A Review of Research on Dropwise Condensation Heat Transfer
Abstract
:1. Introduction
2. The Mechanism of Dropwise Condensation
2.1. Dropwise Condensation and Its Forming Decision
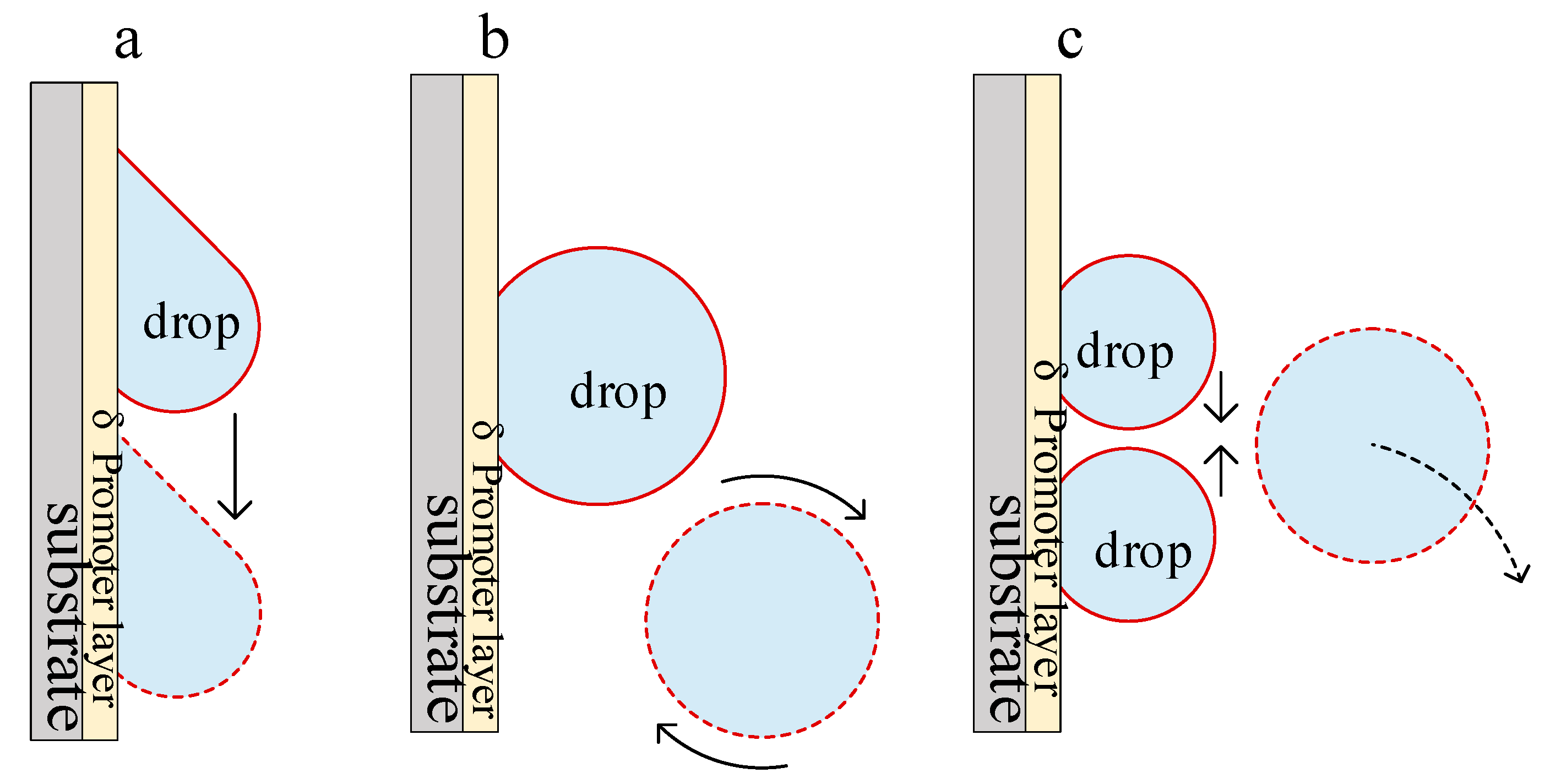
2.2. Research on The Mechanism of Dropwise Condensation
3. Dropwise Condensation Heat Transfer Model
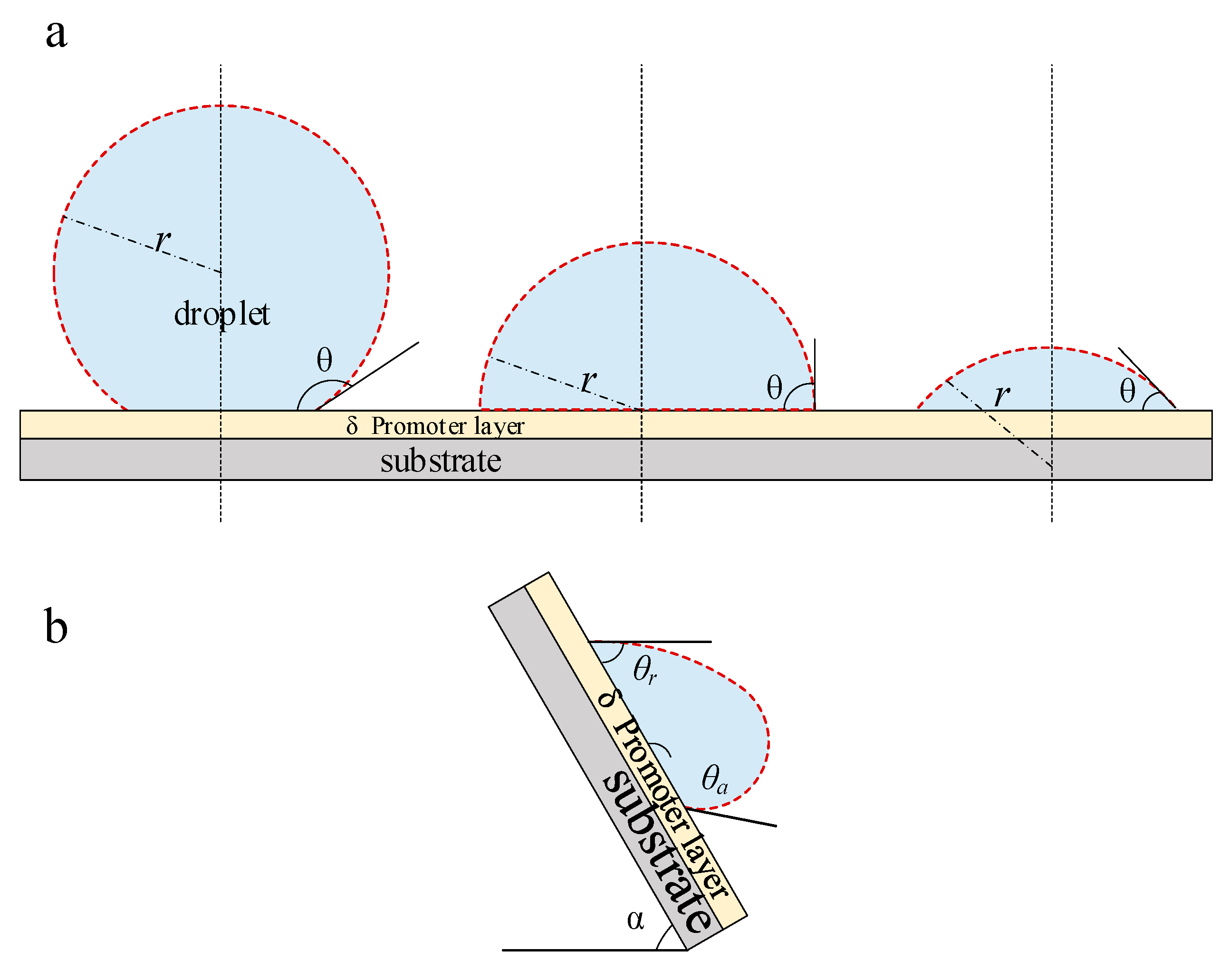
3.1. Surface Integral Heat Transfer Model
- (1)
- Minimum Radius
- (2)
- Effective Radius
- (3)
- Maximum Radius
3.2. Single Drop Heat Transfer Model
3.3. Drop Size Distribution
4. Methods, Problems, and Solutions for Promoting Dropwise Condensation
4.1. Methods for Promoting Dropwise Condensation
4.1.1. Low Free Energy Surfaces
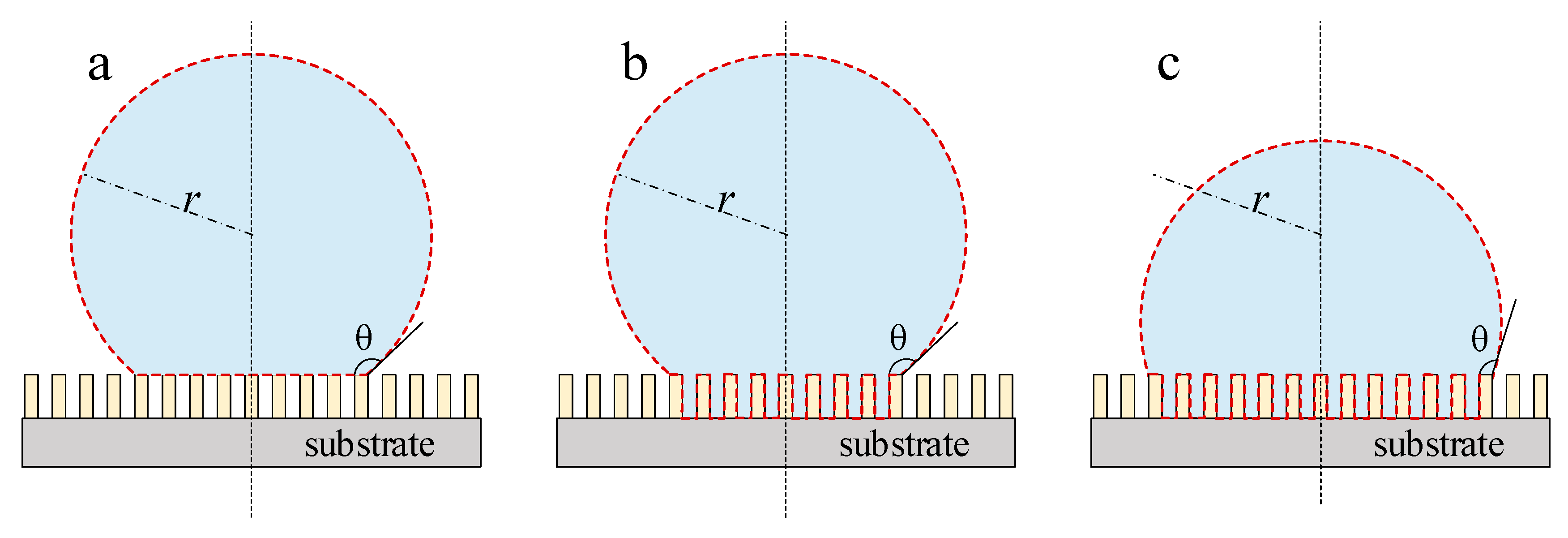
4.1.2. Micro-Nano Structure Surface
4.2. The Main Problem of Enhancing the Heat Transfer of Dropwise Condensation
4.3. Solutions to Main Problems
4.3.1. Composite Surface Method
4.3.2. Increased Driving Force Promotes Droplet Shedding
5. Conclusions
- (1)
- A series of problems in the process of dropwise condensation restrict the effect of heat transfer. For dropwise condensation, increasing droplet departure rate is the key to further enhancing heat transfer.
- (2)
- The hydrophilicity and hydrophobicity surface have a great influence on the nucleation and departure of droplets, respectively. The effect of enhancing heat transfer of dropwise condensation can be changed by setting the hydrophilicity and hydrophobicity of the surface.
- (3)
- As for theoretical research on dropwise condensation heat transfer, the dropwise condensation heat transfer model established at present is still based on the nucleation sites theory, and the modeling of the surface with droplet migration and the patterned surface is still blank, and further study should continue in the future.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| area of filmwise condensation surface, m2 | |
| area of dropwise condensation surface, m2 | |
| acceleration of gravity, m/s2 | |
| heat transfer coefficient on surface, W/(m2·K) | |
| latent heat of condensation, J/kg1 | |
| interfacial heat transfer coefficient, W/(m2·K) | |
| thermal conductivity of condensation, W/(m2·K) | |
| thermal conductivity of the coating material, W/(m·K) | |
| population density of small drops, m−3 | |
| population density of large drops, m−3 | |
| Number of nucleation sites per unit area m−2 | |
| PTFE | Polytetrafluoroethylene |
| q | heat flux, w/m2 |
| heat flux through the drop, w/m2 | |
| r | Droplet radius, m |
| Minimum droplet radius, m | |
| Effective radius, m | |
| Maximum droplet radius, m | |
| T | temperature, K |
| vapor saturation temperature, K | |
| ΔT | surface subcooling temperature, K |
| ΔRi | the vapor-liquid interfacial resistance, m2·K/W |
| ΔRd | the resistance due to the conduction through the drop, m2·K/W |
| ΔRp | the resistance from the promoter layer, m2·K/W |
| ΔRc | he resistance due to the curvature of the drop of liquid–vapor interface, m2·K/W |
| Greek symbol | |
| angle in Figure 1, deg. | |
| θ | contact angle, deg. |
| receding contact angle, deg. | |
| advancing contact angle, deg. | |
| surface tension, N/m | |
| thickness of promoter layer, m | |
| γ | surface free energy, J/m2 |
| Φ | Heat transfer rate, W |
| Δγ | surface free energy difference, J/m2 |
| density of the condensate, kg/m3 | |
| sweeping period, s | |
| Subscripts | |
| l | liquid |
| s | solid |
| f | surface |
| d | droplet |
References
- Nakhchi, M.; Esfahani, J.A. CFD approach for two-phase CuO nanofluid flow through heat exchangers enhanced by double perforated louvered strip insert. Powder Technol. 2020, 367, 877–888. [Google Scholar] [CrossRef]
- Nakhchi, M.; Esfahani, J. Numerical investigation of heat transfer enhancement inside heat exchanger tubes fitted with perforated hollow cylinders. Int. J. Therm. Sci. 2020, 147, 106153. [Google Scholar] [CrossRef]
- Nakhchi, M.; Hatami, M.; Rahmati, M. A numerical study on the effects of nanoparticles and stair fins on performance improvement of phase change thermal energy storages. Energy 2021, 215, 119112. [Google Scholar] [CrossRef]
- Schmidt, E.; Schurig, W.; Sellschopp, W. Versuche über die Kondensation von Wasserdampf in Film- und Tropfenform. Tech. Mech. Thermodyn. 1930, 1, 53–63. [Google Scholar] [CrossRef]
- Mirkovich, V.V.; Missen, R.W. Non-filmwise condensation of binary vapors of miscible liquids. Can. J. Chem. Eng. 1961, 39, 86–87. [Google Scholar] [CrossRef]
- Ali, H.; Kamran, M.; Imran, S. Condensation heat transfer enhancement using steam-ethanol mixtures on horizontal finned tube. Int. J. Therm. Sci. 2019, 140, 87–95. [Google Scholar] [CrossRef]
- Wang, X.; Yan, S.; Liu, Q. Experiment for drop-wise condensation heat transfer by infrared thermal imager. Chin. J. Space Sci. 2016, 36, 520–524. (In Chinese) [Google Scholar]
- Davies, G.; Mojtehedi, W.; Ponter, A. Measurement of contact angles under condensation conditions. The prediction of dropwise-filmwise transition. Int. J. Heat Mass Transf. 1971, 14, 709–713. [Google Scholar] [CrossRef]
- Preston, D.J.; Mafra, D.L.; Miljkovic, N.; Kong, J.; Wang, E.N. Scalable Graphene Coatings for Enhanced Condensation Heat Transfer. Nano Lett. 2015, 15, 2902–2909. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Chen, J.; Xu, D.; Lin, J. Surface free energy difference criterion for condensation modes. J. Chem. Ind. Eng. 2002, 5, 457–460. (In Chinese) [Google Scholar]
- Jakob, M. Heat transfer in evaporation and condensation II. Mech. Eng. 1936, 58, 729–739. [Google Scholar]
- McCormick, J.L.; Westwater, J.W. Nucleation sites for dropwise condensation. Chem. Eng. Sci. 1965, 20, 1021–1036. [Google Scholar] [CrossRef]
- Mikic, B. On mechanism of dropwise condensation. Int. J. Heat Mass Transf. 1969, 12, 1311–1323. [Google Scholar] [CrossRef]
- McCormick, J.L.; Baer, E. On the mechanism of heat transfer in dropwise condensation. J. Colloid Sci. 1963, 18, 208–216. [Google Scholar] [CrossRef]
- Yongji, S.; Dunqi, X.; Jifang, L.; Siexong, T. A study on the mechanism of dropwise condensation. Int. J. Heat Mass Transf. 1991, 34, 2827–2831. [Google Scholar] [CrossRef]
- Ma, X.; Wang, B.; Xu, D.; Lin, J. Filmwise Condensation Heat Transfer Enhancement with Dropwise and Filmwise Coexisting Condensation Surfaces. J. Chem. Ind. Eng. 1998, 6, 95–102. [Google Scholar]
- Ma, X.; Xu, D.; Lin, J. Condensation heat transfer is enhanced on the surface of droplet membrane coexistence. J. Chem. Ind. Eng. 1999, 4, 535–540. (In Chinese) [Google Scholar]
- Nusselt, W. Die Oberflachenkodensation des Wasserdampfes. Z. VDI 1916, 60, 569–575. [Google Scholar]
- Wen, H.W.; Jer, R.M. On the heat transfer in dropwise condensation. Chem. Eng. J. 1976, 12, 225–231. [Google Scholar] [CrossRef]
- Graham, C.; Griffith, P. Drop size distributions and heat transfer in dropwise condensation. Int. J. Heat Mass Transf. 1973, 16, 337–346. [Google Scholar] [CrossRef]
- Abu-Orabi, M. Modeling of heat transfer in dropwise condensation. Int. J. Heat Mass Transf. 1998, 41, 81–87. [Google Scholar] [CrossRef]
- Kim, S.; Kim, K.J. Dropwise Condensation Modeling Suitable for Superhydrophobic Surfaces. J. Heat Transf. 2011, 133, 081502. [Google Scholar] [CrossRef]
- Hu, H.; Tang, G. Theoretical investigation of stable dropwise condensation heat transfer on a horizontal tube. Appl. Therm. Eng. 2014, 62, 671–679. [Google Scholar] [CrossRef]
- Xie, J.; Xu, J.; Shang, W.; Zhang, K. Dropwise condensation on superhydrophobic nanostructure surface, part II: Mathematical model. Int. J. Heat Mass Transf. 2018, 127, 1170–1187. [Google Scholar] [CrossRef]
- Rose, J. Dropwise condensation theory. Int. J. Heat Mass Transf. 1981, 24, 191–194. [Google Scholar] [CrossRef]
- Miljkovic, N.; Enright, R.; Wang, E.N. Modeling and Optimization of Superhydrophobic Condensation. J. Heat Transf. 2013, 135, 111004. [Google Scholar] [CrossRef]
- Lee, S.; Yoon, H.K.; Kim, K.J.; Kim, S.; Kennedy, M.; Zhang, B.J. A dropwise condensation model using a nano-scale, pin structured surface. Int. J. Heat Mass Transf. 2013, 60, 664–671. [Google Scholar] [CrossRef]
- Xiao, X.; Fan, X. Heat transfer and growth model of droplet on nano-structured surface. Hebei J. Ind. Sci. Technol. 2018, 3, 164–170. (In Chinese) [Google Scholar]
- Bahrami, H.R.T.; Saffari, H. Theoretical study of stable dropwise condensation on an inclined micro/nano-structured tube. Int. J. Refrig. 2017, 75, 141–154. [Google Scholar] [CrossRef]
- Niu, D.; Guo, L.; Hu, H.W.; Tang, G.H. Dropwise condensation heat transfer model considering the liquid-solid interfacel thermal resistance. Int. J. Heat Mass Transf. 2017, 112, 324–333. [Google Scholar] [CrossRef]
- Gose, E.E.; Mucciardi, A.; Baer, E. Model for dropwise condensation on randomly distributed sites. Int. J. Heat Mass Transf. 1967, 10, 15–22. [Google Scholar] [CrossRef]
- Maa, J.R. Drop size distribution and heat flux of dropwise condensation. Chem. Eng. J. 1978, 16, 171–176. [Google Scholar] [CrossRef]
- Ma, X.; Song, T.; Lan, Z.; Zhou, X.; Yang, J. Advances in liquid solid interfacial energy difference effect and condensation heat transfer enhancement. J. Chem. Ind. Eng. 2006, 8, 1763–1775. (In Chinese) [Google Scholar]
- Ma, X.; Lan, Z.; Wang, A.; Wang, M.; Wang, S. Experimental investigation of characteristics of steam dropwise condensation heat transfer at low pressure. J. Eng. Thermophys. 2010, 3, 483–486. (In Chinese) [Google Scholar]
- Erb, R.A. Wettability of Metals under Continuous Condensing Conditions. J. Phys. Chem. 1965, 69, 1306–1309. [Google Scholar] [CrossRef]
- Erb, R.; Thelen, E. PROMOTING PERMANENT DROPWISE CONDENSATION. Ind. Eng. Chem. 1965, 57, 49–52. [Google Scholar] [CrossRef]
- Wilkins, D.G.; Bromley, L.A.; Read, S.M. Dropwise and filmwise condensation of water vapor on gold. AIChE J. 1973, 19, 119–123. [Google Scholar] [CrossRef]
- Holden, K.M.; Wanniarachchi, A.S.; Marto, P.J.; Boone, D.H.; Rose, J.W. The Use of Organic Coatings to Promote Dropwise Condensation of Steam. J. Heat Transf. 1987, 109, 768–774. [Google Scholar] [CrossRef]
- Finnicum, S.S.; Westwater, J. Dropwise vs filmwise condensation of steam on chromium. Int. J. Heat Mass Transf. 1989, 32, 1541–1549. [Google Scholar] [CrossRef]
- Qi, Z.; Dongchang, Z.; Jifang, L. Surface materials with dropwise condensation made by ion implantation technology. Int. J. Heat Mass Transf. 1991, 34, 2833–2835. [Google Scholar] [CrossRef]
- Zhao, Q.; Burnside, B. Dropwise condensation of steam on ion implanted condenser surfaces. Heat Recover. Syst. CHP 1994, 14, 525–534. [Google Scholar] [CrossRef]
- Kim, K.; Lee, Y.; Jeong, J.H. Dropwise condensation induced on chromium ion implanted aluminum surface. Nucl. Eng. Technol. 2019, 51, 84–94. [Google Scholar] [CrossRef]
- Tanasawa, I. Advances in Condensation Heat Transfer. Heat Transfer in Nuclear Reacter Safety 1991, 21, 55–139. [Google Scholar] [CrossRef]
- Ma, X.; Xu, D.; Lin, J. Dropwise condensation on surperthin polymer surface. J. Chem. Ind. Eng. 1993, 2, 165–170. (In Chinese) [Google Scholar]
- Liu, X.; Xu, D. Study of enhancement of condensation heat transfer on Cu-BTA film surface. J. Dalian Univ. Technol. 1996, 2, 162–164. (In Chinese) [Google Scholar]
- Ma, X.; Li, H.; Xu, D. A Study of Dropwise Condensation Heat Transfer on the Superthin Polymer Surfaces. J. Chem. Eng. Chin. Univ. 1993, 4, 309–316. (In Chinese) [Google Scholar]
- Das, A.K.; Kilty, H.P.; Marto, P.J.; Andeen, G.B.; Kumar, A. The Use of an Organic Self-Assembled Monolayer Coating to Promote Dropwise Condensation of Steam on Horizontal Tubes. J. Heat Transf. 1999, 122, 278–286. [Google Scholar] [CrossRef]
- Ma, X.; Xu, D.; Lin, J. Superthin polymer surface for dropwise condensation heat transfer. J. Chem. Ind. Eng. 1993, 3, 277–281. [Google Scholar]
- Paxson, A.T.; Yague, J.L.; Gleason, K.K.; Varanasi, K.K. Stable Dropwise Condensation for Enhancing Heat Transfer via the Initiated Chemical Vapor Deposition (iCVD) of Grafted Polymer Films. Adv. Mater. 2014, 26, 418–423. [Google Scholar] [CrossRef] [Green Version]
- He, P.; Wang, L.; Wang, H. Experimental study on enhancement of condensation heat transfer for surface deposited PTFE. J. Chem. Ind. Eng. 2000, 1, 330–334. (In Chinese) [Google Scholar]
- Yang, C.; Li, Y.; Mei, X. Study on the fabrication of titanium surface texture by nanosecond laser and its wettability. J. Hebei Univ. Sci. Technol. 2016, 37, 315–321. (In Chinese) [Google Scholar]
- Luo, B.; Shum, P.; Zhou, Z.; Li, K. Preparation of hydrophobic surface on steel by patterning using laser ablation process. Surf. Coat. Technol. 2010, 204, 1180–1185. [Google Scholar] [CrossRef]
- Mirhosseini, N.; Crouse, P.L.; Schmidth, M.J.J.; Li, L.; Garrod, D. Laser surface micro-texturing of Ti–6Al–4V substrates for improved cell integration. Appl. Surf. Sci. 2007, 253, 7738–7743. [Google Scholar] [CrossRef]
- Nam, Y.; Ju, Y.S. A comparative study of the morphology and wetting characteristics of micro/nanostructured Cu surfac-es for phase change heat transfer applications. J. Adhes. Sci. Technol. 2013, 27, 2163–2176. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Song, Y. Porous Aligned Carbon Nanotube Films for Ultrahydrophobic Surfaces. Chem. J. Chin. Univ. 2001, 5, 759–761. (In Chinese) [Google Scholar]
- Song, Y.; Ren, X.; Ren, S. Condensation heat transfer of steam on superhydrophobic sufaces. J. Eng. Thermophys. 2007, 1, 95–97. (In Chinese) [Google Scholar]
- Athauda, T.J.; Hari, P.; Ozer, R.R. Tuning Physical and Optical Properties of ZnO Nanowire Arrays Grown on Cotton Fibers. ACS Appl. Mater. Interfaces 2013, 5, 6237–6246. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Wei, M.; Cao, M. Preparation of Superhydrophobic Cu Mesh with Corrosin Resistance and Applications in Oil-water Separation. J. Mater. Eng. 2018, 46, 92–98. (In Chinese) [Google Scholar]
- Torresin, D.; Tiwari, M.K.; del Col, D.; Poulikakos, D. Flow Condensation on Copper-Based Nanotextured Superhydro-phobic Surfaces. Langmuir 2013, 29, 840–848. [Google Scholar] [CrossRef]
- Geng, W.; Hu, A.; Li, M. Super-hydrophilicity to super-hydrophobicity transition of a surface with Ni micro–nano cones array. Appl. Surf. Sci. 2012, 263, 821–824. [Google Scholar] [CrossRef]
- Toma, M.; Loget, G.; Corn, R.M. Flexible Teflon Nanocone Array Surfaces with Tunable Superhydrophobicity for Self-Cleaning and Aqueous Droplet Patterning. ACS Appl. Mater. Interfaces 2014, 6, 11110–11117. [Google Scholar] [CrossRef]
- Fang, X.; Gao, S.; Li, W. Growth and photocatalytic properties of ZnO nanodarrays/p-diamond heterojunction. China Sci. 2015, 10, 2662–2665. (In Chinese) [Google Scholar]
- Pahari, D.; Das, N.; Das, B.; Chattopadhyay, K.K.; Banerjee, D. Tailoring the optical and hydrophobic property of zinc oxide nanorod by coating with amorphous graphene. Phys. E: Low-Dimens. Syst. Nanostructures 2016, 83, 47–55. [Google Scholar] [CrossRef]
- Li, X.; Qi, B.; Miljkovic, N. Experimental study on condensation heat transfer of copper-based nano-structure hydropbobic surfaces. China Sci. 2017, 12, 1220–1224. (In Chinese) [Google Scholar]
- Miljkovic, N.; Enright, R.; Nam, Y.; Lopez, K.; Dou, N.G.; Sack, J.H.; Wang, E.N. Jumping-Droplet-Enhanced Condensation on Scalable Superhydrophobic Nanostructured Surfaces. Nano Lett. 2012, 13, 179–187. [Google Scholar] [CrossRef]
- Masuda, Y.; Ohji, T.; Kato, K. Tin Oxide Nanosheet Assembly for Hydrophobic/Hydrophilic Coating and Cancer Sensing. ACS Appl. Mater. Interfaces 2012, 4, 1666–1674. [Google Scholar] [CrossRef]
- Tarwal, N.; Patil, P. Superhydrophobic and transparent ZnO thin films synthesized by spray pyrolysis technique. Appl. Surf. Sci. 2010, 256, 7451–7456. [Google Scholar] [CrossRef]
- Azimi, G.; Dhiman, R.; Kwon, H.-M.; Paxson, A.T.; Varanasi, K.K. Hydrophobicity of rare-earth oxide ceramics. Nat. Mater. 2013, 12, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, Z.; Zhang, M.; Meng, A. Super-hydrophobic surfaces of SiO2-coated SiC nanowires: Fabrication, mechanism and ultraviolet-durable super-hydrophobicity. J. Colloid Interface Sci. 2015, 444, 33–37. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Wang, K. The super hydrophobicity of ZnO nanorods fabricated by electrochemical deposition method. Appl. Surf. Sci. 2011, 257, 6590–6594. [Google Scholar] [CrossRef]
- Chatterjee, A.; Derby, M.M.; Peles, Y.; Jensen, M.K. Condensation heat transfer on patterned surfaces. Int. J. Heat Mass Transf. 2013, 66, 889–897. [Google Scholar] [CrossRef]
- Baojin, Q.; Li, Z.; Hong, X.; Yan, S. Experimental study on condensation heat transfer of steam on vertical titanium plates with different surface energies. Exp. Therm. Fluid Sci. 2011, 35, 211–218. [Google Scholar] [CrossRef]
- Kim, H.; Nam, Y. Condensation behaviors and resulting heat transfer performance of nano-engineered copper surfaces. Int. J. Heat Mass Transf. 2016, 93, 286–292. [Google Scholar] [CrossRef]
- Miljkovic, N.; Enright, R.; Wang, E.N. Effect of Droplet Morphology on Growth Dynamics and Heat Transfer during Condensation on Superhydrophobic Nanostructured Surfaces. ACS Nano 2012, 6, 1776–1785. [Google Scholar] [CrossRef]
- Yin, L.; Wang, Y.; Ding, J.; Wang, Q.; Chen, Q. Water condensation on superhydrophobic aluminum surfaces with different low-surface-energy coatings. Appl. Surf. Sci. 2012, 258, 4063–4068. [Google Scholar] [CrossRef]
- Anand, S.; Paxson, A.T.; Dhiman, R.; Smith, J.D.; Varanasi, K.K. Enhanced Condensation on Lubricant-Impregnated Nan-otextured Surfaces. ACS Nano 2012, 6, 10122–10129. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Niu, D.; Tang, S. Condensation heat transfer performance of heat transfer tubes with diffenrent wettabilities in presence of a larger amount of noncondensable gas. J. Xi’an Jiaotong Univ. 2015, 49, 30–36. (In Chinese) [Google Scholar]
- Varanasi, K.K.; Hsu, M.; Bhate, N.; Yang, W.; Deng, T. Spatial control in the heterogeneous nucleation of water. Appl. Phys. Lett. 2009, 95, 094101. [Google Scholar] [CrossRef]
- Ma, X.; Du, B.; Hu, S. Effect of contact angle hysteresis on droplet dynamic behaviors for hybrid surface. J. Eng. Thermophys. 2017, 38, 855–861. (In Chinese) [Google Scholar]
- Ma, X.; Liu, X.; Luo, S. Experimental Investigation of mix-vapor condensation with hydrophobic-superhydrophobic hybrid surface on inclined tubes. J. Eng. Thermophys. 2018, 39, 860–865. (In Chinese) [Google Scholar]
- Peng, B.; Lan, Z.; Xu, W. Experimental Investigation of dropwise condensation of steam on hydrophobic-superhydrophobic hybrid surface. J. Eng. Thermophys. 2014, 35, 2036–2040. (In Chinese) [Google Scholar]
- Peng, B.; Ma, X.; Lan, Z. Steam condensation heat transfer enhancement through droplet properties manipulation with hybrid surface. CIESC J. 2015, 66, 3826–3833. (In Chinese) [Google Scholar]
- Zhou, D.; Ji, X.; Dai, C. Steam condensation heat transfer enhancement on superhydropbilic-hydropbobic hybrid vertical surface. J. Mech. Eng. 2018, 54, 182–187. (In Chinese) [Google Scholar] [CrossRef]
- Varanasi, K.K.; Deng, T. Controlling nucleation and growth of water using hybrid hydrophobic-hydrophilic surfaces. In Proceedings of the 2010 12th Ieee Intersociety Conference on Thermal and Thermomechanical Phenomena in Electronic Systems, Las Vegas, NV, USA, 2–5 June 2010; pp. 1–5. [Google Scholar]
- Chen, X.; Wu, J.; Ma, R.; Hua, M.; Koratkar, N.; Yao, S.; Wang, Z. Nanograssed Micropyramidal Architectures for Continu-ous Dropwise Condensation. Adv. Funct. Mater. 2011, 21, 4617–4623. [Google Scholar] [CrossRef]
- Hou, Y.; Yu, M.; Chen, X.; Wang, Z.; Yao, S. Recurrent Filmwise and Dropwise Condensation on a Beetle Mimetic Surface. ACS Nano 2015, 9, 71–81. [Google Scholar] [CrossRef]
- Cheng, J.; Vandadi, A.; Chen, C.-L. Condensation heat transfer on two-tier superhydrophobic surfaces. Appl. Phys. Lett. 2012, 101, 131909. [Google Scholar] [CrossRef]
- Daniel, S.; Chaudhury, M.K.; Chen, J.C. Fast Drop Movements Resulting from the Phase Change on a Gradient Surface. Science 2001, 291, 633–636. [Google Scholar] [CrossRef] [Green Version]
- Boreyko, J.B.; Chen, C.-H. Self-Propelled Dropwise Condensate on Superhydrophobic Surfaces. Phys. Rev. Lett. 2009, 103, 184501. [Google Scholar] [CrossRef] [Green Version]
- Chu, F.; Wu, X.; Zhu, Y. Theoretical model for multidroplet coalescence induced droplet jumping on superhydrophobic surfaces. J. Mech. Eng. 2017, 38, 352–357. (In Chinese) [Google Scholar]
- Li, G.; Alhosani, M.H.; Yuan, S.; Liu, H.; Al Ghaferi, A.; Zhang, T. Microscopic Droplet Formation and Energy Transport Analysis of Condensation on Scalable Superhydrophobic Nanostructured Copper Oxide Surfaces. Langmuir 2014, 30, 14498–14511. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Zhang, Q.; Zeng, X.; Cui, D.; Chen, J.; Li, H.; Wang, J.; Song, Y. Hierarchical Porous Surface for Efficiently Control-ling Microdroplets’ Self-Removal. Adv. Mater. 2013, 25, 2291–2295. [Google Scholar] [CrossRef] [PubMed]
- Miljkovic, N.; Preston, D.J.; Enright, R.; Wang, E.N. Electrostatic charging of jumping droplets. Nat. Commun. 2013, 4, 2517. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Li, J.; Li, L.; Huang, Z.; Wang, F.; Wei, Y. Droplet condensation on superhydrophobic surfaces with enhanced dewetting under a tangential AC electric field. Appl. Phys. Lett. 2016, 109, 161601. [Google Scholar] [CrossRef]
- Zang, D.; Yu, Y.; Chen, Z.; Li, X.; Wu, H.; Geng, X. Acoustic levitation of liquid drops: Dynamics, manipulation and phase transitions. Adv. Colloid Interface Sci. 2017, 243, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Boreyko, J.B.; Baker, C.H.; Poley, C.R.; Chen, C.-H. Wetting and Dewetting Transitions on Hierarchical Superhydrophobic Surfaces. Langmuir 2011, 27, 7502–7509. [Google Scholar] [CrossRef]
- Boreyko, J.B.; Chen, C.-H. Restoring Superhydrophobicity of Lotus Leaves with Vibration-Induced Dewetting. Phys. Rev. Lett. 2009, 103, 174502. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Methods | Materials Used | Manufacturing Process | Advantages | Disadvantages |
|---|---|---|---|---|
| Metallization | Gold. Silver. Palladium. Rhodium (Noble metals) | Electroless plating, Electroplate | Long life. No additional thermal resistance. Simple processing. No pollution | Difficult to maintain continuous dropwise condensation under pure steam. High cost. Difficult to industrialize. Effect decreases after oxidation |
| Chromium | Electroplate. Ion plating | Low cost. Good thermal conductivity | Easy to be oxidized. Difficult to maintain dropwise condensation in pure steam. Electroplating may cause pollution | |
| Ion implantation | Nitrogen. Argon, Helium. Hydrogen. Chromium et al. | Ions bombard surface | Enhanced heat transfer effect is obvious. Easy to industrialize | Will destroy the substrate. Anti-erosion is not guaranteed |
| Organic polymer coating | Benzotriazoles. Polymer of Fluorocarbon. Silica gel. Hexamethyldisiloxane | Vacuum deposition. Spray technology. Plasma polymerization. Self-assembled coating. iCVD | Good thermal stability. Lower surface energy. Anti-erosion. Low cost. Mature technology | Low thermal conductivity. High thermal resistance. Alternate cooling and heating easy to crack off |
| Nanostructure | Reference | Methods | Substrate | Deposition Materials | Contact Angle |
|---|---|---|---|---|---|
| Nanowires (nanoneedles) | [55] | Pyrolysis | Quartz glass | Carbon nanotube | 161° |
| [57] | Chemical deposition | Cotton | ZnO | 156° | |
| [58] | Spray | Copper | carbon nanotubes | 162° | |
| [69] | Chemical treatment | — | SiO2-coated SiC | 153° | |
| Nanocones | [60] | Electro-deposition | Copper | Ni | 153.6° |
| [61] | Oxygen plasma etching | PTFE | Polystyrene | 144° | |
| Nanorods | [57] | Chemical deposition | Cotton | ZnO | 167.5° |
| [63] | Chemical deposition | Glass and silicon | ZnO-a-Gs | 120.8 | |
| [70] | Electro-deposition | Zinc | ZnO | 167 | |
| Nanosheet | [64] | Electro-deposition | Copper | C36H70CuO4 | 130.5° |
| Hydrothermal | Copper | CuO | 124.7° | ||
| [65] | Hydrothermal | Copper | CuO | 172° | |
| [66] | Chemical deposition | Fluorine doped tin oxide | SnO2 | 125° | |
| Nanoblock | [9] | Chemical vapor deposited (CVD) | Copper | Nano graphene particles | 87° |
| [67] | Spray pyrolysis | Glass | ZnO | 154° | |
| [68] | Direct melt | Ceramic | Rare Earth Oxide | 100°–120° |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Yi, Q.; Kong, X.; Wang, J. A Review of Research on Dropwise Condensation Heat Transfer. Appl. Sci. 2021, 11, 1553. https://doi.org/10.3390/app11041553
Hu X, Yi Q, Kong X, Wang J. A Review of Research on Dropwise Condensation Heat Transfer. Applied Sciences. 2021; 11(4):1553. https://doi.org/10.3390/app11041553
Chicago/Turabian StyleHu, Xuechao, Qiujie Yi, Xiangqiang Kong, and Jianwei Wang. 2021. "A Review of Research on Dropwise Condensation Heat Transfer" Applied Sciences 11, no. 4: 1553. https://doi.org/10.3390/app11041553
APA StyleHu, X., Yi, Q., Kong, X., & Wang, J. (2021). A Review of Research on Dropwise Condensation Heat Transfer. Applied Sciences, 11(4), 1553. https://doi.org/10.3390/app11041553






