The Kinetics of Pollutant Removal through Biofiltration from Stormwater Containing Airport De-Icing Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Model
2.2. Analytical Procedures
2.3. Kinetics
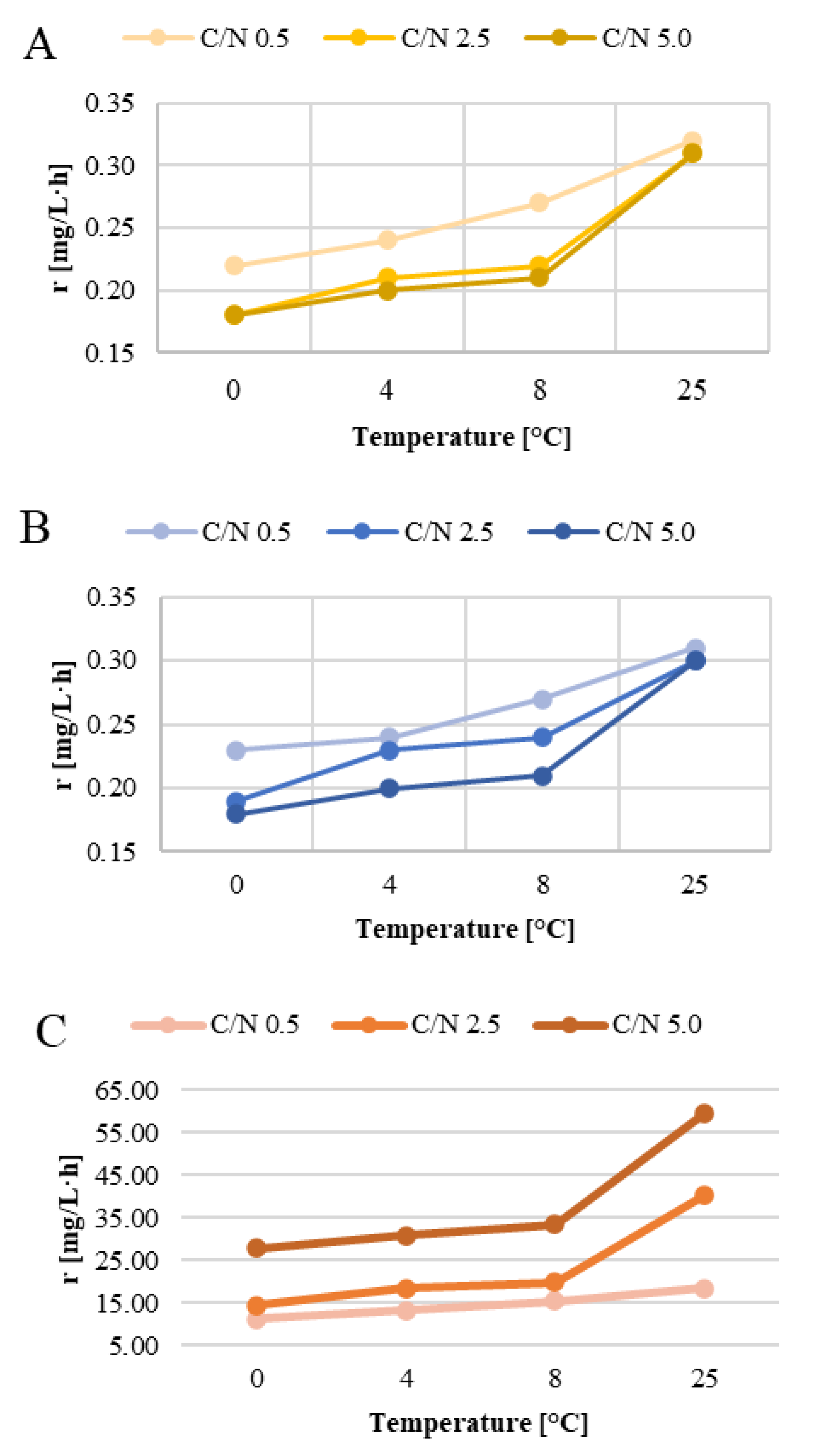
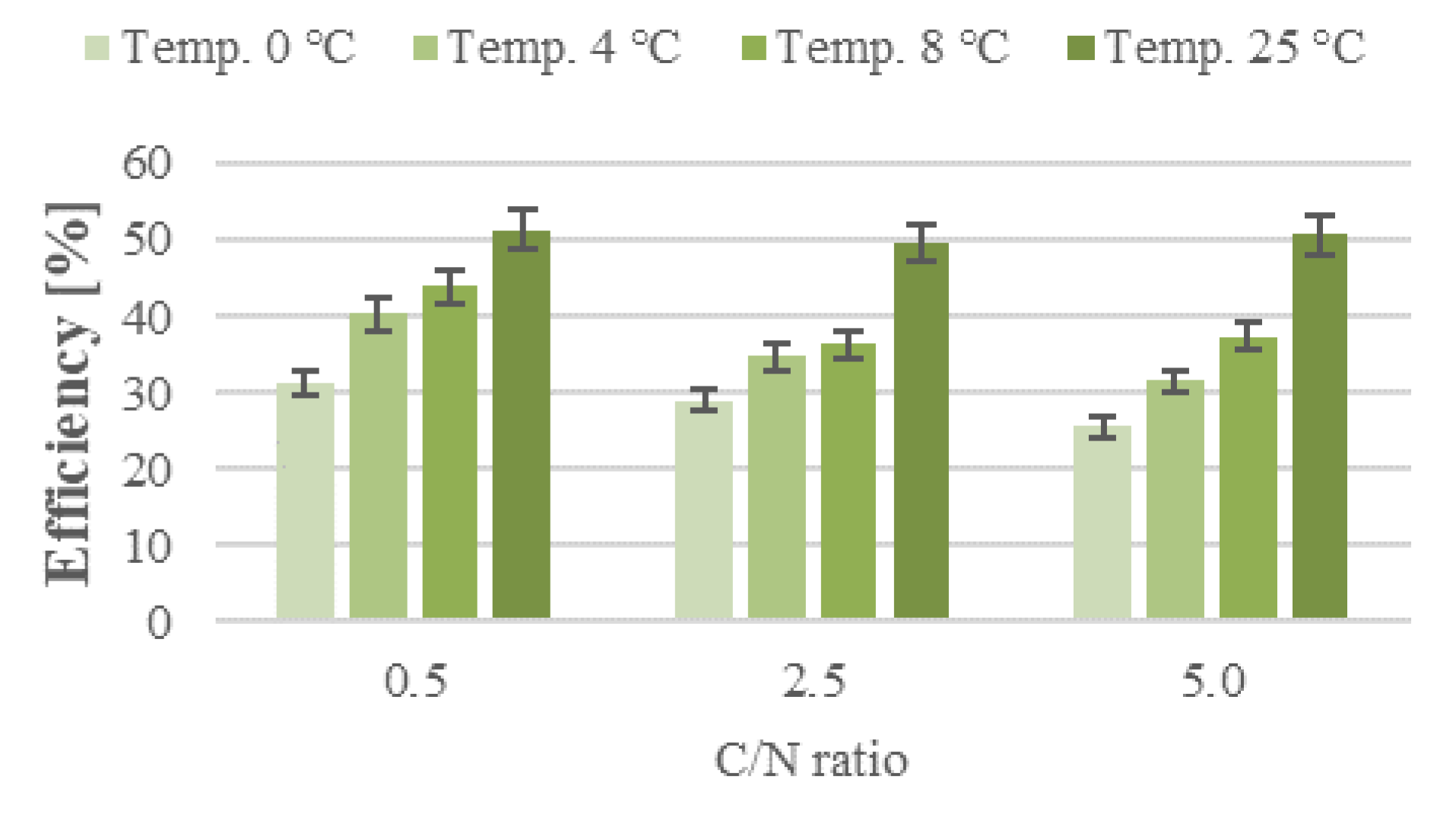
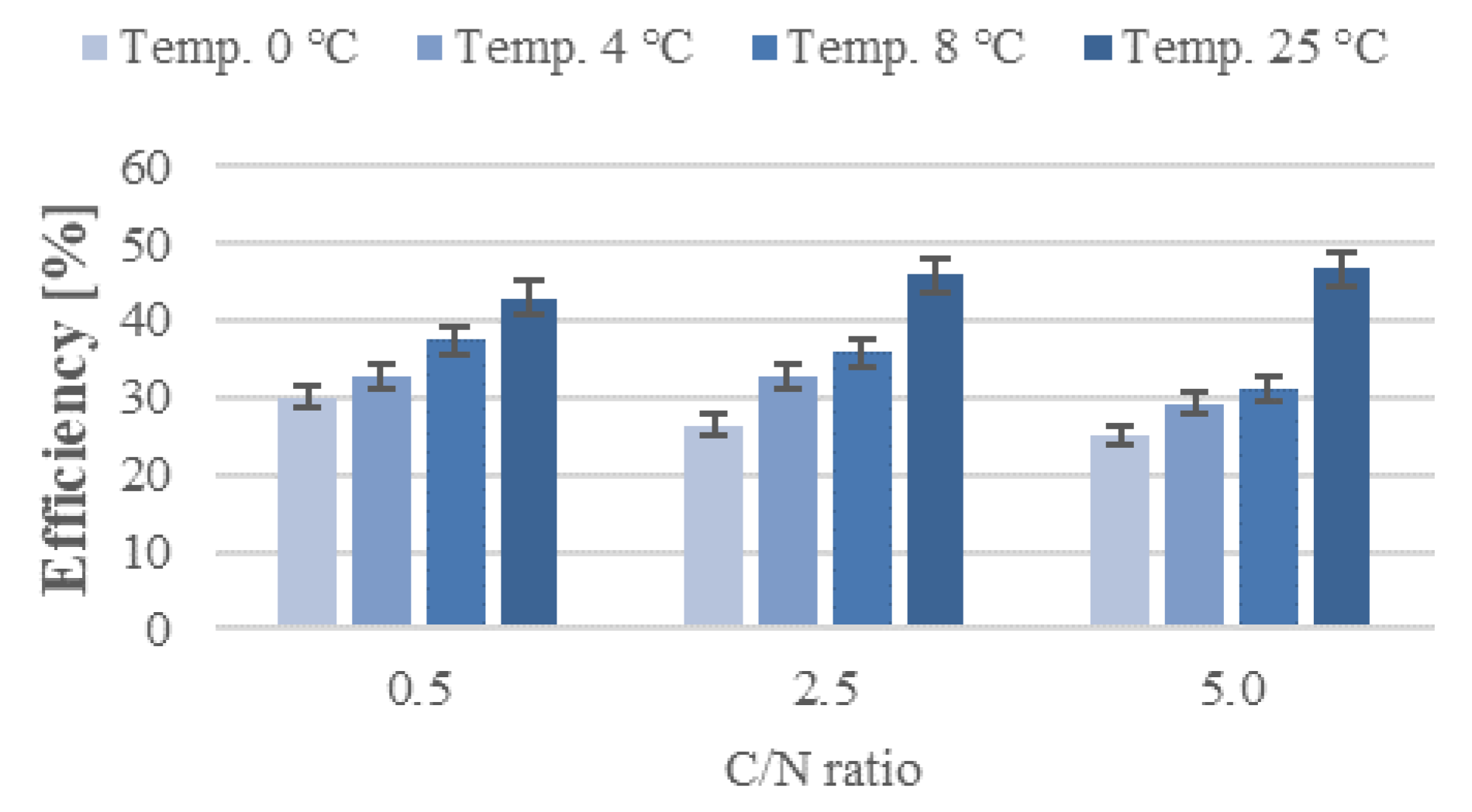
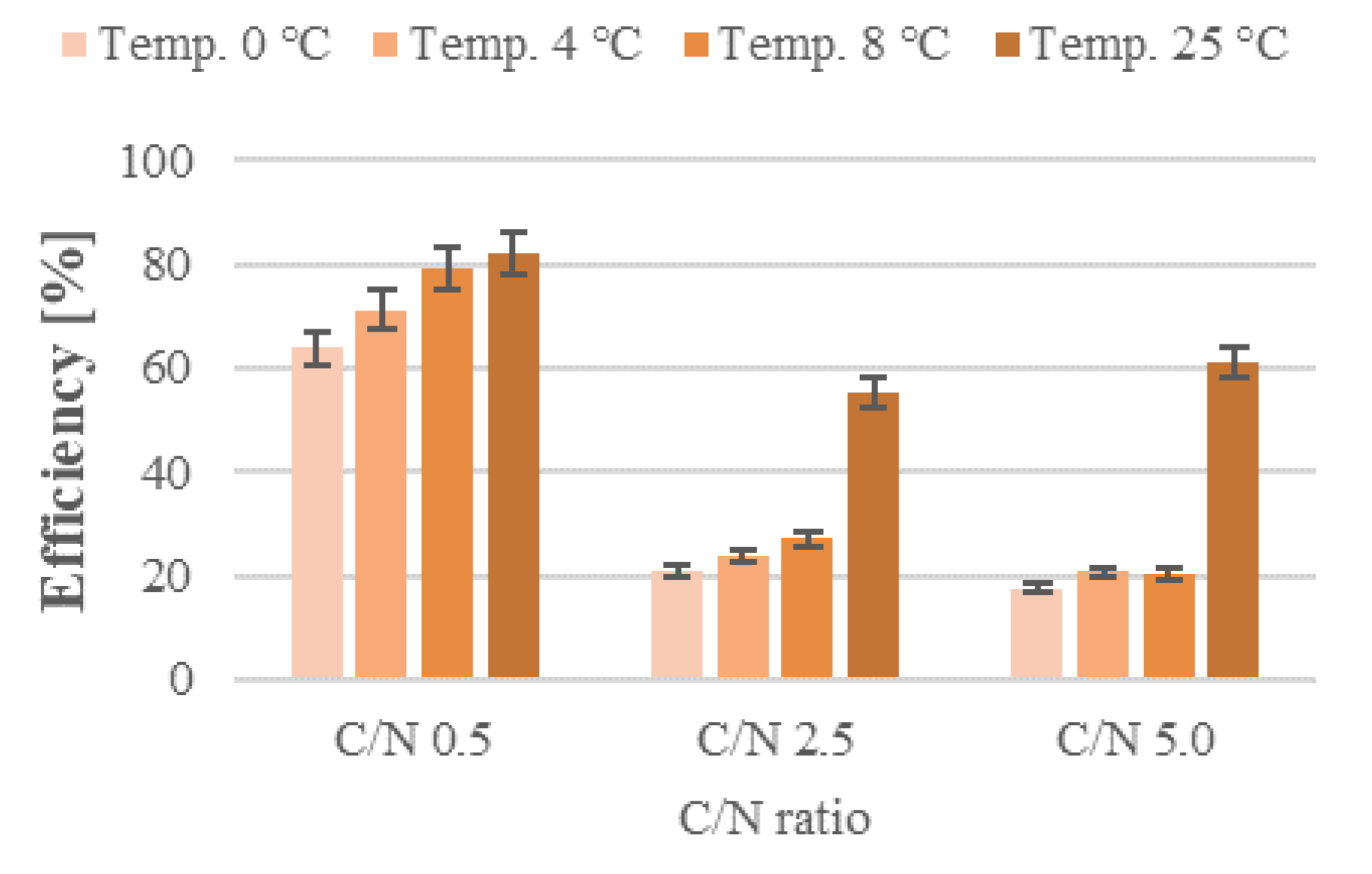
3. Results and Discussion
3.1. Nitrification
3.2. Nitrogen Removal
3.3. Organic Removal Rate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sulej, A.; Polkowska, Z.; Namieśnik, J. Contaminants in airport runoff water in the vicinities of two international airports in Poland. Polish J. Environ. Stud. 2012, 21, 725–739. [Google Scholar]
- Switzenbaum, M.S.; Veltman, S.; Mericas, D.; Wagoner, B.; Schoenberg, T. Best management practices for airport deicing stormwater. Chemosphere 2001, 43, 1051–1062. [Google Scholar] [CrossRef]
- Kinyage, J.P.H.; Pedersen, L.F. Impact of temperature on ammonium and nitrite removal rates in RAS moving bed biofilters. Aquac. Eng. 2016, 75, 51–55. [Google Scholar] [CrossRef]
- Yu, G.; Peng, H.; Fu, Y.; Yan, X.; Du, C.; Chen, H. Enhanced nitrogen removal of low C/N wastewater in constructed wetlands with co-immobilizing solid carbon source and denitrifying bacteria. Bioresour. Technol. 2019, 280, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Rodziewicz, J.; Mielcarek, A.; Janczukowicz, W.; Bryszewski, K.; Ostrowska, K. Treatment of wastewater containing runway de-icing agents in biofilters as a part of airport environment management system. Sustainability 2020, 12, 3608. [Google Scholar] [CrossRef]
- Rodziewicz, J.; Mielcarek, A.; Janczukowicz, W.; Ostrowska, K.; Jóźwiakowski, K.; Bugajski, P.; Jucherski, A. Biofilter with innovative filling for low-temperature treatment of sewage from de-icing airport runways. Sep. Purif. Technol. 2020, 242, 116761. [Google Scholar] [CrossRef]
- Katipoglu-Yazan, T.; Ubay Cokgor, E.; Insel, G.; Orhon, D. Is ammonification the rate limiting step for nitrification kinetics? Bioresour. Technol. 2012, 114, 117–125. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Sahrawat, K.L.; Nakahara, K.; Ishikawa, T.; Kishii, M.; Rao, I.M.; Hash, C.T.; George, T.S.; Srinivasa Rao, P.; Nardi, P.; et al. Biological nitrification inhibition-a novel strategy to regulate nitrification in agricultural systems. In Advances in Agronomy; Elsevier Inc.: Amsterdam, The Netherlands, 2012; Volume 114, pp. 249–302. [Google Scholar]
- Sahrawat, K.L. Factors affecting nitrification in soils. Commun. Soil Sci. Plant Anal. 2008, 39, 1436–1446. [Google Scholar] [CrossRef] [Green Version]
- Ghafari, S.; Hasan, M.; Aroua, M.K. Bio-electrochemical removal of nitrate from water and wastewater-A review. Bioresour. Technol. 2008, 99, 3965–3974. [Google Scholar] [CrossRef] [PubMed]
- Van Rijn, J.; Tal, Y.; Schreier, H.J. Denitrification in recirculating systems: Theory and applications. Aquac. Eng. 2006, 34, 364–376. [Google Scholar] [CrossRef]
- Wang, J.; Chu, L. Biological nitrate removal from water and wastewater by solid-phase denitrification process. Biotechnol. Adv. 2016, 34, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Chen, S. Effects of organic carbon on nitrification rate in fixed film biofilters. Aquac. Eng. 2001, 25, 1–11. [Google Scholar] [CrossRef]
- Rodziewicz, J.; Ostrowska, K.; Janczukowicz, W.; Mielcarek, A. Effectiveness of nitrification and denitrification processes in biofilters treating waste water from de-icing airport runways. Water 2019, 11, 630. [Google Scholar] [CrossRef] [Green Version]
- Rodziewicz, J.; Mielcarek, A.; Janczukowicz, W.; Białowiec, A.; Gotkowska-Płachta, A.; Proniewicz, M. Ammonia Nitrogen Transformations in a Reactor with Aggregate made of Sewage Sludge Combustion Fly Ash. Water Environ. Res. 2016, 88, 715–723. [Google Scholar] [CrossRef]
- Federation, W.E. ; APHA Association. Standard Methods for Examination of Water and Wastewater (Standard Methods for the Examination of Water and Wastewater); American Public Health Association (APHA): Washington, DC, USA, 2012; ISBN 9780875532356. [Google Scholar]
- Mielcarek, A.; Rodziewicz, J.; Janczukowicz, W.; Struk-Sokołowska, J. The impact of biodegradable carbon sources on nutrients removal in post-denitrification biofilm reactors. Sci. Total Environ. 2020, 720, 137377. [Google Scholar] [CrossRef]
- Daija, L.; Selberg, A.; Rikmann, E.; Zekker, I.; Tenno, T.; Tenno, T. The influence of lower temperature, influent fluctuations and long retention time on the performance of an upflow mode laboratory-scale septic tank. Desalin. Water Treat. 2016, 57, 18679–18687. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, S. The impact of temperature on nitrification rate in fixed film biofilters. Aquac. Eng. 2002, 26, 221–237. [Google Scholar] [CrossRef]
- Zhou, H.; Li, X.; Xu, G.; Yu, H. Overview of strategies for enhanced treatment of municipal/domestic wastewater at low temperature. Sci. Total Environ. 2018, 643, 225–237. [Google Scholar] [CrossRef]
- Ge, S.; Wang, S.; Yang, X.; Qiu, S.; Li, B.; Peng, Y. Detection of nitrifiers and evaluation of partial nitrification for wastewater treatment: A review. Chemosphere 2015, 140, 85–98. [Google Scholar] [CrossRef] [PubMed]
- De Prá, M.C.; Kunz, A.; Bortoli, M.; Perondi, T.; Chini, A. Simultaneous removal of TOC and TSS in swine wastewater using the partial nitritation process. J. Chem. Technol. Biotechnol. 2012, 87, 1641–1647. [Google Scholar] [CrossRef]
- Zafarzadeh, A.; Bina, B.; Nikaeen, M.; Attar, H.M.; Khiadani, M.H. Effect of dissolved oxygen and chemical oxygen demand to nitrogen ratios on the partial nitrification/denitrification process in moving bed biofilm reactors. Iran. J. Biotechnol. 2011, 9, 197–205. [Google Scholar]
- Mosquera-Corral, A.; González, F.; Campos, J.L.; Méndez, R. Partial nitrification in a SHARON reactor in the presence of salts and organic carbon compounds. Process Biochem. 2005, 40, 3109–3118. [Google Scholar] [CrossRef]
- Young, B.; Delatolla, R.; Kennedy, K.; Laflamme, E.; Stintzi, A. Low temperature MBBR nitrification: Microbiome analysis. Water Res. 2017, 111, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Peng, Y.; Huang, H.; Wang, S.; Ge, S.; Zhang, J.; Wang, Z. Short- and long-term effects of temperature on partial nitrification in a sequencing batch reactor treating domestic wastewater. J. Hazard. Mater. 2010, 179, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Elgood, Z.; Robertson, W.D.; Schiff, S.L.; Elgood, R. Nitrate removal and greenhouse gas production in a stream-bed denitrifying bioreactor. Ecol. Eng. 2010, 36, 1575–1580. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Reddy, K.R. Temperature Effects in Treatment Wetlands. Water Environ. Res. 2001, 73, 543–557. [Google Scholar] [CrossRef]
- Champagne, P.; Liu, L.; Howell, M. Aerobic Treatment in Cold-Climate Countries. In Current Developments in Biotechnology and Bioengineering: Biological Treatment of Industrial Effluents; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 161–201. ISBN 9780444636768. [Google Scholar]
- Mielcarek, A.; Rodziewicz, J.; Janczukowicz, W.; Dabrowska, D.; Ciesielski, S.; Thornton, A.; Struk-Sokołowska, J. Citric acid application for denitrification process support in biofilm reactor. Chemosphere 2017, 171, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Rodziewicz, J.; Janczukowicz, W.; Mielcarek, A.; Filipkowska, U.; Kłodowska, I.; Ostrowska, K.; Duchniewicz, S. Anaerobic rotating disc batch reactor nutrient removal process enhanced by volatile fatty acid addition. Environ. Technol. 2015, 36, 953–958. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, C.; Rong, H.; Zheng, G.; Zhao, L. The effect of dissolved oxygen concentration (DO) on oxygen diffusion and bacterial community structure in moving bed sequencing batch reactor (MBSBR). Water Res. 2017, 108, 86–94. [Google Scholar] [CrossRef]
- Puznava, N.; Payraudeau, M.; Thornberg, D. Simultaneous nitrification and denitrification in biofilters with real time aeration control. Water Sci. Technol. 2001, 43, 269–276. [Google Scholar] [CrossRef]
- Zinatizadeh, A.A.L.; Ghaytooli, E. Simultaneous nitrogen and carbon removal from wastewater at different operating conditions in a moving bed biofilm reactor (MBBR): Process modeling and optimization. J. Taiwan Inst. Chem. Eng. 2015, 53, 98–111. [Google Scholar] [CrossRef]
- Di Trapani, D.; Christensson, M.; Torregrossa, M.; Viviani, G.; Ødegaard, H. Performance of a hybrid activated sludge/biofilm process for wastewater treatment in a cold climate region: Influence of operating conditions. Biochem. Eng. J. 2013, 77, 214–219. [Google Scholar] [CrossRef]
- Lewandowski, Z.; Boltz, J.P. Biofilms in Water and Wastewater Treatment. In Treatise on Water Science; Elsevier: Amsterdam, The Netherlands, 2011; Volume 4, pp. 529–570. ISBN 9780444531933. [Google Scholar]
- Yao, S.; Ni, J.; Ma, T.; Li, C. Heterotrophic nitrification and aerobic denitrification at low temperature by a newly isolated bacterium, Acinetobacter sp. HA2. Bioresour. Technol. 2013, 139, 80–86. [Google Scholar] [CrossRef] [PubMed]


| C/N Ratio | |||
|---|---|---|---|
| 0.5 | 2.5 | 5.0 | |
| CH4N2O (urea) [mg/L] | 150.00 ± 0.10 | 150.00 ± 0.10 | 150.00 ± 0.10 |
| HCOONa (sodium formate) [mg/L] | 136.00 ± 0.10 | 657.00 ± 0.10 | 1326.00 ± 0.10 |
| CH3COOK (potassium acetate) [mg/L] | 49.00 ± 0.10 | 237.00 ± 0.10 | 478.00 ± 0.10 |
| Parameter | |||
| Nog. [mgN/L] | 71.56 ± 2.20 | 71.56 ± 2.20 | 71.56 ± 2.20 |
| N Kjeldhal [mgN/L] | 70.80 ± 3.02 | 70.80 ± 3.02 | 70.80 ± 3.02 |
| COD [mg/L] | 100.66 ± 1.34 | 386.80 ± 1.94 | 765.50 ± 2.90 |
| pH | 7.47–8.03 | 7.47–8.03 | 7.47–8.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mielcarek, A.; Rodziewicz, J.; Janczukowicz, W.; Ostrowska, K. The Kinetics of Pollutant Removal through Biofiltration from Stormwater Containing Airport De-Icing Agents. Appl. Sci. 2021, 11, 1724. https://doi.org/10.3390/app11041724
Mielcarek A, Rodziewicz J, Janczukowicz W, Ostrowska K. The Kinetics of Pollutant Removal through Biofiltration from Stormwater Containing Airport De-Icing Agents. Applied Sciences. 2021; 11(4):1724. https://doi.org/10.3390/app11041724
Chicago/Turabian StyleMielcarek, Artur, Joanna Rodziewicz, Wojciech Janczukowicz, and Kamila Ostrowska. 2021. "The Kinetics of Pollutant Removal through Biofiltration from Stormwater Containing Airport De-Icing Agents" Applied Sciences 11, no. 4: 1724. https://doi.org/10.3390/app11041724
APA StyleMielcarek, A., Rodziewicz, J., Janczukowicz, W., & Ostrowska, K. (2021). The Kinetics of Pollutant Removal through Biofiltration from Stormwater Containing Airport De-Icing Agents. Applied Sciences, 11(4), 1724. https://doi.org/10.3390/app11041724









