1. Introduction
Electrochemical machining (ECM) is a non-traditional machining technology whereby material removal is accomplished through the controlled anodic dissolution of the workpiece. It has the advantages of high efficiency, no tool wear, and good results in terms of surface quality, regardless of the hardness and toughness of the processed material [
1,
2,
3]. Thus, ECM has become an important processing method for difficult-to-cut material parts and is widely used in the aviation industry [
4,
5,
6]. The current efficiency is an important characteristic parameter that describes the relationship between the dissolution rate of the anode material and the current density in ECM [
7]. The accurate measurement of the current efficiency is the basis for both anode shape prediction and cathode tool design [
8,
9,
10]. However, the dissolution process of alloy materials is affected by elemental composition, electrochemical reaction valence, and material peeling action. Therefore, it is difficult to obtain the current efficiency of alloy materials by theoretical calculations, and it is usually measured by experimental techniques [
11,
12,
13,
14]. Several experimental measurement methods used to obtain the current efficiency of target alloy materials at different current densities have been reported in the literature.
One of these methods is the constant current method. In this measurement technique, the target alloy material is dissolved into electrolyte under a constant current by adjusting the processing voltage; however, it is without any tool feed action. The voltage, the machining time, and the dissolved mass of the anode are recorded. The current efficiency of the target material can be calculated on the basis of the assumption of uniform current distribution on the anode surface [
15,
16,
17]. This method is easy to implement; however, as the ECM process usually adopts a constant voltage machining mode, the cathode tool gets continuously fed during the processing. The variation of the applied voltage may cause a change in the material dissolution behavior. For example, in Lohrengel’s study, it was shown that the machining current of Mn presented three machining states with an increase of machining voltage, and it had the same processing current density at five different processing voltages [
18]. As such, it is difficult to maintain the stability of the measured state in the constant current method. In Haisch’s study, the dissolving properties of steel 100 Cr6 in NaCl and NaNO
3 were measured. The polarization curve indicated a fluctuation in the anode potential when the current density was lower than 3 A/cm
2 [
19]. The above studies show that the anode potential fluctuates significantly at a low current density, which results in errors in the measurement of current efficiency.
Another method used to measure current effiency is the constant voltage method. In this method, the processing voltage is kept constant. The target alloy material is dissolved in the electrolyte under the constant current, which is obtained by adjusting the cathode tool feed rate. This helps the ECM processing to reach the equilibrium state. Given that the tool feed rate is proportional to the material dissolution rate under the ECM equilibrium state, the current efficiency can be calculated from the recorded parameters of the material dissolution rate and the equilibrium current. Tang et al. measured the current efficiency of S-03 material in NaCl, NaNO
3, and mixed solutions, and reported that the NaCl electrolyte had the highest current efficiency [
20]. Zhu and Ge obtained the current efficiency curve of GH4169 in NaNO
3 using the constant voltage method [
21,
22]. Liu et al. explored a constant flow measurement method based on the conventional constant voltage method [
23]. Compared with the constant current method, the measurement conditions of the constant voltage method are more consistent with the ECM process, so this paper mainly discusses the constant voltage method.
In both the constant current and constant voltage methods, the current distribution is assumed to be uniform when calculating the current efficiency. However, in the measurement experiment, the current gets concentrated at the edge of the workpiece, corroding the edge into rounded corners. In light of the edge effect, the current density in the middle of the workpiece is different than that of the edge. The uneven distribution of the electric field can lead to significant measurement errors. To improve the accuracy of the measurement, this paper proposes a current efficiency curve measurement with a casing-type anode in ECM. In this method, the material being measured is divided into two parts: the mandril and the casing. During the measurement, the mandril and the casing are dissolved into the electrolyte at the same time so that the end faces of the mandril and the casing can always be coplanar. The edge effect mainly concentrates on the casing rather than the mandril. Only the current of the mandril is taken into consideration in the calculation of current efficiency. The gap between the mandril and the casing should be kept small enough that the side and edge effect can be reduced because of the electromagnetic shielding of the casing. This measurement mode can improve both the constant voltage method and the constant current method. In reality, the constant voltage machining mode is often used in production, so this paper mainly discusses the application of this mode in the constant voltage method. Several numerical simulations and experiments have been performed to investigate the measurement accuracy and stability of the current efficiency curve obtained by the proposed measurement method.
2. Principles of Casing-Type Anode Measurement
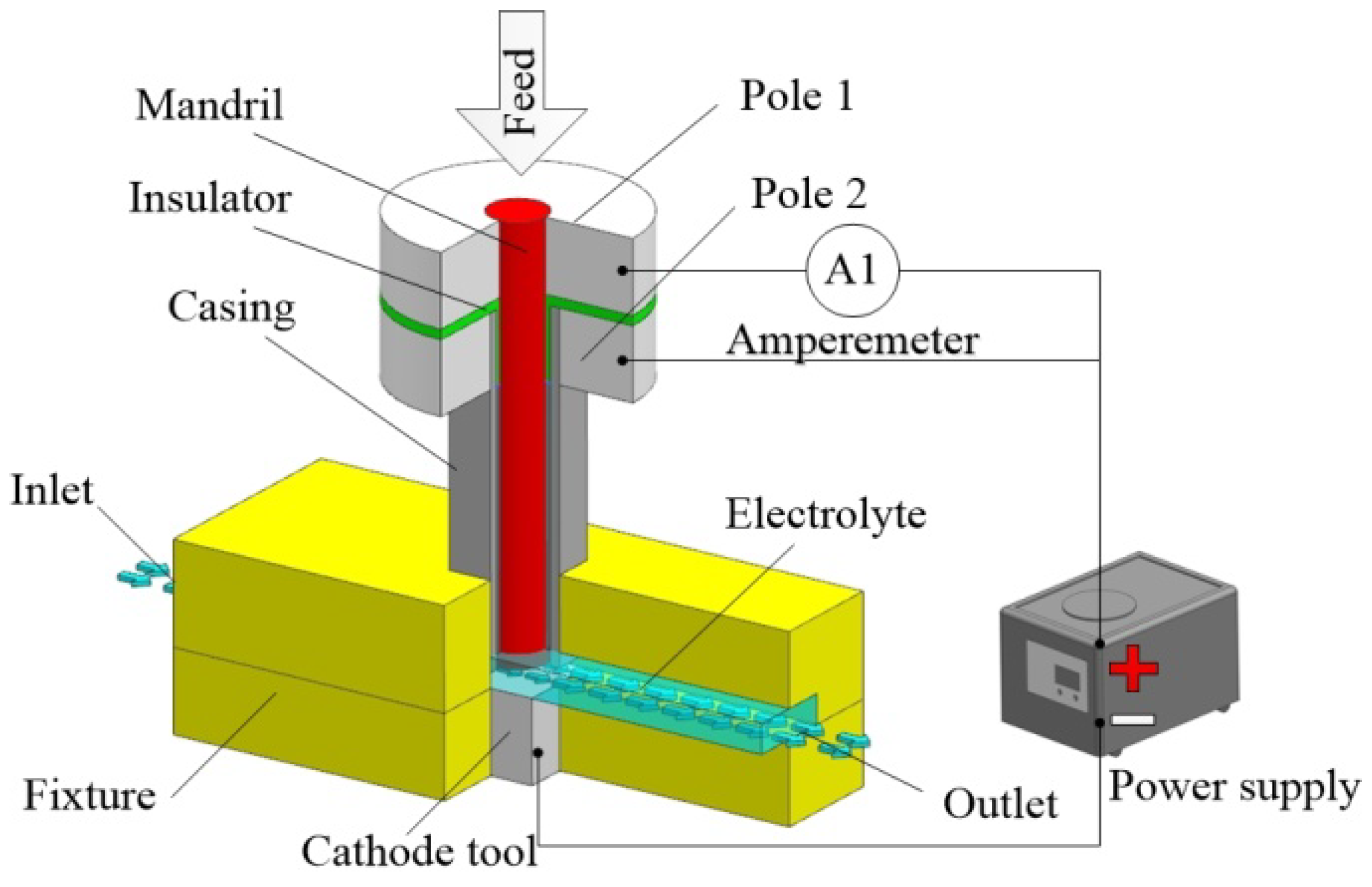
The schematic diagram of the conventional constant voltage method is shown in
Figure 1. The key components of the measuring system include a cathode tool, anode workpiece, constant voltage source, and current measuring equipment. The measurement process is consistent with most of the ECM processes because of the feed action and application of the constant voltage source. In this method, the relationship between the current density and the material removal rate can be obtained by analyzing the recorded data of feed rate
v and the processing current. Obtaining the accurate value of current density distribution on the anode workpiece in an equilibrium state is the key to achieving the accurate current efficiency of the target material.
During the measurement, the high-velocity electrolyte is pumped into the inter-electrode gap, so that bubble and Joule heat can be quickly removed to ensure the purity of the electrolyte. The tool and workpiece are connected to the cathode and anode of the power supply, respectively. The power supply outputs a constant voltage. The current distribution on the anode workpiece can be assumed as uniform based on the use of an electrode with flat end. The cathode tool is fixed in the fixture and static, while the anode workpiece is moved relative to the cathode tool at a constant speed. Under the electrochemical reaction, the anode material dissolves into the electrolyte. The dissolution rate
va is given by Faraday’s law:
where
η is the current efficiency,
ω is the volume electrochemical equivalent, and
i is the current density.
As the process continues, the ECM process enters an equilibrium state. In this state, the shape of the inter-electrode gap and the processing current do not continue to change, and the anode material dissolution rate is equal to the cathode feed rate
v.
The current density
i can be calculated using Equation (3):
where
I is the processing current and
Send is the end face area of the workpiece.
Using Equations (1)–(3), the current efficiency
η can be obtained. However, it is difficult to accurately measure and calculate the volume electrochemical equivalent,
ω, of metal alloys. Thus,
ηω is used to characterize the current efficiency, and it can be obtained from Equation (4). By adjusting the feed rate,
v, the current efficiency at different current densities,
i, can be obtained.
In the above description, the measurement is based on the assumption of uniform current density distribution. However, the edge of the workpiece is processed into fillets as a result of the tip effect of the electric field, as shown in
Figure 2. The current density distribution in the edge area is different from that in the central region. Besides, the sidwall of the workpiece is exposed to the processing zone during the measurement, and the stray current distribution on the sidewall can lead to a higher measurement value than the actual value. According to Equation (4), the current efficiency measured by the conventional constant voltage method is smaller than the actual value.
To reduce the influence of both edge effects and stray current, this study proposes a current efficiency curve measurement with a casing-type anode.
Figure 3 shows the schematic diagram of the measurement setup with a casing-type anode. In this method, the target material is divided into two parts: the mandril and the casing. There is a small gap between the mandril and the casing to ensure that the mandril and the casing do not conduct directly. The electric field at the end of the mandril is less affected by the stray current and the edge because of the shielding effect of the casing, and thus, the current density distribution on the end of the mandril can be considered uniform.
During the measurement, the mandril and the casing are connected to the anode of the power supply through different circuits and will be moved towards the cathode by the same device at the same velocity. Under the electrochemical reaction, the mandril and the casing are dissolved into the electrolyte. Because the mandril and the casing are made of the same material and feed at the same rate, the end face of the mandril and the casing can always be kept coplanar during the ECM process. The casing, working as the regulator, is used to improve the electric field distribution in the mandril. The electric field within the machining gap is uniform and continuous, as shown in
Figure 4. When calculating the current efficiency, only the current of mandril is taken into consideration. The current efficiency can be calculated by Equation (5):
where
Smandril is the end face area of the mandril, and
Imandril is the processing current of the mandril.
3. Model and Numerical Analysis
To analyze the effectiveness of the proposed measurement method, the study conducted a 2D comparison simulation of the conventional and the proposed methods of current efficiency measurement. In the numerical analysis, an alloy of 304SS was selected as the target material. The current efficiency
ηω304SS of 304SS used in both the conventional and proposed method simulations was obtained from our previous studies [
16]. The equilibrium processing current in the two methods was recorded. Using Equations (4) and (5), the measured value of current efficiency was calculated.
Figure 5 shows a schematic view of the models of the two measurement methods. In the analysis, the dissolution behavior of the workpiece was assumed to be primarily determined by the electric field. The following assumptions were made [
24,
25,
26]:
- (1)
The electrolyte flow within the inter-electrode gap is assumed to be ideal and the conductivity κ of the electrolyte is assumed to be uniform.
- (2)
The electrodes are defined as equipotential surfaces.
- (3)
Joule heat, bubbles, and the anode products are negligible, and the current distribution in the inter-electrode gap is determined solely by Ohmic effects.
According to classical electric field theory, the distribution of electric potential
φ can be obtained by solving Laplace’s equation:
where
x is the abscissa and
y is the ordinate.
The evolution of the anode boundary is affected by both the feed and dissolution activities. On the basis of Ohm’s law and Faraday’s law, the evolution rate of the anode boundary can be described as:
where
η is the current efficiency,
ω304SS is the volume electrochemical equivalent of 304SS, and
v is the feed rate of the workpiece.
The steady processing current Ianode of the anode workpiece in the conventional method and the current Imandril of th mandril in the proposed method were recorded. According to Equations (4) and (5), the measured value of current efficiency was obtained.
The transient simulations of current efficiency measurement were carried out using COMSOL Multiphysics, which uses the finite element method as the numerical method. All analyses were calculated for 2500 s which is long enough that the machining processes enter the equilibrium state. In this state, the processing currents no longer change. In order to make the distribution of the electric field in the gap uniform, the gap
G, between the mandril and the casing, should be as small as possible. In this study, 0.1 mm was selected as the size of
G, and nothing was filled in the gap. The thickness
T of the casing was set at 2.5 mm so that the edge effect was kept away from the mandril. The simulated conditions are listed in
Table 1. The relationship between
ηω and
I is listed in
Table 2.
Figure 6 and
Figure 7 show the current and the size of the inter-electrode gap evolutions, respectively. Since the initial size of the inter-electrode gap was the same, the initial processing current under different feed rates was also the same. In the beginning, both processing methods were in a non-equilibrium state. The processing current and the gap changed rapidly at first, and then tended to be stable. Finally, the values of the processing current and the size of the inter-electrode gap did not change any more. This means that the dissolution rate of the anode was the same as the feed rate of the cathode tool.
Figure 8 and
Figure 9 show the simulated contours in the workpiece obtained by the conventional and proposed methods, respectively. The profile of the workpiece was smooth and flat in the central region at the end of the workpiece in both methods. However, there were distinct machined fillets at the edge of the workpiece, especially at a low feed rate. Compared to the conventional method, the fillets of the proposed method can be ignored. The edge effect was also effectively suppressed by using the proposed method.
Figure 10 shows the current density distribution of the conventional and proposed methods with a feed rate of 0.1 mm/min. The current distribution in the central region was consistent with the ideal uniform current density in both of the methods used. However, the current distributions at the edge and the sidewall were different from the ideal current. The current distributions at the fillets and sidewall changed dramatically. The current on the edges was smaller than the ideal current because the surface of the workpiece was far away from the cathode tool at the edge. The current distribution at the sidewall decreased gradually as it moved away from the end and dropped to 0 at the edge of the fixture. The current distribution at the sidewall caused the measured current to be larger, and this was not consistent with the hypothesis of a uniform electric field. In comparison to the ideal current, the influenced area at the end face and the sidewall was 2.160 mm and 3.883 mm in the conventional method, while it was only 0.223 mm and 0.253 mm in the proposed method. This means that the edge effect and sidewall corrosion were significantly inhibited.
The error between the processing current and the ideal current can be obtained by comparing the processing current of the two processes with the ideal current. As shown in
Figure 11, the maximum error of the conventional method was 11.0% when the feed rate was 0.1 mm/min. The error of the conventional method had a tendency to decrease continuously as the feed rate increased. When the feed rate was 1 mm/min, the conventional method had the minimum error at 2.0%. The error between the processing current of the proposed method and the ideal current was almost negligible, differing from the conventional method. In the proposed method, the maximum error was only 0.2% when the feed rate was 0.1 mm/min. The current measured by the proposed method was closer to the ideal current value when compared to the conventional method, which means that the proposed method can obtain a more accurate current efficiency.
From calculations using Equations (3) and (4), the current efficiency was obtained. The current efficiency curves of 304SS were obtained by both method simulations and are shown in
Figure 12. From
Figure 12, it can be observed that the current efficiency measured by the conventional method was significantly smaller than the value obtained from the measurements by the proposed method when the current density was smaller than 50 A/cm
2. The maximum difference occurred when the current density was about 10 A/cm
2. This difference decreased with the increase in the current density. As the current density increased over 50 A/cm
2, the difference is negligible.
5. Results and Discussion
The current efficiency curves of 304SS measured by the conventional and proposed methods are shown in
Figure 15. It was observed that the value of
ηω measured by the conventional method was significantly smaller than that of the proposed method. When the current density was over 50 A/cm
2, the difference was negligible and the value of
ηω measured by both the methods was maintained at about 1.20 mm
3/Amin. For current density values smaller than 50 A/cm
2, both the methods showed the same trend. The value of current efficiency increased with the increase in current density. However, the deviation between the conventional method and the proposed method grew with the decrease in current density. The results of the experiment are consistent with the analysis from the simulation. Furthermore, the stability of the measurement process improved by using the proposed measurement method. In the conventional method, the maximum fluctuation of
ηω was 0.042 mm
3/Amin, while it was 0.030 mm
3/Amin in the proposed method.
Figure 16 shows the specimens with a feed rate of 0.1 mm/min. In the conventional constant voltage method, the corrosion of the workpiece on the side was visible and had a bevel angle of about 11°, which was potentially caused by intense scour and electrochemical corrosion. The rounded corner at the edge of the workpiece was about 0.622 mm. This means that the non-uniform current distribution at the side and edge was present and had a severe influence on the measurement. In the casing-type anode measurement, the condition of the casing was consistent with the workpiece used in the conventional method. However, the mandril showed an entirely different appearance: the sidewall of the mandril maintained the metallic luster and the edge was very sharp. The electric field shielding of the casing contributed to this appearance. These results were also consistent with the analysis from the simulation, meaning that the recorded current of the mandril was less influenced by the side current and edge effects, and the accuracy of the current efficiency curve measurement improved.