
| Form of results | concentrations of compounds and relation to each other | intensities of flavours | comparison plots, highlighting the differences |
| Difficulty of data interpretation | high | low | medium |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daute, M.; Jack, F.; Baxter, I.; Harrison, B.; Grigor, J.; Walker, G. Comparison of Three Approaches to Assess the Flavour Characteristics of Scotch Whisky Spirit. Appl. Sci. 2021, 11, 1410. https://doi.org/10.3390/app11041410
Daute M, Jack F, Baxter I, Harrison B, Grigor J, Walker G. Comparison of Three Approaches to Assess the Flavour Characteristics of Scotch Whisky Spirit. Applied Sciences. 2021; 11(4):1410. https://doi.org/10.3390/app11041410
Chicago/Turabian StyleDaute, Martina, Frances Jack, Irene Baxter, Barry Harrison, John Grigor, and Graeme Walker. 2021. "Comparison of Three Approaches to Assess the Flavour Characteristics of Scotch Whisky Spirit" Applied Sciences 11, no. 4: 1410. https://doi.org/10.3390/app11041410
APA StyleDaute, M., Jack, F., Baxter, I., Harrison, B., Grigor, J., & Walker, G. (2021). Comparison of Three Approaches to Assess the Flavour Characteristics of Scotch Whisky Spirit. Applied Sciences, 11(4), 1410. https://doi.org/10.3390/app11041410